COVID-19 Pandemic: Prevention and Protection Measures to Be Adopted at the Workplace
Abstract
1. Introduction
2. Materials and Methods
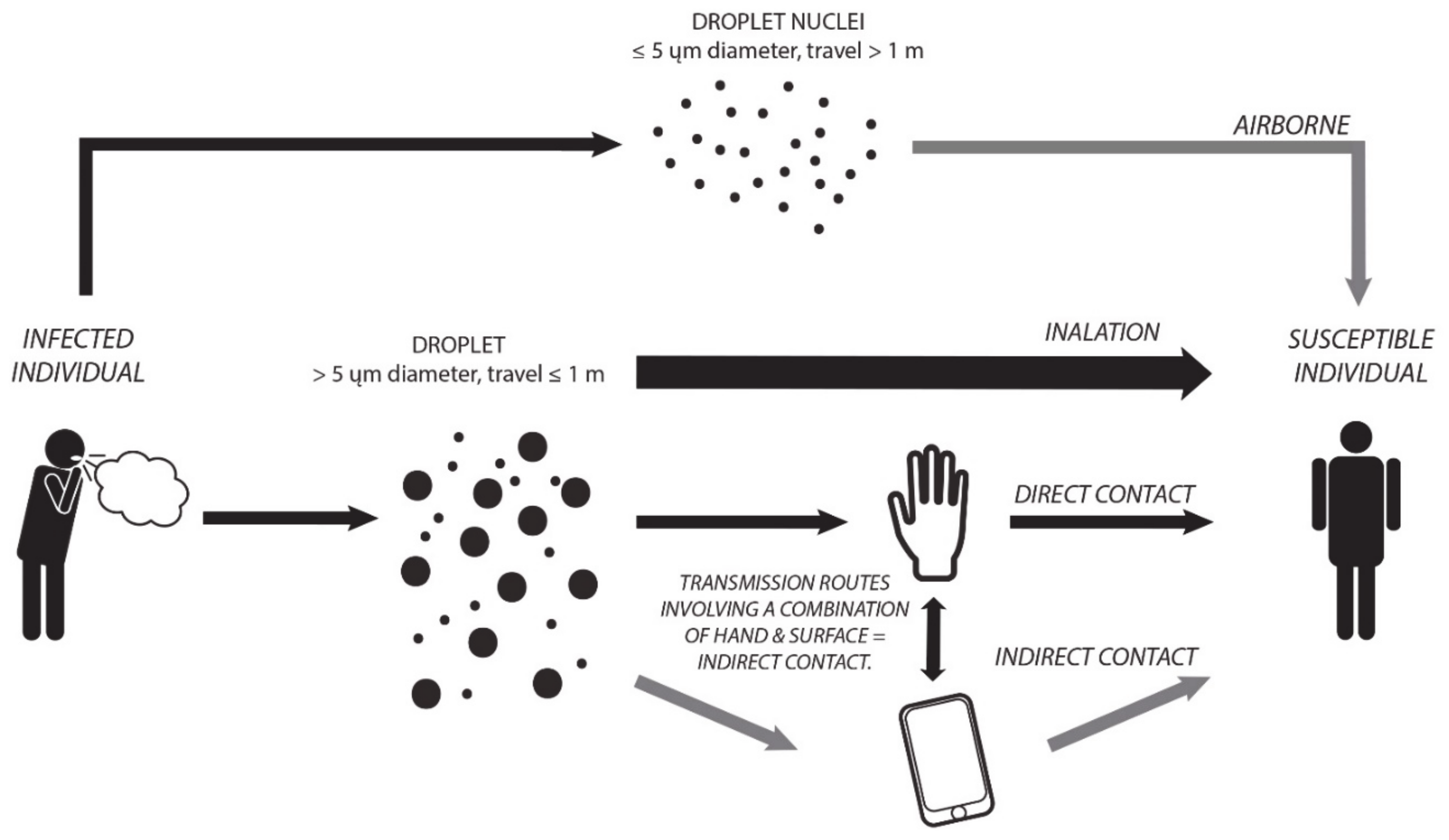
2.1. Transmission
2.2. Epidemiology
2.3. Testing and Pharmacological Approach
3. Prevention and Protection
3.1. Organizational Measures
- Blocking all trips to and from all areas defined as “red”, in which cases of COVID-19 infections have already been ascertained.
- Possible 14-day home quarantine for those who live, work or return from these areas.
- Selective control and measurement of body temperature of all suppliers and external collaborators.
- Reduction of the number of operators within each confined environment.
- Prioritize, where possible, work from home (smart working).
- Composing, if possible, two or more closed and independent working groups, to be alternated every 14 days to work in the company or in smart working.
- Predisposition and maximum adherence to PPE dressing and undressing protocols.
3.2. Environmental Measures
- Reduce direct physical contact (for example, shake hands);
- Avoid direct unprotected contact with secretions (esp. coughing, touching used paper tissues with bare hands);
- Avoid direct contact within 2 m and >15 min;
3.3. Personal Measures
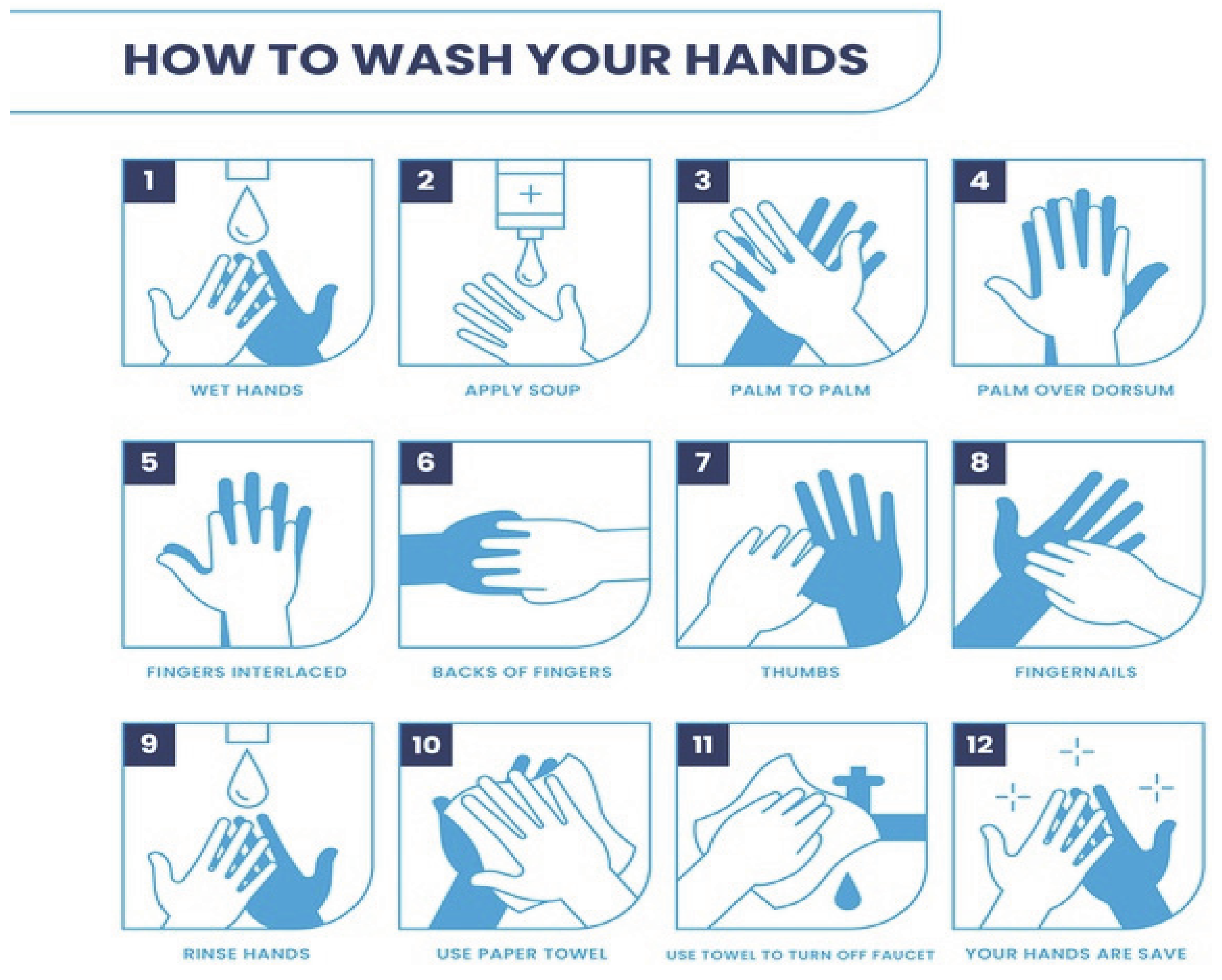
3.3.1. Hand Washing
- Before starting work, especially if this involves contact with the public;
- Frequently during the work shift, especially after contact with other staff or customers;
- After contact with secretions, excretions, biological liquids;
- After contact with potentially contaminated objects (gloves, clothing, masks, used tissues, waste);
- Immediately after removing gloves and other protective equipment.
3.3.2. Personal Protective Equipment (PPE)
Gloves
- Must be clean gloves and they must cover the wrist well;
- Must be removed immediately after completing the procedures that they were used for; in particular, great care must be taken not to touch clean surfaces with contaminated gloves;
- Must be absolutely changed if dirty or not perfectly intact;
- Glove decontamination prior to glove removal with hypochlorite [60], after every contact with different inanimate surface, and during doffing procedures;
- Must not be reused or washed.
Disposable Masks/Respirators
Filter Masks
Disposable Surgical Masks (Facemasks)
- For those who work in contact with subjects with suspected airborne disease (flu syndrome, chicken pox, measles);
- In activities for which there is the possibility of generating splatters or splashes of blood or other body fluids;
- In technical and administrative support activities;
- By doctors, nurses, biologists, midwives and all healthcare personnel;
- By the staff of contracting firms (e.g., cleaning);
- By public assistance staff.
- Read the manufacturer’s instructions to verify the correct adhesion of the facial filter;
- Wear the mask, taking care that there are no structural alterations;
- Check adhesion by exhaling (if the mask has an exhalation valve) or inhaling (if the mask does not have an exhalation valve), thus checking for any abnormal passage of air.
Safety Goggles and Splash Guard Visor
- Removing the gloves by rolling them down from the wrist, without touching the skin;
- Removing the protective clothing, being careful to fold it with the contaminated external part inside and disposing of it in a container with a lid;
- Hand washing;
- Removing the safety goggles or the splash guard goggles;
- Removing the facemask/respirator, taking care to touch only the strings and not the contaminated surface, and disposing of it in a container with a lid.
- Avoid close contact with the sick person; if possible, accompany the subject in the isolation room especially prepared;
- If available, provide the person with a surgical facemask and disposable nitrile gloves which he must put on by himself;
- Wash your hands thoroughly;
- Pay particular attention to the body surfaces that have possibly come into contact with the patient’s fluids (respiratory secretions, urine, faeces);
- Have the subject eliminate, directly in a waterproof bag, both the masks and the gloves, as well as any used paper tissues. The bag will then be disposed of in an appropriate manner, together with the infected materials used during the medical assistance procedure offered by the healthcare personnel.
Isolation Gowns
4. Discussion
- Companies with a low probability level of infection spreading:
- ○
- Located in areas where there are no reported cases of disease contamination in the entire province;
- ○
- With a maximum of 10 employees;
- ○
- Which mainly carry out office activities with a limited flow of customers.
- Companies with a medium probability level of infection spreading:
- ○
- Located in areas where there are reported cases of disease contamination in the province;
- ○
- With a maximum number of 50 employees;
- ○
- Which mainly carry out commercial activities;
- ○
- Which expose employees to sporadic contact with customers.
- Companies with a high probability level of infection spreading:
- ○
- Located in areas in which in the neighboring cities or in the same city of the workplace, there are clear cases of disease contamination;
- ○
- With a maximum number of over 50 employees;
- ○
- Which carry out front-office activities in continuous contact with customers;
- ○
- With travelling staff;
- ○
- Which operate in the health sector.
- Companies with a very high probability level of infection spreading
- ○
- Very-high-exposure-risk jobs are those with high potential for exposure to known or suspected sources of COVID-19 during specific medical, post-mortem, or laboratory procedures. Workers in this category include:
- ■
- Healthcare workers (doctors, nurses, dentists, paramedics, emergency medical technicians) performing aerosol-generating procedures (e.g., intubation, cough induction procedures, bronchoscopies, some dental procedures and exams, or invasive specimen collection) on known or suspected COVID-19 patients.
- ■
- Healthcare or laboratory personnel collecting or handling specimens from known or suspected COVID-19 patients (manipulating cultures from known or suspected COVID-19 patients).
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yin, Y.; Wunderink, R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology 2017, 23, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.S.E.I.A.; Madani, T.A. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020, 91, 264–266. [Google Scholar] [CrossRef]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.; Lau, E.H.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Phelan, A.L.; Katz, R.; Gostin, L.O. The Novel Coronavirus Originating in Wuhan, China. JAMA 2020, 323, 709. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Harding, A.; Lanese, N. The 12 Deadliest Viruses on Earth. Marzo 2020. Articolo Pubblicato su “Livescience”. Available online: https://www.livescience.com/56598-deadliest-viruses-on-earth.html#xenforo-comments-1182. (accessed on 10 March 2020).
- Guo, Y.; Cao, Q.; Hong, Z.; Tan, Y.; Chen, S.; Jin, H.; Tan, K.; Wang, D. The origin Yan Yan, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—An update on the status. Mil. Med. Res. 2020, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Li, Q.; Wu, J.; Zhao, C.; Hao, H.; Liu, H.; Zhang, L.; Nie, L.; Qin, H.; Wang, M.; et al. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. J. Emerg. Microbes Infect. 2020, 9, 680–686. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, W.; Zhu, J.; Li, B.; Xing, J.; Liao, M.; Qi, W. Insights into the cross-species evolution of 2019 novel coronavirus. J. Infect. 2020. [Google Scholar] [CrossRef]
- Ceraolo, C.; Giorgi, F.M. Genomic variance of the 2019-nCoV coronavirus. J. Med. Virol. 2020, 92, 522–528. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Zhang, M.Q.; Wang, X.H.; Chen, Y.L.; Zhao, K.L.; Cai, Y.Q.; An, C.L.; Lin, M.G.; Mu, X.D. Clinical features of 2019 novel coronavirus pneumonia in the early stage from a fever clinic in Beijing. Chin. J. Tuberc. Respir. Dis. 2020, 43, 13. [Google Scholar]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Cannizzaro, E.; Ramaci, T.; Cirrincione, L.; Plescia, F. Work-Related Stress, Physio-Pathological Mechanisms, and the Influence of Environmental Genetic Factors. Int. J. Environ. Res. Public Health 2019, 16, 4031. [Google Scholar] [CrossRef]
- Cannizzaro, E.; Plescia, F.; Cirrincione, L.; Lo Pinto, E.; Plescia, F. Sport for job. differences in cortisol levels in a water polo team at different times of workout. Euromediterranean Biomed. J. 2018, 13, 181–184. [Google Scholar]
- Cannizzaro, E.; Cirrincione, L.; Mazzucco, W.; Scorciapino, A.; Catalano, C.; Ramaci, T.; Ledda, C.; Plescia, F. Night-Time Shift Work and Related Stress Responses: A Study on Security Guards. Int. J. Environ. Res. Public Health 2020, 17, 562. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.R.; Cao, Q.D.; Hong, Z.S.; Tan, Y.Y.; Chen, S.D.; Jin, H.J.; Tan, K.S.; Wang, D.J.; Yan, Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—An update on the status. Mil. Med. Res. 2020, 7, 1–10. [Google Scholar] [CrossRef]
- WHO Novel Coronavirus (2019-nCoV); Situation Report—12; WHO: Geneva, Switzerland, 2020.
- Singhal, T. A Review of Coronavirus Disease-2019 (COVID-19). Indian J. Pediatr. 2020, 87, 281–286. [Google Scholar] [CrossRef]
- Tellier, R.; Li, Y.; Cowling, B.J.; Tang, J.W. Recognition of aerosol transmission of infectious agents: A commentary. BMC Infect. Dis. 2019, 19, 101. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.; Gamble, A.; Williamson, B.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.; et al. Aerosol and surface stability of HCoV-19 (SARS-CoV-2) compared to SARS-CoV-1. N. Eng. J. Med. 2020. [Google Scholar] [CrossRef]
- Peng, X.; Xu, X.; Li, Y.; Cheng, L.; Zhou, X.; Ren, B. Transmission routes of 2019-nCoV and controls in dental practice. Int. J. Oral Sci. 2020, 12, 1–6. [Google Scholar] [CrossRef]
- WHO Coronavirus Disease 2019 (COVID-19); Situation Report; WHO: Geneva, Switzerland, 2020.
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Zhonghua, L.; Xing, B.; Xue, Z.Z. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Chin. J. Epidemiol. 2020, 41, 145–151. [Google Scholar]
- World Health Organization. Laboratory Testing for Coronavirus Disease (COVID-19) in Suspected Human Cases. Interim guidance 19 March 2020. Available online: https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117 (accessed on 20 March 2020).
- LabCorp COVID-19 RT-PCR test EUA Summary. Accelerated Emergency Use Authorization (EUA) Summary COVID-19 RT-PCR Test (Laboratory Corporation of America). Available online: www.fda.gov (accessed on 20 March 2020).
- Won, J.; Lee, S.; Park, M.; Kim, T.Y.; Park, M.G.; Choi, B.Y.; Kim, D.; Chang, H.; Kim, V.N.; Lee, V.N.K.A.C.J. Development of a Laboratory-safe and Low-cost Detection Protocol for SARS-CoV-2 of the Coronavirus Disease 2019 (COVID-19). Exp. Neurobiol. 2020. [Google Scholar] [CrossRef]
- Agostini, M.L.; Andres, E.L.; Sims, A.C.; Graham, R.L.; Sheahan, T.P.; Lu, X.; Smith, E.C.; Case, J.B.; Feng, J.Y.; Jordan, R.; et al. Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. mBio 2018, 9, e00221-18. [Google Scholar] [CrossRef] [PubMed]
- Warren, T.K.; Jordan, R.; Lo, M.K.; Ray, A.S.; Mackman, R.L.; Soloveva, V.; Siegel, D.; Perron, M.; Bannister, R.; Hui, H.C.; et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 2016, 531, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Sheahan, T.P.; Sims, A.C.; Leist, S.R.; Schäfer, A.; Won, J.; Brown, A.J.; Montgomery, S.A.; Hogg, A.; Babusis, D.; Clarke, M.O.; et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020, 11, 222–314. [Google Scholar] [CrossRef]
- Siegel, D.; Hui, H.C.; Doerffler, E.; Clarke, M.O.; Chun, K.; Zhang, L.; Neville, S.; Carra, E.; Lew, W.; Ross, B.; et al. Discovery and Synthesis of a Phosphoramidate Prodrug of a Pyrrolo[2,1-f][triazin-4-amino] Adenine C-Nucleoside (GS-5734) for the Treatment of Ebola and Emerging Viruses. J. Med. Chem. 2017, 60, 1648–1661. [Google Scholar] [CrossRef]
- Lo, M.K.; Jordan, R.; Arvey, A.; Sudhamsu, J.; Shrivastava-Ranjan, P.; Hotard, A.L.; Flint, M.; McMullan, L.K.; Siegel, D.; Clarke, M.O.; et al. GS-5734 and its parent nucleoside analog inhibit Filo-, Pneumo-, and Paramyxoviruses. Sci. Rep. 2017, 7, 43395. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Gordon, C.J.; Tchesnokov, E.P.; Feng, J.Y.; Porter, D.P.; Gotte, M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J. Biol. Chem. 2020, 295, 4773–4779. [Google Scholar] [CrossRef]
- Ye, X.T.; Luo, Y.L.; Xia, S.C.; Sun, Q.-F.; Ding, J.-G.; Zhou, Y.; Chen, W.; Wang, X.-F.; Zhang, W.W.; Du, W.J.; et al. Clinical efficacy of lopinavir/ritonavir in the treatment of Coronavirus disease 2019. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3390–3396. [Google Scholar] [CrossRef] [PubMed]
- Sheahan, T.P.; Sims, A.C.; Graham, R.L.; Menachery, V.D.; Gralinski, L.E.; Case, J.B.; Leist, S.R.; Pyrc, K.; Feng, J.Y.; Trantcheva, I.; et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017, 9, eaal3653. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Zhang, Q.; Xiong, T. The treatment of malaria. N. Engl. J. Med. 1996, 335, 800–806. [Google Scholar]
- Wu, K.; Zhang, Q.; Wu, X.; Lu, W.; Tang, H.; Liang, Z.; Gu, Y.; Song, S.; Ayon, R.J.; Wang, J.; et al. Chloroquine is a potent pulmonary vasodilator that attenuates hypoxia-induced pulmonary hypertension. Br. J. Pharmacol. 2017, 174, 4155–4172. [Google Scholar] [CrossRef]
- Gao, J.; Tian, Z.; Yang, X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends 2020, 14, 72–73. [Google Scholar] [CrossRef]
- Yao, X.; Ye, F.; Zhang, M.; Cui, C.; Huang, B.; Niu, P.; Liu, X.; Zhao, L.; Dong, E.; Song, C.; et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Furuta, Y.; Komeno, T.; Nakamura, T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 449–463. [Google Scholar] [CrossRef]
- Xu, X.; Han, M.; Li, T.; Sun, W.; Wang, D.; Fu, B.; Zhou, Y.; Zheng, X.; Yang, Y.; Li, X.; et al. Effective Treatment of Severe COVID-19 Patients with Tocilizumab. ChinaXiv Prepr. 2020. [Google Scholar]
- Wang, C.; Li, W.; Drabek, D.; Okba, N.M.A.; Van Haperen, R.; Osterhaus, A.D.; Van Kuppeveld, F.J.; Haagmans, B.L.; Grosveld, F.; Bosch, B.J. A Human Monoclonal Antibody Blocking SARS-CoV-2 Infection; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 2020. [Google Scholar]
- Batlle, D.; Wysocki, J.; Satchell, K. Soluble angiotensin-converting enzyme 2: A potential approach for coronavirus infection therapy? Clin. Sci. 2020, 134, 543–545. [Google Scholar] [CrossRef]
- Du, L.; He, Y.; Zhou, Y.; Liu, S.; Zheng, B.J.; Jiang, S. The spike protein of sars-cov–a target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009, 7, 226–236. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Z.; Wang, Y.; Zhou, Y.; Ma, Y.; Zuo, W. Single-cell rna expression profiling of ace2, the putative receptor of wuhan 2019-ncov. medRxiv 2020, 2, 11. [Google Scholar]
- Imai, Y.; Kuba, K.; Ohto-Nakanishi, T.; Penninger, J.M. Angiotensin-converting enzyme 2 (ACE2) in disease pathogenesis. Circ. J. 2010, 74, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, J.; Schulze, A.; Batlle, D. Novel Variants of Angiotensin Converting Enzyme-2 of Shorter Molecular Size to Target the Kidney Renin Angiotensin System. Biomology 2019, 9, 886. [Google Scholar] [CrossRef] [PubMed]
- Consiglio, d.M. Comunicato Stampa Consiglio dei Ministrin 31. Febbraio 2020. Articolo Pubblicato su “Governo.it”. Available online: http://www.governo.it/it/articolo/comunicato-stampa-del-consiglio-dei-ministri-n-31/14163 (accessed on 1 March 2020).
- La Sindrome Acuta Respiratoria Severa–Sars Raccomandazioni Per La Prevenzione E Il Controllo. 2003. Articolo Pubblicato su: “Epicentro”. Available online: https://www.epicentro.iss.it/territorio/sars/Documento%20SARS.pdf (accessed on 27 February 2020).
- Sun, Z.; Thilakavathy, K.; Kumar, S.; He, G.; Liu, S. Potential Factors Influencing Repeated SARS Outbreaks in China. Int. J. Environ. Res. Public Health 2020, 17, 1633. [Google Scholar] [CrossRef] [PubMed]
- Otter, J.; Donskey, C.; Yezli, S.; Douthwaite, S.; Goldenberg, S.D.; Weber, D. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: The possible role of dry surface contamination. J. Hosp. Infect. 2016, 92, 235–250. [Google Scholar] [CrossRef]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef]
- Walker, C.M.; Ko, G. Effect of Ultraviolet Germicidal Irradiation on Viral Aerosols. Environ. Sci. Technol. 2007, 41, 5460–5465. [Google Scholar] [CrossRef]
- Chan, K.H.; Peiris, J.S.M.; Lam, S.Y.; Poon, L.L.M.; Yuen, K.-Y.; Seto, W.H. The Effects of Temperature and Relative Humidity on the Viability of the SARS Coronavirus. Adv. Virol. 2011, 2011, 1–7. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control An agency of the European Union. Case Definition and European Surveillance for COVID-19, as of 2 March 2020. Available online: https://www.ecdc.europa.eu/en/case-definition-and-european-surveillance-human-infection-novel-coronavirus-2019-ncov (accessed on 15 March 2020).
- Direzione Generale Della Prevenzione Sanitaria. FAQ—Covid-19, Domande e Risposte. Marzo 2020. Articolo Pubblicato su “Governo.it”. Available online: http://www.salute.gov.it/portale/malattieInfettive/dettaglioFaqMalattieInfettive.jsp?lingua=italiano&id=228 (accessed on 15 March 2020).
- Norm EN 374 2016. Available online: https://www.uni3servizi.it/2019/07/23/en-iso-374-1-2016/ (accessed on 3 March 2020).
- Casanova, L.M.; Teal, L.J.; Sickbert-Bennett, E.E.; Anderson, D.J.; Sexton, D.J.; Rutala, W.A.; Weber, D.J.; Program, T.C.P.E. Assessment of Self-Contamination During Removal of Personal Protective Equipment for Ebola Patient Care. Infect. Control Hosp. Epidemiol. 2016, 37, 1156–1161. [Google Scholar] [CrossRef]
- Shiu, E.Y.; Leung, N.H.L.; Cowling, B.J.; Tada, H.; Nohara, A.; Kawashiri, M.-A. Controversy around airborne versus droplet transmission of respiratory viruses. Curr. Opin. Infect. Dis. 2019, 32, 372–379. [Google Scholar] [CrossRef]
- Lee, S.-A.; Grinshpun, S.A.; Reponen, T. Respiratory Performance Offered by N95 Respirators and Surgical Masks: Human Subject Evaluation with NaCl Aerosol Representing Bacterial and Viral Particle Size Range. Ann. Occup. Hyg. 2008, 52, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Chellamani, K.P.; Veerasubramanian, D.; Vignesh Balaji, R.S. Surgical Face Masks: Manufacturing Methods and Classification. J. Acad. Ind. Res. 2013, 2, 6. [Google Scholar]
- Booth, C.M.; Clayton, M.; Crook, B.; Gawn, J. Effectiveness of surgical masks against influenza bioaerosols. J. Hosp. Infect. 2013, 84, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Van Der Sande, M.A.B.; Teunis, P.; Sabel, R. Professional and Home-Made Face Masks Reduce Exposure to Respiratory Infections among the General Population. PLoS ONE 2008, 3. [Google Scholar] [CrossRef]
- Sui Huang COVID-19: Why We Should All Wear Masks—There Is New Sciencific Rational. 2020. Available online: https://medium.com/@Cancerwarrior/covid-19-why-we-should-all-wear-masks-there-is-new-scientific-rationale-280e08ceee71 (accessed on 10 March 2020).
- Norm EN 166. 2004. Available online: https://www.univet.it/app/media/Marcature.pdf (accessed on 25 February 2020).
- Kilinc, F.S. A Review of Isolation Gowns in Healthcare: Fabric and Gown Properties. J. Eng. Fibers Fabr. 2015, 10, 180–190. [Google Scholar] [CrossRef]
- Ravanelli, A.; Di Lorenzo, F.; Aguzzi, I. Valutazione del Rischio Biologico. Relazione Sulla Valutazione del Rischio Biologico Correlato All’Improvvisa Emergenza Legata alla Diffusione del Virus SARS-CoV-2 (Cosiddetto ‘Coronavirus’) Causa della Malattia Covid-19 (Art. 271 del D.Lgs. 9 Aprile 2008, n. 81 e s.m.i.). 2020. Articolo pubblicato su “punto sicuro”. Available online: https://www.puntosicuro.it/sicurezza-sul-lavoro-C-1/tipologie-di-contenuto-C-6/valutazione-dei-rischi-C-59/come-fare-la-valutazione-dei-rischi-relativi-al-nuovo-coronavirus-AR-19858/ (accessed on 8 March 2020).
- OSHA. Guidance on Preparing Workplaces for COVID-19; OSHA: Washington, DC, USA, 2020. [Google Scholar]
- Deng, S.-Q.; Peng, H.-J. Characteristics of and Public Health Responses to the Coronavirus Disease 2019 Outbreak in China. J. Clin. Med. 2020, 9, 575. [Google Scholar] [CrossRef]
- WHO Novel Coronavirus (2019-nCoV); Situation Report—73 2; WHO: Geneva, Switzerland, 2020.
- Verbeek, J.H.; Rajamaki, B.; Ijaz, S.; Sauni, R.; Toomey, E.; Blackwood, B.; Tikka, C.; Ruotsalainen, J.H.; Balci, F.S.K. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database Syst. Rev. 2020, 4, CD011621. [Google Scholar] [CrossRef]
| LESS LETHAL KNOWN CORONAVIRUSES | MOST LETHAL KNOWN CORONAVIRUSES |
|---|---|
| 229E (the alpha coronavirus) | MERS-CoV (the beta coronavirus that causes the Middle East Respiratory Syndrome) |
| NL63 (the alpha coronavirus) | SARS-CoV (the beta coronavirus that causes the Severe Acute Respiratory Syndrome) |
| OC43 (the beta coronavirus) | 2019 new coronavirus (SARS-CoV-2) |
| HKU1 (the beta coronavirus) |
| SURFACE MATERIAL | TITER OF VIABLE VIRUS | THE HALF-LIFE OF VIABLE VIRUS |
|---|---|---|
| Stainless steel | 48 h | 5 h |
| Plastic | 72 h | 7 h |
| Copper | 8 h | 1 h |
| Cardboard | 48 h | 3 h |
| DISINFECTING SUBSTANCE | APPLICATION SCOPE |
|---|---|
| Alcohol | Cutaneous antisepsis Disinfection of small surfaces |
| Chlorine compounds (chloramine, hypochlorite) | Cutaneous and wound antisepsis Water treatmentSurface disinfection |
| Glutaraldehyde | Disinfection of inanimate objects |
| Hydrogen peroxide | Cutaneous antisepsis |
| Iodophors | Skin and wound antisepsis |
| Acetic acid | Disinfection of inanimate objects |
| RISK L. | ORGANIZATIONAL MEASURES | ENVIRONMENTAL MEASURES | INDIVIDUAL MEASURES |
|---|---|---|---|
| Low |
|
|
|
| Medium | All measures indicated for the previous level
| All measures indicated for the previous level
| All measures indicated for the previous level
|
| High | All measures indicated for the previous level
| All measures indicated for the previous level
| All measures indicated for the previous level
|
| Very high | All measures indicated for the previous level
| All measures indicated for the previous level
| All measures indicated for the previous level
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirrincione, L.; Plescia, F.; Ledda, C.; Rapisarda, V.; Martorana, D.; Moldovan, R.E.; Theodoridou, K.; Cannizzaro, E. COVID-19 Pandemic: Prevention and Protection Measures to Be Adopted at the Workplace. Sustainability 2020, 12, 3603. https://doi.org/10.3390/su12093603
Cirrincione L, Plescia F, Ledda C, Rapisarda V, Martorana D, Moldovan RE, Theodoridou K, Cannizzaro E. COVID-19 Pandemic: Prevention and Protection Measures to Be Adopted at the Workplace. Sustainability. 2020; 12(9):3603. https://doi.org/10.3390/su12093603
Chicago/Turabian StyleCirrincione, Luigi, Fulvio Plescia, Caterina Ledda, Venerando Rapisarda, Daniela Martorana, Raluca Emilia Moldovan, Kelly Theodoridou, and Emanuele Cannizzaro. 2020. "COVID-19 Pandemic: Prevention and Protection Measures to Be Adopted at the Workplace" Sustainability 12, no. 9: 3603. https://doi.org/10.3390/su12093603
APA StyleCirrincione, L., Plescia, F., Ledda, C., Rapisarda, V., Martorana, D., Moldovan, R. E., Theodoridou, K., & Cannizzaro, E. (2020). COVID-19 Pandemic: Prevention and Protection Measures to Be Adopted at the Workplace. Sustainability, 12(9), 3603. https://doi.org/10.3390/su12093603









