1. Introduction
Although there seems to be sufficient evidence to suggest total sleep deprivation can have a significant negative impact on sports performance, the effects of partial sleep deprivation are more contradictory [
1]. Sleep deprivation can have a major impact on the athlete’s preparation in sport, and in many situations, sleep is disturbed before sporting events (e.g., change of time zones, stress) [
2]. Accordingly, it is important to know how sleep deprivation effect on specific kind of physical effort.
Azboy and Kaygisiz [
3] suggest that one-night sleep deprivation may reduce exercise performance by decreasing exercise minute ventilation (VE) and time to exhaustion. They also report that sleep loss may decrease the performance of volleyball players more than that of runners. One night of sleep deprivation does not have a statistically significant effect on the anaerobic capacity. No differences were found for both mean and peak anaerobic capacity [
2]. On the other hand, Souissi et al. [
4] showed that the sleepless period is important because the peak anaerobic capacity does not change after 24 h without sleep, but it significantly decreases after 36 h of insomnia. Rodgers et al. [
5] investigated the effect of 48 h of sleep deprivation on the performance of selected physical tasks with a load of 30–45% VO2 max. Moreover, they evaluated the influence of continuous physical work during 48 h of sleep deprivation in the control group. This study showed that after 48 h with no sleep, the performance of all tasks set for the surveyed group significantly deteriorated. Additionally, a variety of conclusions have been reported for the effects of sleep restriction on muscular strength and power. Sleep deprivation and lack of sleep combined with physical effort had no influence on muscle contractile properties, anaerobic capacity, resting glucose and lactate levels in blood. Only people with sleep deprivation alone showed a decrease in cardiopulmonary function. Chen and Tang [
6] observed that after 30-h sleep deprivation in patients with respiratory disorders the inspiratory muscle endurance deteriorated, whereas the respiratory muscle strength did not change. A decrease in isokinetic strength after 30 h of sleep deprivation in Marine Corps soldiers was noted by Bulbulian et al. [
7]. Instead, Reilly and Piercy [
8] indicate that submaximal lifting tasks are more affected by sleep loss than are maximal efforts, particularly for the first two nights of successive sleep restriction. Other studies show that sleep deprivation had little effect on muscle strength during resistance exercises. In contrast, consecutive nights of sleep restriction could reduce the force output of multi-joint, but not single-joint movements [
9].
There is significant individual variability in response to sleep deprivation [
10]. People who lead more active lifestyles show fewer negative effects of sleep deprivation and are more tolerant of poorer quality sleep than inactive people. In addition, gender can also influence the changes caused by sleep deprivation. Sleep deprivation can have different effects on risk-taking in men and women [
11]. Older women have better regenerative sleep after 36 h of sleep deprivation than men. These observations point to the importance of gender in determining sleep patterns [
12]. In mice, homeostasis responses to 6-h sleep deprivation do not differ in terms of sexes, but the way sleep is affected and disturbed by the environment may be sex-specific [
13]. However, a study of differences in the sympathetic system after 24-h total sleep deprivation showed that the activity of the sympathetic nervous system decreased in men but not in women [
14].
Analysis of the research shows the effects of sleep restriction on exercise performance are mixed. Sleep restriction does not appear to affect singular bouts of aerobic performance or maximal measures of strength, although, admittedly, conflicting results still exist. It is still not clear whether sleep is critical to performance for all athletes who experience small one-off sleep restriction periods [
1].
The aim of this experiment was to investigate the influence of the 24-h sleep deprivation on the knee muscle strength of extensors and flexors in healthy, young and physically fit physical education students. Additionally, in this study we tested the influence of the dominant lower limb on the changes in strength of flexor and extensor muscles under the influence of sleep deprivation.
The value of our present study compared to the past studies is that we researched impact of sleep deprivation on muscle strength and took into consideration leg dominance. These are not frequently considered in studies on the effects of sleep deprivation on muscle strength.
2. Materials and Methods
The study material consisted of 67 physical education students (38 women and 29 men) (age 21.52 ± 1.58 years). The surveyed students were healthy, without injury or signs of fatigue. Their BMI was at the level of 23.43 ± 2.34 kg/m
2. Students were randomly divided into a control and experimental group subjected to a 24-h sleep deprivation. The control group was composed of 23 subjects: 15 women (Age: 21.20 ± 1.08 years; BMI: 23.15 ± 2.25 kg/m
2) and 8 men (Age: 21.63 ± 1.69 years; BMI: 24.69 ± 1.99 kg/m
2). The experimental group was composed of 44 subjects, 23 women (Age: 21.78 ± 2.17 years; BMI: 22.33 ± 2.45 kg/m
2) and 21 men (Age: 21.43 ± 1.03 years; BMI: 24.34 ± 1.88 kg/m
2). This number of subjects was considered to be sufficient [
2,
7,
15]. Participants gave written informed consent. The study protocol was approved by the Local Committee of Ethics in Research (no 1261/18). Before the experiment, we assessed the ‘daytime sleepiness’ of the participants using the Epworth Sleepiness Scale (ESS). The Epworth Sleepiness Scale (ESS) is a widely used scale for daytime sleepiness in adults [
16,
17]. The Epworth Sleepiness Scale determines the respondent’s sleepiness for the last 4 weeks. The experimental group obtained an average score of 7.84 ± 3.54, whereas those in the control group obtained 8.43 ± 4.34. There is no significant differences between control and experimental group (
p > 0.05).
All students of the physical education university have to undergo a medical examination and obtain the doctor’s permission to study at this university. Therefore, all subjects must be in good health and physically fit. Students at the University of Physical Education participate in many sports classes required by the curriculum. In addition, applicants have to pass an entrance exam to check their physical fitness. The study included students who comply with the following inclusion criteria: bachelor’s or master’s degree students; average sleep duration 7–8 h a day; resignation from alcoholic beverages and beverages containing caffeine on the day of the test. Exclusion criteria: sleep and psychiatric disorders which were clinically diagnosed in the past; taking sleep medication; travel far away or change of time zone in the past 3 weeks.
The experiment began with an introductory study to allow both the experimental and control groups to familiarize themselves with the tests. The next day students have participated in standard lectures at the University all day long. They had lunch and dinner but they could not sleep or relax in bed. During the night, students from control group slept in their own houses whereas students from experimental group were in the room at the university under the care of researchers. The respondents did not perform any additional physical activity. They could read books, play board games, listen to music, and communicate with each other. All students were obliged not to drink beverages with caffeine. The last meal could be consumed at the latest at 8 p.m. At 7 a.m., after 24-h sleep deprivation, the sleep deprivation tests began.
Measurements of muscle strength of flexors and extensors of the knee were taken on a UPR-02 A/S chair with Moment II by Sumer software. The device is equipped with a strain gauge torque transducer with an electronic measuring shaft. A short warm-up was performed before measurements. The study was performed in a sitting position, with stabilization of the thighs and trunk. Isometric knee extension torque was measured in both settings, with a knee joint angle of 45° and a hip angle of 85° (0° supine position). At the signal, the patient pressed the lever placed next to her tarsal joint for a period of 10 s, first in the direction of knee extension and then in the direction of knee flexion (which resulted in isometric tension). The lower limb underwent flexion and extension two times in the knee joint. The best result was analyzed. The results, namely maximum moments of force for knee extensors and flexors in the right and left limb, were expressed in kilograms (kg).
Statistical analyses were performed using the Statistica 10 software (StatSoft Inc., OK, USA) and statistical significance was defined at the level of
p ≤ 0.05. ANOVA (analysis of variance) was used to determine the significance of differences between group “E” (experimental) and group “C” (control), and for the covariation analysis of pre-test and post-test results. The analysis with two levels of the first factor (within-subject factor: “deprivation”—pre-test, post-test) and two levels of second factor (between-subject factor: “group”—experimental or control) was performed. ANCOVA (analysis of covariance) was used to determine the significance of initial performance level on changes in muscle strength under the influence of sleep deprivation [
18]. Likewise, analyses by sex and leg dominance were performed. For interaction effects, the eta-squared (η
2p) effect size was calculated. The effect size indicates the percent of variance explained by the particular effects of the dependent variable.
3. Results
First, the influence of experimental interactions on the strength of right knee extensor muscles was analyzed. The analysis of variance shows that the results for the experimental group decreased after sleep deprivation (F = 17.74,
p < 0.001, η
2p = 0.21) (
Figure 1). These changes were statistically significant for both women (F = 14.36,
p < 0.001, η
2p = 0.29) and men (F = 8.22,
p = 0.008, η
2p = 0.23).
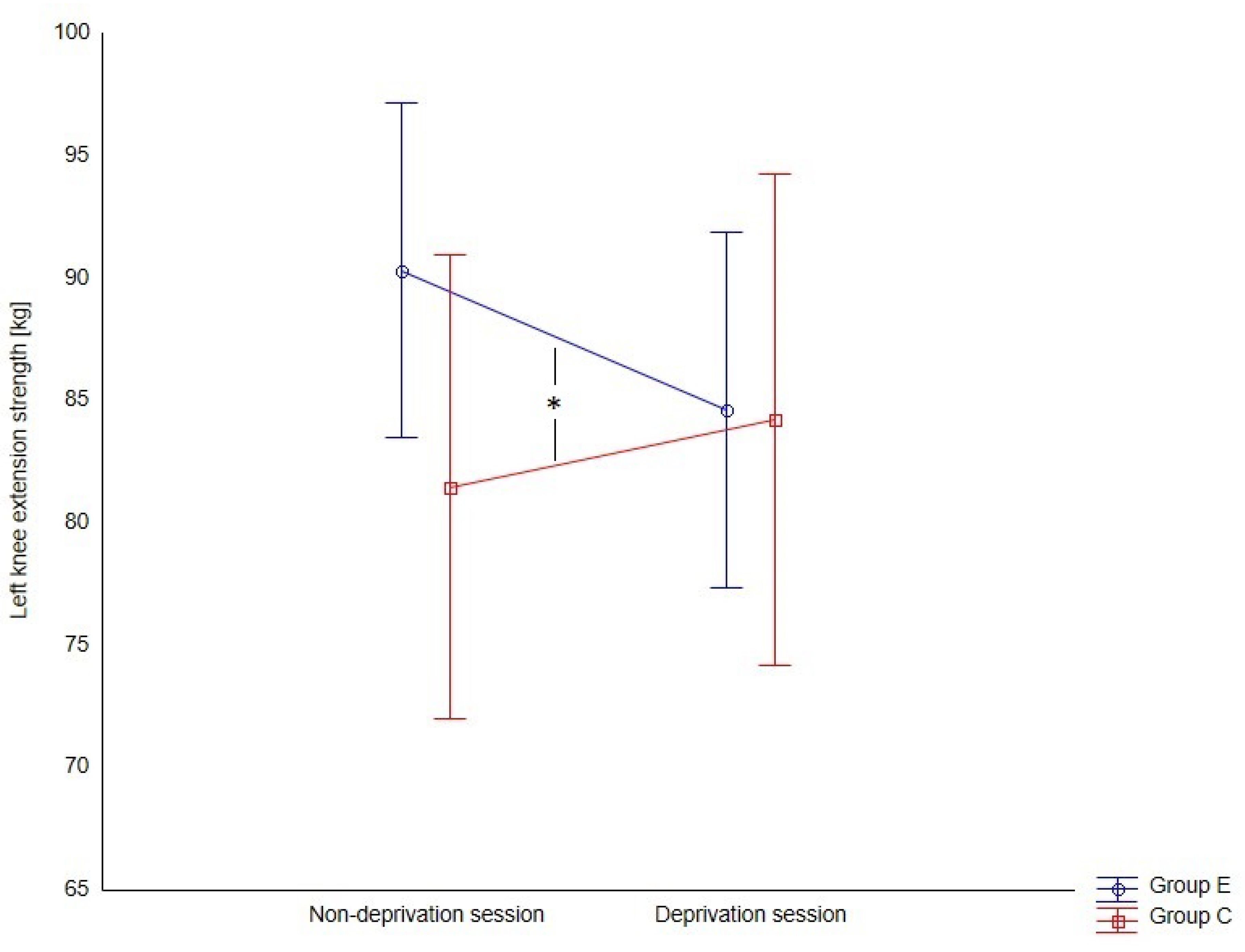
Next, the influence of experimental interactions on the strength of left knee extensor muscles was analyzed. The variance analysis shows that the average strength of left knee extensor muscles decreased due to sleep deprivation in the experimental group, and the difference between the control and experimental groups was statistically significant (F = 6.61,
p = 0.012, η
2p = 0.09) (
Figure 2). However, these changes were statistically significant for women (F = 9.90,
p = 0.003, η
2p = 0.22), but not for men (F = 1.39,
p = 0.248, η
2p = 0.05).
The influence of the 24-h sleep deprivation on the changes in strength of the right and left knee flexor muscles was calculated. Changes in strength were not statistically significant for the right (F = 1.71, p = 0.196, η2p = 0.03) or the left (F = 0.45, p = 0.50, η2p = 0.01) knee. Additionally, these changes were not statistically significant for either women (Right knee: F = 0.95, p = 0.335, η2p = 0.03; Left knee: F = 0.0001, p = 0.991, η2p = 0.000003) or men (Right knee: FF = 0.50, p = 0.486, η2p = 0.02; Left knee: F = 0.56, p = 0.459, η2p = 0.02).
Next, the influence of the dominant lower limb on changes in strength of flexor and extensor muscles under the influence of sleep deprivation in the experimental group was analyzed. Significant changes in the strength of the right knee extensor muscles were observed in patients with dominant left lower limbs (F = 16.20,
p < 0.001, η
2p = 0.29). In these respondents, the strength decreased under the influence of sleep deprivation (
Figure 3).
However, the changes in the strength of right knee extensor muscles were insignificant for the respondents with dominant right lower limbs (F = 3.06, p = 0.093, η2p = 0.11).
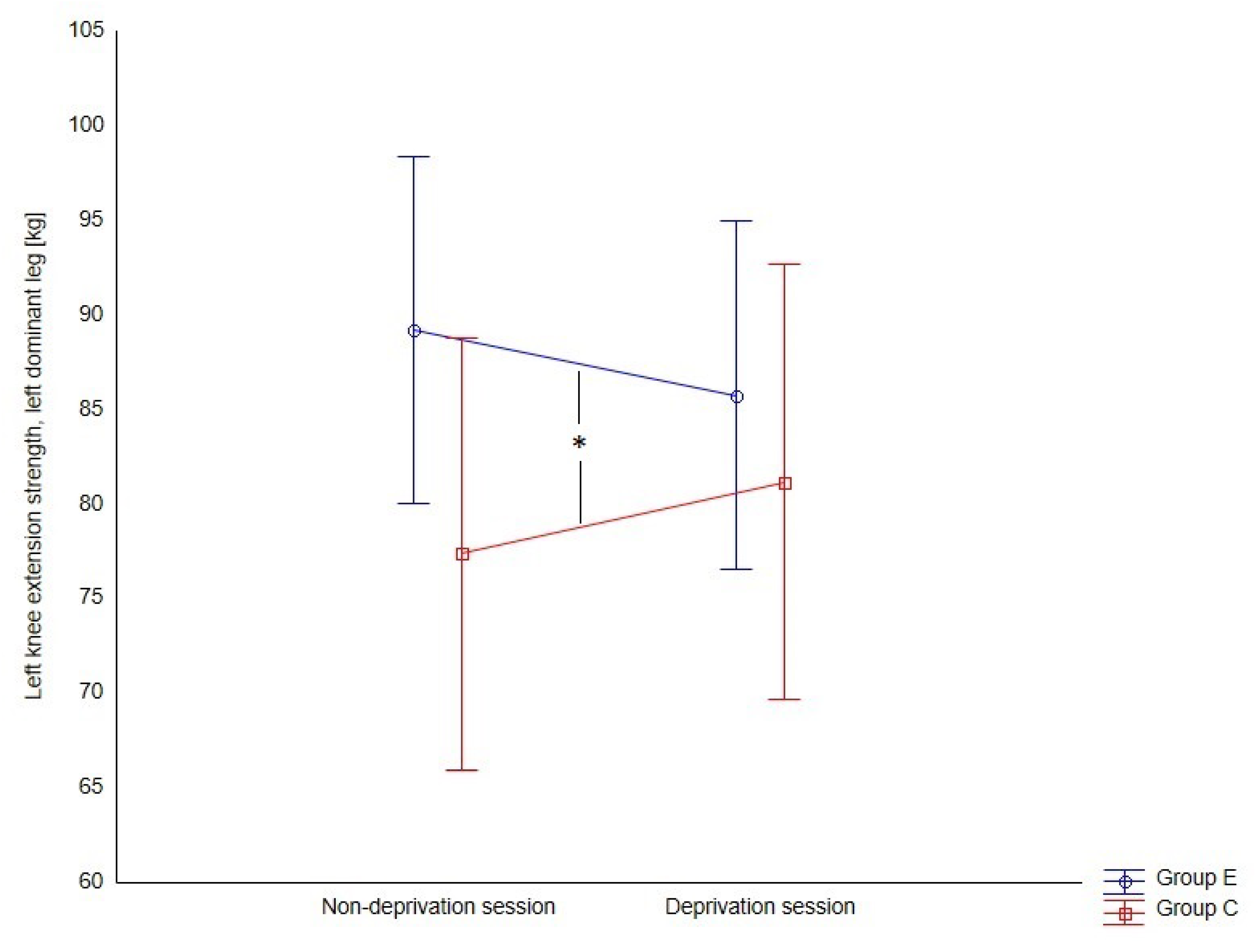
Changes in the strength of left knee extensor muscles under the influence of sleep deprivation were important for respondents who indicated their left lower limb as dominant (F = 7.67,
p = 0.009, η
2p = 0.16) (
Figure 4).
However, these changes were irrelevant for those who indicated the right lower limb as dominant (F = 1.30, p = 0.266, η2p = 0.05). In the case of knee flexor muscles, the dominant limb does not affect the significance of changes.
ANCOVA revealed a statistically significant effect of initial performance level (non-deprivation session) on dependent variables. The analysis of covariance shows that the results of initial performance level impacted on the strength changes of the right knee extensor muscle under the influence of sleep deprivation (F = 12.32, η2p = 0.16, p = 0.001). These changes were statistically significant for both women (F = 10.77, η2p = 0.24, p = 0.002) and men (F = 4.44, η2p = 0.15, p = 0.045).
Next, the influence of initial performance level on the strength of left knee extensor muscles was analyzed. The covariance analysis shows that the initial average strength of the left knee extensor muscles significantly affected strength changes caused by sleep deprivation. (F = 5.28, η2p = 0.08, p = 0.025). However, these impacts were statistically significant for women (F = 8.77, η2p = 0.20, p = 0.005), but not for men (F = 1.00, η2p = 0.04, p = 0.325).
The influence of initial performance level on the changes in strength of the right and left knee flexor muscles after 24-h sleep deprivation was calculated. Changes in strength were not statistically significant for the right (F = 0.53, η2p = 0.01, p = 0.47) and left (F = 0.20, η2p = 0.003, p = 0.653) knee. Additionally, these changes were not statistically significant for either women (Right knee: F = 0.55, η2p = 0.02, p = 0.463; Left knee: F = 0.04, η2p = 0.001, p = 0.845) or men (Right knee: F = 0.04, η2p = 0.001, p = 0.851; Left knee: F = 0.66, η2p = 0.03, p = 0.423).
Next, the influence of the initial performance level of strength in the dominant lower limb on the changes in strength of flexor and extensor muscles under the influence of sleep deprivation was analyzed. A significant impact of initial performance level of strength of the right knee extensor muscles was observed in patients with dominant left lower limbs (F = 12.21, η2p = 0.24, p = 0.001). The influence of initial performance level on changes in strength of right knee extensor muscles was insignificant for the respondents with dominant right lower limbs (F = 2.50, η2p = 0.10, p = 0.128). The influence of initial performance level on changes in the strength of left knee extensor muscles under the influence of sleep deprivation were important for respondents who indicated their left lower limb as dominant (F = 5.87, η2p = 0.13, p = 0.020). However, the impact of initial performance level on these changes was irrelevant for those who indicated the right lower limb as dominant (F = 1.24, η2p = 0.05, p = 0.277).
4. Discussion
This study examined the effect of 24-h sleep deprivation on students’ strength task performance. The findings suggest that 24-h sleep deprivation impact on knee muscle strength but with a different effect on knee extensors and flexors. The strength level of the right and left lower limb extensor muscles decreased statistically in the experimental group. However, the strength level of lower limb flexor muscles did not change significantly. The results obtained in this study show that the influence of sleep deprivation on the muscle may be different for extensor and flexor muscles, as well as that it can differ between women and men. Additionally, the dominance of limbs may influence the changes. Changes in extensor muscle strength are significant for students with left dominant legs. The effects of sleep loss on muscle strength performance is equivocal [
1] and studies report different results. Research conducted among physically fit soldiers also shows that 24 h of sleep deprivation do not affect the grip strength of hand muscles [
19], which is usually measured for the dominant hand side. Combined measurements of both limbs do not give such unambiguous results. However, Chen, Cui, Chen and Wu [
20] even observed a significant relation between the sleep duration and grip strength of hand muscles. Men who slept for less than 6 h a day had less grip strength than those who slept for 7–8 h a day. Therefore, these authors pointed out that short sleeping times can constitute a risk factor for muscle strength reduction. Studies conducted by Blumert et al. [
21] did not show any adverse influence of 24-h sleep deprivation on the results of weight lifting among men. However, Bulbulian et al. [
7] observed a decrease in isokinetic strength of lower limbs in soldiers after 30 h of sleep deprivation. Therefore, the results obtained among students are a part of the discussion on the influence of sleep deprivation on muscle strength, but subsequent analyses should aim at explaining the mechanisms of possible changes.
Currently, there are several different factors that may determine changes in muscle strength as a result of sleep deprivation. Auyeung et al. [
22] demonstrated an inverted U-shaped relation between sleep duration and muscle mass and function, but also between sleep duration and testosterone levels in men. In addition, they noted that testosterone levels increased with the duration of sleep up to almost 10 h, after which they decreased. On the other hand, no correlation between insomnia and testosterone levels has been shown. After additional adjustment of testosterone levels, muscle strength did not change significantly, thus the authors assume that testosterone levels are not responsible for such changes in muscle strength.
Disruption of circadian rhythms may imply changes in skeletal muscles: changes in fiber type, changes in sarcomeric structure, decreased mitochondrial respiration and impaired muscle function [
23]. Mitochondrial oxidative capacity in skeletal muscles occurs at a circadian rhythm—oxidative capacity reaches its peak late in the evening and is lowest in the early afternoon hours [
24]. Disruption of this rhythm by limiting sleep may have a negative effect on the results of muscle strength measurements. Studies on rats deprived of sleep for 96 h have shown that sleep deprivation can even reduce the weight and cross-section of muscle fiber, while on the other hand, the next 96 h are enough to partially restore the changed morphology [
25].
The daily rhythm, which is an important determinant of the strength generated by the muscle, is similar to that of deep body temperature [
26]. Racinas et al. [
27] also demonstrated the relation between the temperature of the skin surface and the body and strength generated by the muscle. Higher internal muscle temperature causes, among other things, faster conduction of action potentials in muscle fibers and acceleration of energy metabolism in muscle tissue [
28]. In the study conducted by Vaara et al. [
29], the body temperature decreased after 60 h of sleep deprivation. Although the results of deep body temperature measurement are strongly influenced by the type of the applied measurement method [
30], changes in body temperature under the impact of a circadian rhythm disturbance seem to be a significant factor explaining the influence of sleep deprivation on muscle strength of the studied physical education students.
Changes in the concentration of anabolic and catabolic hormones are another effect of sleep deprivation, which may be associated with a change in muscle strength [
31]. It can be assumed that sleep duration disturbs the physiology of muscles and weakens their regeneration due to increased stimulation of protein degradation, which is harmful to protein synthesis and promotes muscle atrophy [
32]. This thesis has been confirmed by a previous study conducted by Kant et al. [
33], in which a slight decrease in cortisol levels in urine after 72 h of sleep deprivation and a significant increase in urea was observed. This indicates increased muscle proteolysis due to sleep deprivation. The Everson and Crowley [
34] studies show that, under sleep deprivation conditions, the IGF-1 hormone concentration in rats decreases. IGF-1 is an anabolic hormone that has an important role in protein synthesis and thus in muscle mass maintenance [
32]. The results of Bucci et al. [
35] show that the loss of strength is associated with lower IGF-1 levels in humans. Similar conclusions were reached by Cappola et al. [
36]. Among the population studied, low levels of IGF-1 were associated with weak strength of knee extensor muscles, low walking speed and difficulties in independent movement. These data suggest that a decrease in concentration of this hormone may also be the cause of muscle strength changes after sleepless nights in healthy and able-bodied students of physical education.
Furthermore, our research suggests that domination of lower limbs may be associated with changes in the strength of right lower limb extensor muscles due to sleep deprivation. Differences in the strength of extensor muscles under the influence of deprivation between the experimental and control groups were found to be significant for those who indicated the lower left limb as dominant. Other studies also indicate that limb muscle strength is related to, among others, limb domination [
37]. Lanshammar and Ribom [
38] report that women experience a significant asymmetry of lower limb muscle strength when bending the non-dominant limb and straightening the dominant lower limb. In this group of respondents, the strength of muscles in the dominant lower limb was on average 8.6% weaker in knee flexors, but 5.3% stronger in knee extensors than in the non-dominant lower limb. Meanwhile, the results of Williams et al. [
39] show significantly greater failure in voluntary activation and neuromuscular propagation with sustained activity for the non-dominant compared with the dominant side, and no effect of dominance on MVC torque, endurance time, and fatigue-induced changes in EMG median frequency and elicited torques. These results suggest that the preferential use of elbow flexor muscles with the dominant arm leads to more fatigue resistance in certain structures/mechanisms of the neuromuscular system, but not in others [
39]. Zijdewind et al. [
40] studied mechanical and electrical properties for the first dorsal interosseous muscle of the dominant and non-dominant hand. At the end of the fatigue test, burst force had decreased to about the same extent in the both hands. The final decline in first M-wave (muscle action potential) amplitude was, however, significantly more pronounced for the non-dominant hand than for the dominant hand. Comparisons among the non-dominant hand of various individuals demonstrated the presence of significant inter-individual differences in fatigue-related force-drop without any associated differences in M-wave decline. Intra-individual variability was similar for fatigue-related force-drop and M-wave decline. However, Incel et al. [
41] concluded that the dominant hand is significantly stronger in right-handed subjects, but no such significant difference between sides could be documented for left-handed people. The percentage of stronger nondominant hand grips was 10.93% and 33.33% for right- and left-handed groups respectively. This may be the explanation of why, in our studies, strength changes are not significant for people with right dominant limbs. This may be because the dominant limb is stronger in right-footed subjects, and therefore the stronger muscle reacts less to sleep deprivation. Nevertheless, this issue requires further investigation.
Each of the above-mentioned changes in the physiology and morphology of muscles may constitute a predictive factor explaining the effect of the 24-h sleep deprivation on muscle strength. However, it may be difficult to identify the main factor responsible for changes or lack of changes in muscle strength after sleep deprivation, as this may be the result of overall changes in the muscles, which may differ individually.
There are some limitations in our research that should be acknowledged. Primarily, sleeping behavior of the participants during the day was not controlled. Secondly, tests have been conducted on a small group of people. The subjects were young, healthy and physically fit. All this can affect the fact that 24 h of sleep deprivation are not enough to observe considerable changes in this population. In such studies, a blind test cannot be carried out for obvious organizational reasons. On this basis, it is possible to determine future directions. When the subjects are young and physically fit people, we should apply no less than 48 h of sleep deprivation to observe significant changes in test results. When we conduct tests, we should motivate the participants to partake in the effort as much as possible. An interesting direction of further research in one study would be to compare the impact of sleep deprivation on physically fit people (but not athletes) and those who do not undertake additional physical activity. This will allow evaluation of how the initial level of fitness of the subjects affects the decrease in physical fitness under the influence of sleep deprivation.