Population Dynamics of the ‘Golden Tides’ Seaweed, Sargassum horneri, on the Southwestern Coast of Korea: The Extent and Formation of Golden Tides
Abstract
1. Introduction
2. Materials and Methods
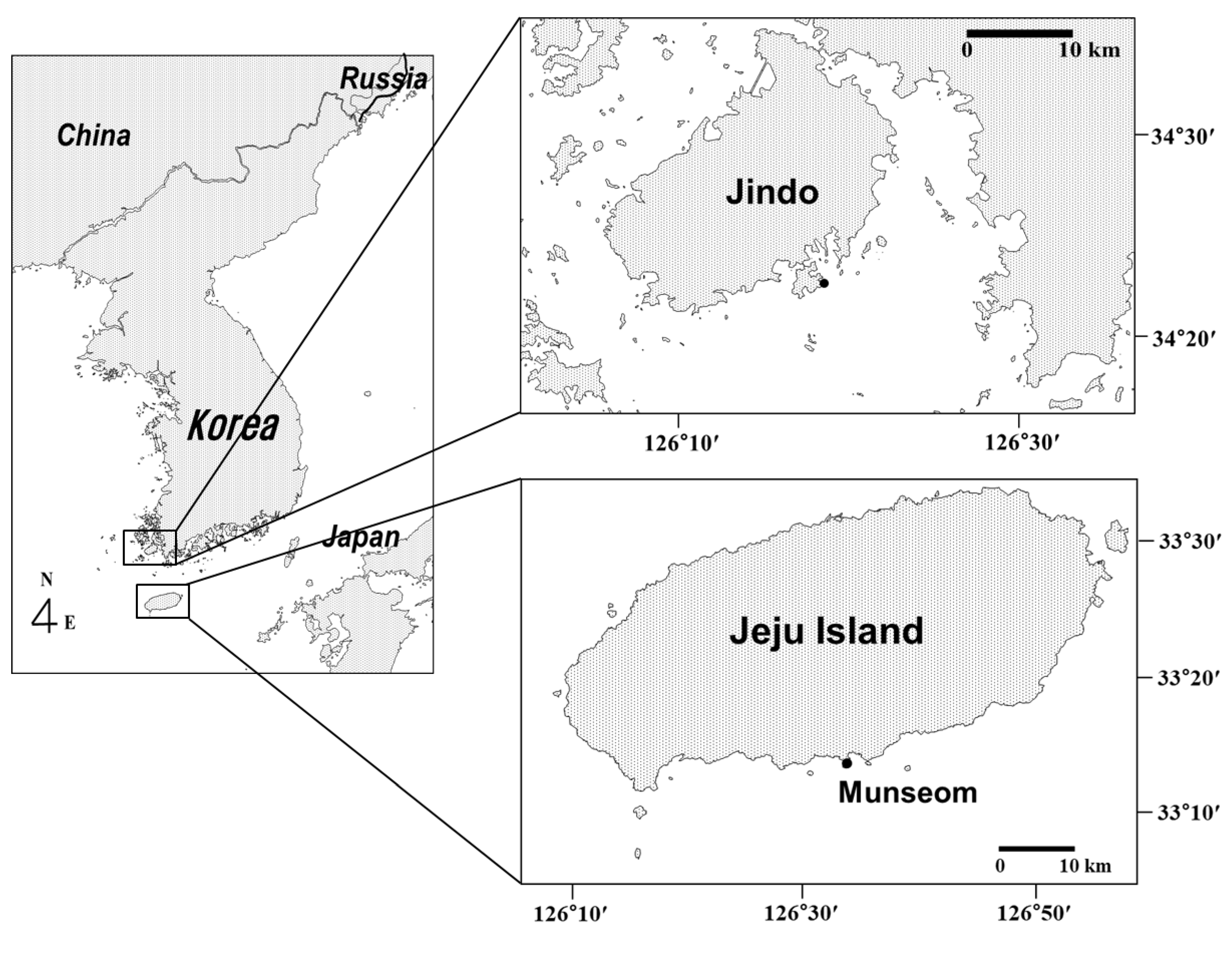
2.1. Study Area
2.2. Sampling Design
2.3. Data Collection
2.4. Statistical Analyses
3. Results
3.1. Environmental Factors
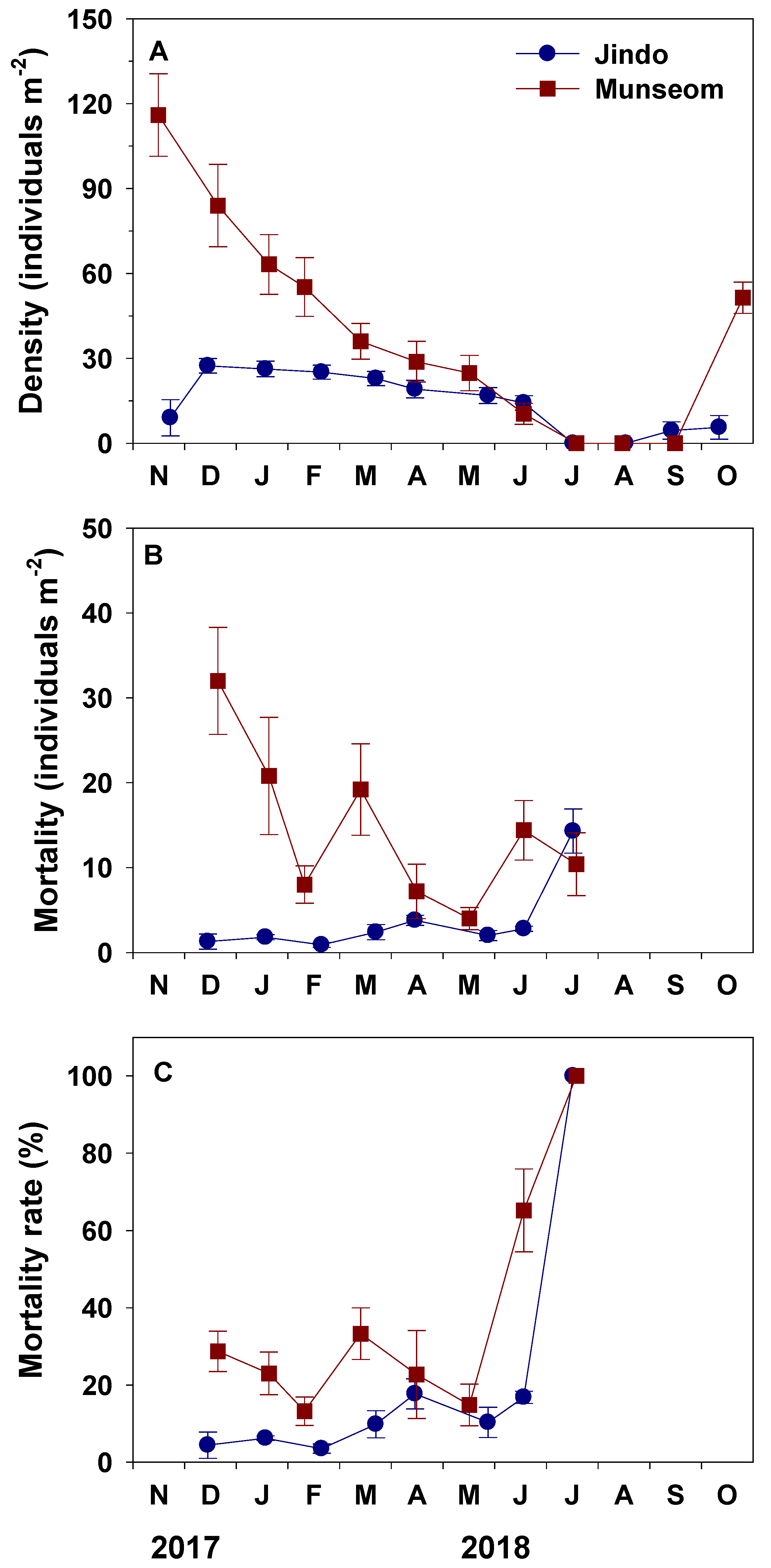
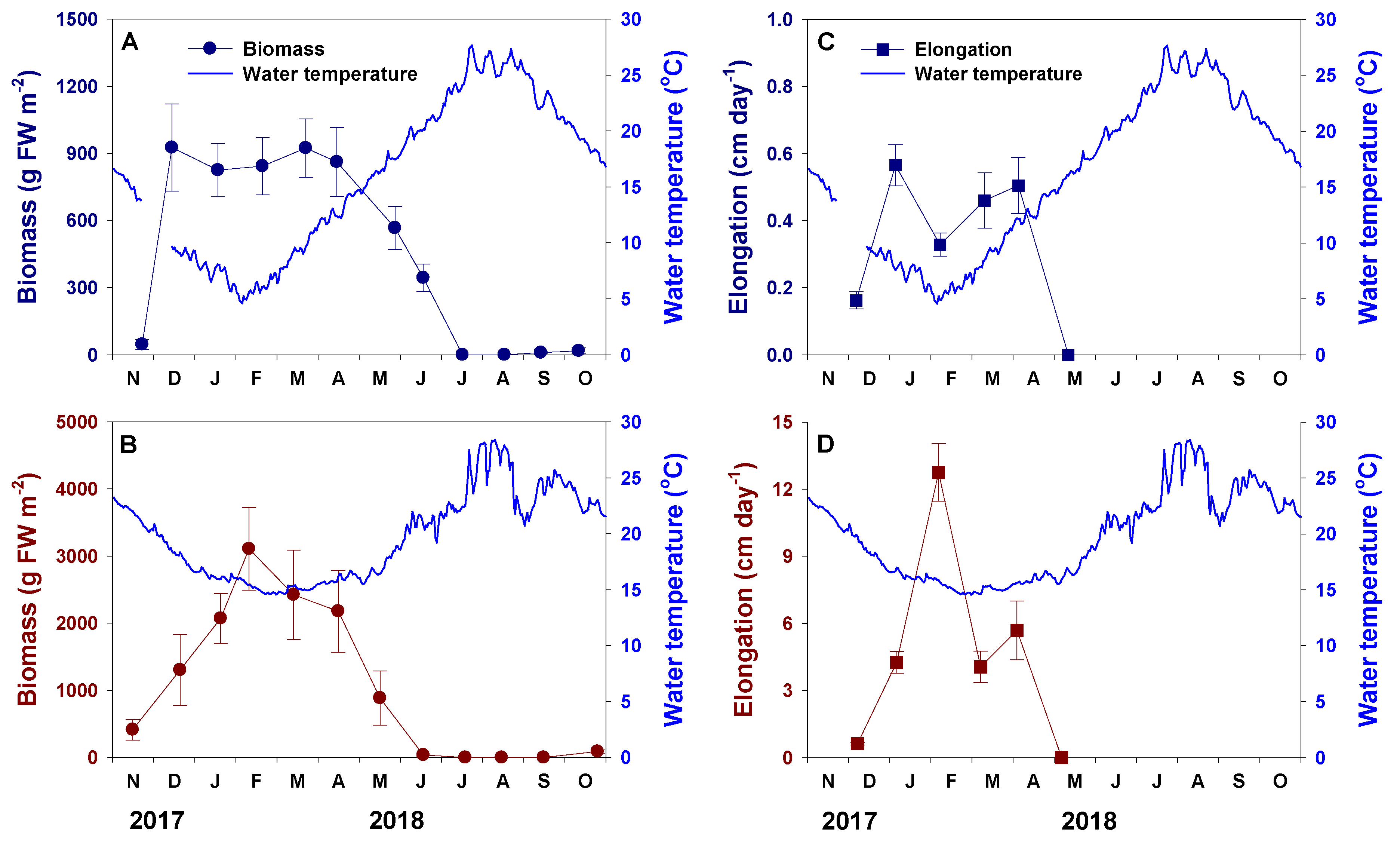
3.2. Density and Mortality
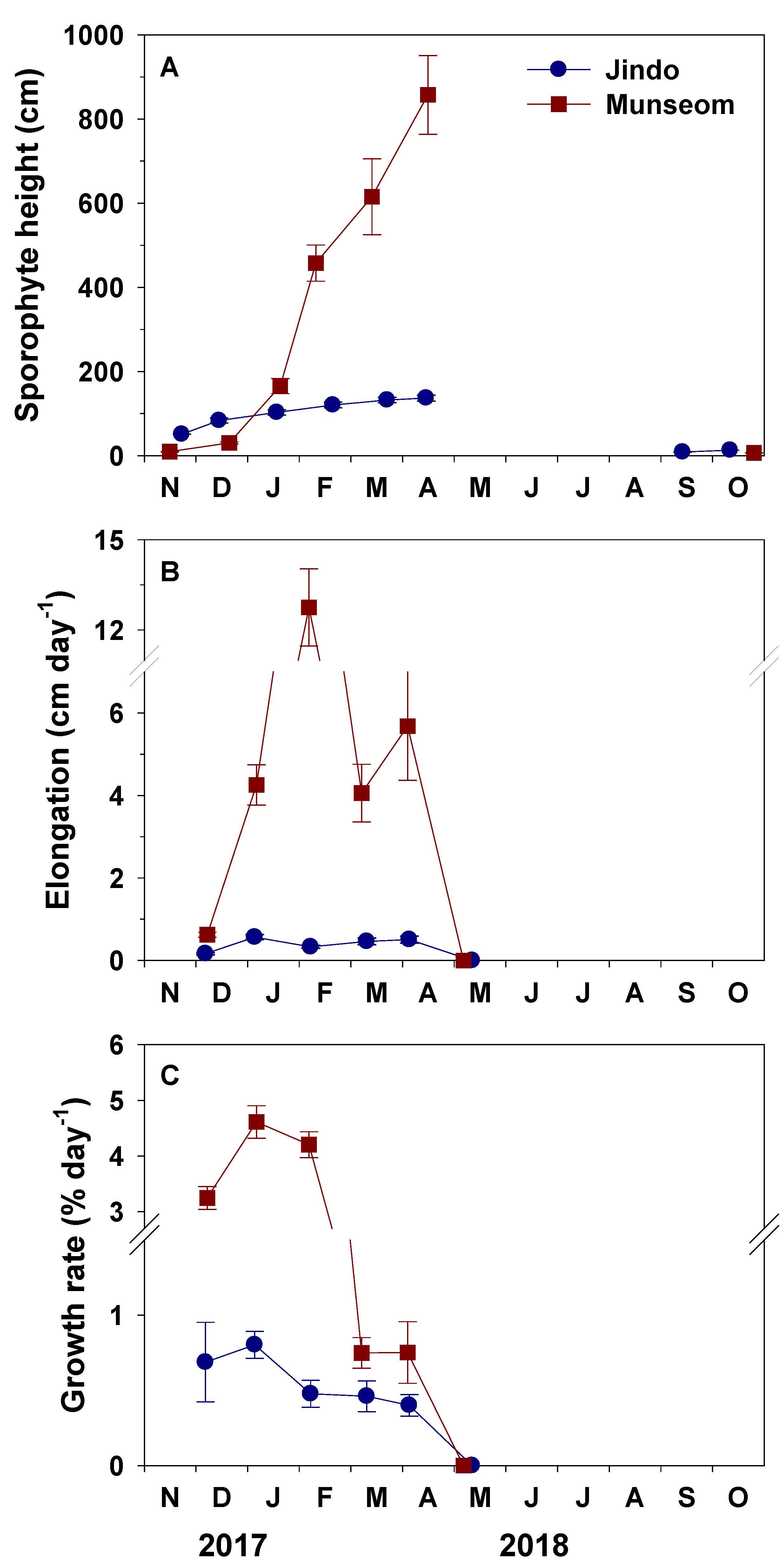
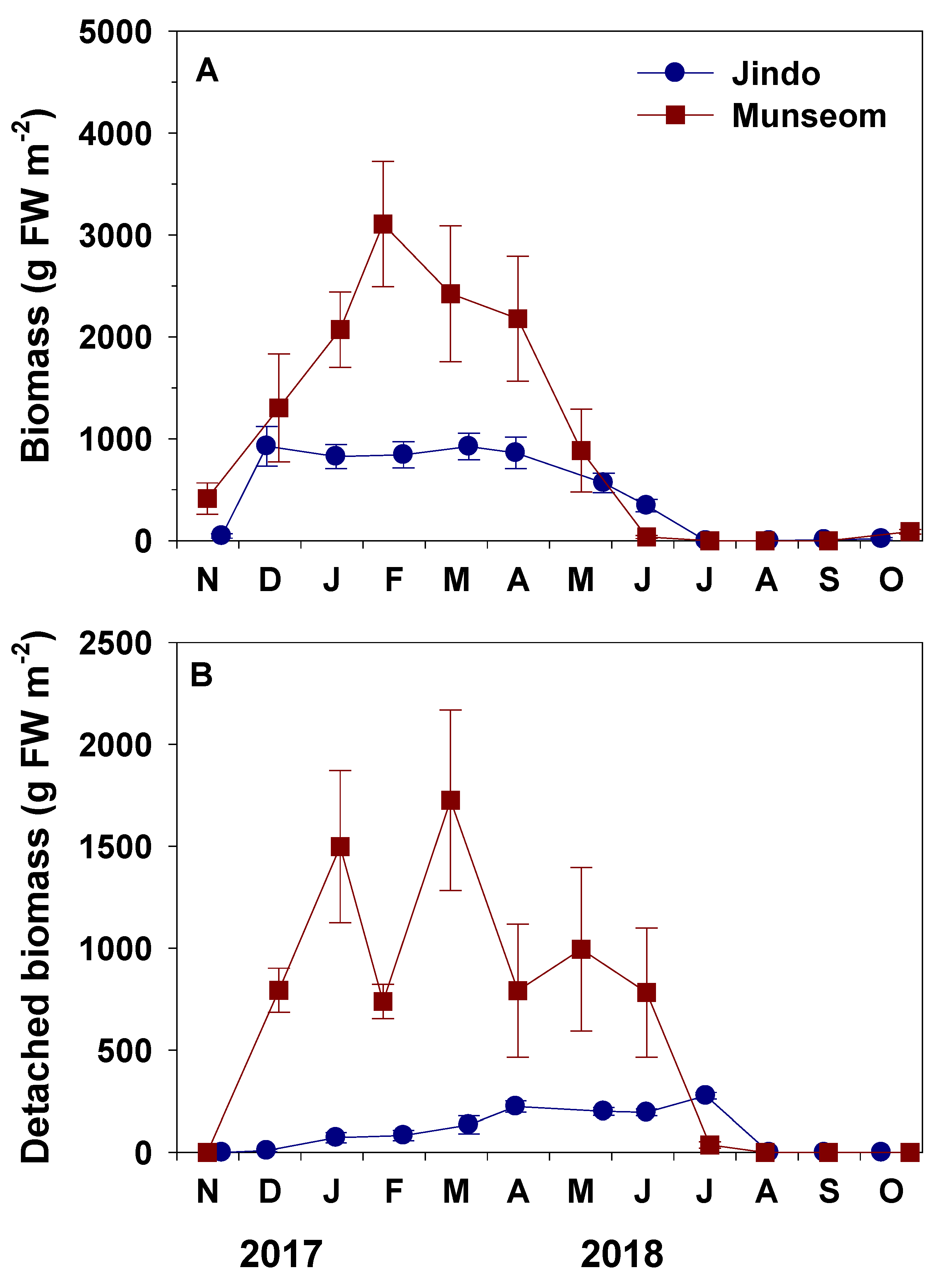
3.3. Height, Growth and Biomass
3.4. Relationship between Water Temperature and Thallus Biomass
4. Discussion
4.1. Population Dynamics of S. horneri Populations
4.2. Contribution of Mortality to the Formation of Golden Tides
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smetacek, V.; Zingone, A. Green and golden seaweed tides on the rise. Nature 2013, 504, 84–88. [Google Scholar] [CrossRef]
- Wang, M.; Hu, C.; Barnes, B.B.; Mitchum, G.; Lapointe, B.; Montoya, J.P. The great Atlantic Sargassum belt. Science 2019, 365, 83–87. [Google Scholar] [CrossRef]
- Lapointe, B.E. A comparison of nutrient-limited productivity in Sargassum natans from neritic vs. oceanic waters of the western North Atlantic Ocean. Limnol. Oceanogr. 1995, 40, 625–633. [Google Scholar] [CrossRef]
- Fletcher, R.L. The occurrence of “green tides”—A review. In Marine Benthic Vegetation; Springer: Berlin, Germany, 1996; pp. 7–43. [Google Scholar]
- Qi, L.; Hu, C.; Wang, M.; Shang, S.; Wilson, C. Floating algae blooms in the East China Sea. Geophys. Res. Lett. 2017, 44, 11501–11509. [Google Scholar] [CrossRef]
- Cho, S.-H.; Myoung, J.-G.; Kim, J.-M.; Lee, J.H. Fish fauna associated with drifting seaweed in the coastal area of Tongyeong, Korea. Trans. Am. Fish. Soc. 2001, 130, 1190–1202. [Google Scholar] [CrossRef]
- Thiel, M.; Gutow, L. The ecology of rafting in the marine environment. I. The floating substrata. In Oceanography and Marine Biology: An Annual Review; Gibson, R.N., Atkinson, R.J.A., Gordon, J.D.M., Eds.; CRC Press: Boca Raton, FL, USA, 2005; Volume 42, pp. 181–264. [Google Scholar]
- Witherington, B.; Hirama, S.; Hardy, R. Young sea turtles of the pelagic Sargassum-dominated drift community: Habitat use, population density, and threats. Mar. Ecol. Prog. Ser. 2012, 463, 1–22. [Google Scholar] [CrossRef]
- Ha, D.S.; Yoo, H.I.; Chang, S.J.; Hwang, E.K. Bloom of a filamentous green alga Cladophora vadorum (Areschoug) Kützing and nutrient levels at Shangrok beach, Buan, Korea. Korean J. Fish. Aquat. Sci. 2016, 49, 241–246. [Google Scholar]
- Wang, M.; Hu, C.; Cannizzaro, J.; English, D.; Han, X.; Naar, D.; Lapointe, B.; Brewton, R.; Hernandez, F. Remote sensing of Sargassum biomass, nutrients, and pigments. Geophys. Res. Lett. 2018, 45, 12359–12367. [Google Scholar] [CrossRef]
- Komatsu, T.; Tatsukawa, K.; Filippi, J.B.; Sagawa, T.; Matsunaga, D.; Mikami, A.; Ishida, K.; Ajisaka, T.; Tanaka, K.; Aoki, M. Distribution of drifting seaweeds in eastern East China Sea. J. Mar. Syst. 2007, 67, 245–252. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, X.; Zhuang, M.; Wang, S.; Chen, L.; Shen, H.; He, P. An increase in new Sargassum (Phaeophyceae) blooms along the coast of the East China Sea and Yellow Sea. Phycologia 2019, 58, 374–381. [Google Scholar] [CrossRef]
- Liu, F.; Liu, X.; Wang, Y.; Jin, Z.; Moejes, F.W.; Sun, S. Insights on the Sargassum horneri golden tides in the Yellow Sea inferred from morphological and molecular data. Limnol. Oceanogr. 2018, 63, 1762–1773. [Google Scholar] [CrossRef]
- Byeon, S.Y.; Oh, H.-J.; Kim, S.; Yun, S.H.; Kang, J.H.; Park, S.R.; Lee, H.J. The origin and population genetic structure of the ‘golden tide’seaweeds, Sargassum horneri, in Korean waters. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.M.; Uwai, S.; Yu, S.H.; Komatsu, T.; Ajisaka, T.; Duan, D.L. Phylogeographic heterogeneity of the brown macroalga Sargassum horneri (Fucaceae) in the northwestern Pacific in relation to late Pleistocene glaciation and tectonic configurations. Mol. Ecol. 2011, 20, 3894–3909. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.J.; Liu, F.; Shan, T.F.; Gao, S.Q.; Zhang, Z.H. Cultivation of the brown alga Sargassum horneri: Sexual reproduction and seedling production in tank culture under reduced solar irradiance in ambient temperature. J. Appl. Phycol. 2009, 21, 413–422. [Google Scholar] [CrossRef]
- Xie, X.; Wang, G.; Pan, G.; Sun, J.; Li, J. Development of oogonia of Sargassum horneri (Fucales, Heterokontophyta) and concomitant variations in PSII photosynthetic activities. Phycologia 2014, 53, 10–14. [Google Scholar] [CrossRef][Green Version]
- Xu, M.; Sakamoto, S.; Komatsu, T. Attachment strength of the subtidal seaweed Sargassum horneri (Turner) C. Agardh varies among development stages and depths. J. Appl. Phycol. 2016, 28, 3679–3687. [Google Scholar] [CrossRef]
- Marks, L.M.; Reed, D.C.; Holbrook, S.J. Life history traits of the invasive seaweed Sargassum horneri at Santa Catalina Island, California. Aquat. Invasions 2018, 13, 339–350. [Google Scholar] [CrossRef]
- Mizuno, S.; Ajisaka, T.; Lahbib, S.; Kokubu, Y.; Alabsi, M.; Komatsu, T. Spatial distributions of floating seaweeds in the East China Sea from late winter to early spring. J. Appl. Phycol. 2014, 26, 1159–1167. [Google Scholar] [CrossRef]
- Yatsuya, K. Floating period of Sargassacean thalli estimated by the change in density. J. Appl. Phycol. 2008, 20, 797–800. [Google Scholar] [CrossRef]
- Yoshida, G.; Arima, S.; Terawaki, T. Growth and maturation of the ‘autumn-fruiting type’of Sargassum horneri (Fucales, Phaeophyta) and comparisons with the ‘spring-fruiting type’. Phycol. Res. 1998, 46, 183–189. [Google Scholar] [CrossRef]
- Komatsu, T.; Ariyama, H.; Nakahara, H.; Sakamoto, W. Spatial and temporal distributions of water temperature in a Sargassum forest. J. Oceanogr. Soc. Jpn. 1982, 38, 63–72. [Google Scholar] [CrossRef]
- Choi, C.G.; Kim, H.G.; Sohn, C.H. Transplantation of young fronds of Sargassum horneri for construction of seaweed beds. Korean J. Fish. Aquat. Sci. 2003, 36, 469–473. [Google Scholar]
- Miller, K.A.; Engle, J.M.; Uwai, S.; Kawai, H. First report of the Asian seaweed Sargassum filicinum Harvey (Fucales) in California, USA. Biol. Invasions 2007, 9, 609. [Google Scholar] [CrossRef]
- Marks, L.M.; Salinas-Ruiz, P.; Reed, D.C.; Holbrook, S.J.; Culver, C.S.; Engle, J.M.; Kushner, D.J.; Caselle, J.E.; Freiwald, J.; Williams, J.P. Range expansion of a non-native, invasive macroalga Sargassum horneri (Turner) C. Agardh, 1820 in the eastern Pacific. Bioinvasions Rec. 2015, 4, 243–248. [Google Scholar] [CrossRef]
- Yamauchi, K. The formation of Sargassum beds on artificial substrata by Transplanting Seedlings of S. horneri (Turner) C. Agardh and S. muticum (Yendo) Fensholt. Bull. Jpn. Soc. Sci. Fish. 1984, 50, 1115–1123. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Bi, Y.X.; Wu, Z.L. Spatial distribution pattern of Sargassum horneri around Gouqi Island, Shengsi, China. J. Fish. China 2013, 37, 884–893. [Google Scholar] [CrossRef]
- Kim, S.; Kang, Y.H.; Kim, T.-H.; Lee, H.J.; Park, S.R. Use of morphological characteristics for calculating individual biomass in the kelp Ecklonia cava. J. Appl. Phycol. 2017, 29, 2587–2593. [Google Scholar] [CrossRef]
- Mikami, A.; Komatsu, T.; Aoki, M.; Yokohama, Y. Seasonal changes in growth and photosynthesis-light curves of Sargassum horneri (Fucales, Phaeophyta) in Oura Bay on the coast of central Honshu, Japan. La mer 2006, 44, 109–118. [Google Scholar]
- Yong, Y.S.; Yong, W.T.L.; Anton, A. Analysis of formulae for determination of seaweed growth rate. J. Appl. Phycol. 2013, 25, 1831–1834. [Google Scholar] [CrossRef]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Chemical and Biological Methods for Seawater Analysis; Pergamon Press: Oxford, UK, 1984; p. 173. [Google Scholar]
- Umezaki, I. Ecological studies of Sargassum horneri (Turner) C. Agardh in Obama Bay, Japan Sea. Bull. Jpn. Soc. Sci. Fish. 1984, 50, 1193–1200. [Google Scholar] [CrossRef]
- Murakami, K.; Yamaguchi, Y.; Sugawa-Katayama, Y.; Katayama, M. Effect of water depth on seasonal variation in the chemical composition of Akamoku, Sargassum horneri (Turner) C. Agardh. Nat. Resour. 2016, 7, 147–156. [Google Scholar]
- Fulton, C.J.; Depczynski, M.; Holmes, T.H.; Noble, M.M.; Radford, B.; Wernberg, T.; Wilson, S.K. Sea temperature shapes seasonal fluctuations in seaweed biomass within the Ningaloo coral reef ecosystem. Limnol. Oceanogr. 2014, 59, 156–166. [Google Scholar] [CrossRef]
- Yoshida, G.; Murase, N.; Arai, S.; Terawaki, T. Ecotypic differentiation in maturation seasonality among Sargassum horneri (Fucales, Phaeophyta) populations in Hiroshima Bay, Seto Inland Sea, Japan. Phycologia 2004, 43, 703–710. [Google Scholar] [CrossRef]
- Yu, J.; Li, J.; Wang, Q.; Liu, Y.; Gong, Q. Growth and resource accumulation of drifting Sargassum horneri (fucales, phaeophyta) in response to temperature and nitrogen supply. J. Ocean Univ. China 2019, 18, 1216–1226. [Google Scholar] [CrossRef]
- Hales, J.M.; Fletcher, R.L. Studies on the recently introduced brown alga Sargassum muticum (Yendo) Fensholt. V. Receptacle initiation and growth, and gamete release in laboratory culture. Bot. Mar. 1990, 33, 241–250. [Google Scholar] [CrossRef]
- Borchardt, M.A. Nutrients. In Algal Ecology: Freshwater Benthic Ecosystems; Stevenson, R.J., Bothwell, M.L., Lowe, R.L., Eds.; Academic Press: San Diego, CA, USA, 1996; pp. 183–227. [Google Scholar]
- Larned, S.T. Nitrogen-versus phosphorus-limited growth and sources of nutrients for coral reef macroalgae. Mar. Biol. 1998, 132, 409–421. [Google Scholar] [CrossRef]
- Gagné, J.A.; Mann, K.H.; Chapman, A.R.O. Seasonal patterns of growth and storage in Laminaria longicruris in relation to differing patterns of availability of nitrogen in the water. Mar. Biol. 1982, 69, 91–101. [Google Scholar] [CrossRef]
- Vuki, V.C.; Price, I.R. Seasonal changes in the Sargassum populations on a fringing coral reef, Magnetic Island, Great Barrier Reef region, Australia. Aquat. Bot. 1994, 48, 153–166. [Google Scholar] [CrossRef]
- Kregting, L.; Blight, A.J.; Elsäßer, B.; Savidge, G. The influence of water motion on the growth rate of the kelp Laminaria digitata. J. Exp. Mar. Biol. Ecol. 2016, 478, 86–95. [Google Scholar] [CrossRef]
- Yoshida, G.; Yoshikawa, K.; Terawaki, T. Growth and maturation of two populations of Sargassum horneri (Fucales, Phaeophyta) in Hiroshima Bay, the Seto Inland Sea. Fish. Sci. 2001, 67, 1023–1029. [Google Scholar] [CrossRef]
- Engelen, A.H.; Åberg, P.; Olsen, J.L.; Stam, W.T.; Breeman, A.M. Effects of wave exposure and depth on biomass, density and fertility of the fucoid seaweed Sargassum polyceratium (Phaeophyta, Sargassaceae). Eur. J. Phycol. 2005, 40, 149–158. [Google Scholar] [CrossRef]
- Harder, D.L.; Hurd, C.L.; Speck, T. Comparison of mechanical properties of four large, wave-exposed seaweeds. Am. J. Bot. 2006, 93, 1426–1432. [Google Scholar] [CrossRef] [PubMed]
- Engledow, H.R.; Bolton, J.J. Seaweed α-diversity within the lower eulittoral zone in Namibia: The effects of wave action, sand inundation, mussels and limpets. Bot. Mar. 1994, 37, 267–276. [Google Scholar] [CrossRef]
- Díez, I.; Santolaria, A.; Gorostiaga, J.M. The relationship of environmental factors to the structure and distribution of subtidal seaweed vegetation of the western Basque coast (N Spain). Estuar. Coast. Shelf Sci. 2003, 56, 1041–1054. [Google Scholar] [CrossRef]
- Konishi, Y. Drifting seaweeds coming from China too. Seikai Fish. Res. Instit. News 2000, 103, 11–15. [Google Scholar]
- Lin, S.-M.; Huang, R.; Ogawa, H.; Liu, L.-C.; Wang, Y.-C.; Chiou, Y. Assessment of germling ability of the introduced marine brown alga, Sargassum horneri, in Northern Taiwan. J. Appl. Phycol. 2017, 29, 2641–2649. [Google Scholar] [CrossRef]
- Kim, K.; Shin, J.; Kim, K.Y.; Ryu, J.-H. Long-Term Trend of Green and Golden Tides in the Eastern Yellow Sea. J. Coast. Res. 2019, 90, 317–323. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Wang, L.; Wang, W.D. Algal communities at Gouqi Island in the Zhoushan archipelago, China. J. Appl. Phycol. 2008, 20, 853–861. [Google Scholar] [CrossRef]
- Cruz-Trejo, G.I.; Ibarra-Obando, S.E.; Aguilar-Rosas, L.E.; Poumian-Tapia, M.; Solana-Arellano, E. Presence of Sargassum horneri at Todos Santos Bay, Baja California, Mexico: Its effects on the local macroalgae community. Am. J. Plant. Sci. 2015, 6, 2693–2707. [Google Scholar] [CrossRef]
- Hwang, E.K.; Lee, S.J.; Ha, D.S.; Park, C.S. Sargassum golden tides in the Shinan-gun and Jeju Island, Korea. Korean J. Fish. Aquat. Sci. 2016, 49, 689–693. [Google Scholar]

| Parameter | Source | DF | MS | F-ratio | p-value |
|---|---|---|---|---|---|
| Density | Site | 1 | 96.660 | 70.664 | <0.001 |
| Time | 11 | 58.254 | 42.587 | <0.001 | |
| Site × Time | 11 | 18.271 | 13.357 | <0.001 | |
| Mortality density | Site | 1 | 59.968 | 53.806 | <0.001 |
| Time | 7 | 4.347 | 3.901 | =0.002 | |
| Site × Time | 7 | 6.295 | 5.648 | <0.001 | |
| Mortality rate | Site | 1 | 0.614 | 23.220 | <0.001 |
| Time | 7 | 2.020 | 76.386 | <0.001 | |
| Site × Time | 7 | 0.090 | 3.401 | =0.004 | |
| Height | Site | 1 | 1670.037 | 151.698 | <0.001 |
| Time | 6 | 1885.748 | 171.292 | <0.001 | |
| Site × Time | 6 | 888.421 | 80.700 | <0.001 | |
| Elongation | Site | 1 | 110.785 | 294.097 | <0.001 |
| Time | 4 | 12.143 | 32.236 | <0.001 | |
| Site × Time | 4 | 11.902 | 31.595 | <0.001 | |
| Growth rate | Site | 1 | 0.380 | 192.995 | <0.001 |
| Time | 4 | 0.059 | 30.065 | <0.001 | |
| Site × Time | 4 | 0.039 | 19.674 | <0.001 | |
| Biomass | Site | 1 | 1253.824 | 17.002 | <0.001 |
| Time | 11 | 2441.508 | 33.106 | <0.001 | |
| Site × Time | 11 | 258.725 | 3.508 | <0.001 | |
| Detached biomass | Site | 1 | 3032.415 | 67.151 | <0.001 |
| Time | 11 | 924.001 | 20.462 | <0.001 | |
| Site × Time | 11 | 393.697 | 8.718 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.K.; Oh, H.-J.; Yun, S.-H.; Lee, H.J.; Lee, K.; Han, Y.S.; Kim, S.; Park, S.R. Population Dynamics of the ‘Golden Tides’ Seaweed, Sargassum horneri, on the Southwestern Coast of Korea: The Extent and Formation of Golden Tides. Sustainability 2020, 12, 2903. https://doi.org/10.3390/su12072903
Choi SK, Oh H-J, Yun S-H, Lee HJ, Lee K, Han YS, Kim S, Park SR. Population Dynamics of the ‘Golden Tides’ Seaweed, Sargassum horneri, on the Southwestern Coast of Korea: The Extent and Formation of Golden Tides. Sustainability. 2020; 12(7):2903. https://doi.org/10.3390/su12072903
Chicago/Turabian StyleChoi, Sun Kyeong, Hyun-Ju Oh, Suk-Hyun Yun, Hyuk Je Lee, Kyounghoon Lee, Young Seok Han, Sangil Kim, and Sang Rul Park. 2020. "Population Dynamics of the ‘Golden Tides’ Seaweed, Sargassum horneri, on the Southwestern Coast of Korea: The Extent and Formation of Golden Tides" Sustainability 12, no. 7: 2903. https://doi.org/10.3390/su12072903
APA StyleChoi, S. K., Oh, H.-J., Yun, S.-H., Lee, H. J., Lee, K., Han, Y. S., Kim, S., & Park, S. R. (2020). Population Dynamics of the ‘Golden Tides’ Seaweed, Sargassum horneri, on the Southwestern Coast of Korea: The Extent and Formation of Golden Tides. Sustainability, 12(7), 2903. https://doi.org/10.3390/su12072903






