Relationship between Leg Strength and Balance and Lean Body Mass. Benefits for Active Aging
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Instruments
- Do not eat or drink for at least 4 h prior to testing.
- Do not practice any PA for at least 4 h prior to testing.
- Do not consume alcohol for at least 24 h prior to testing.
- Empty one’s bladder 30 min before testing.
- Remove any metal objects, such as jewellery and watches, prior to testing.
2.3. Procedure
2.4. Statistical Analysis
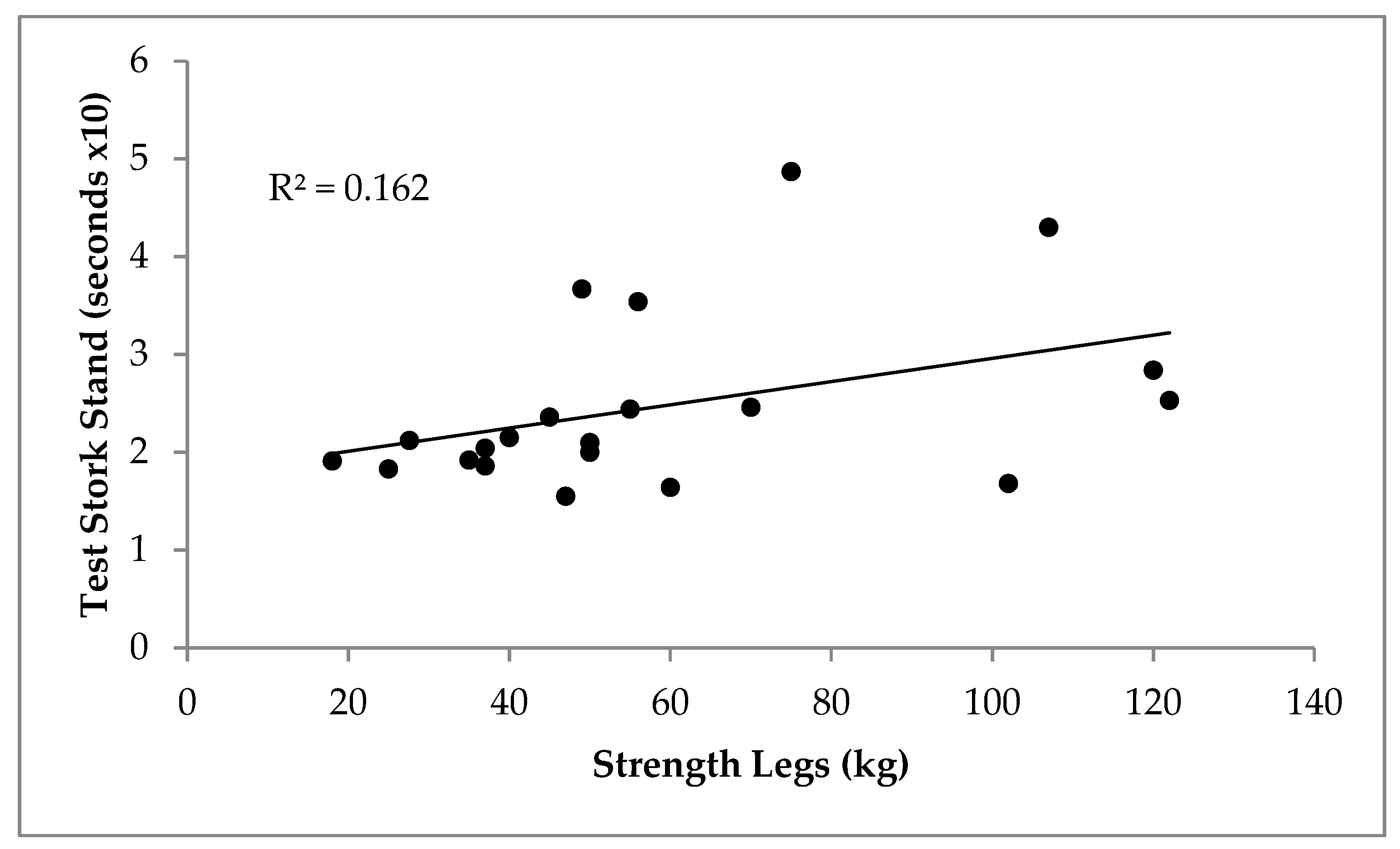
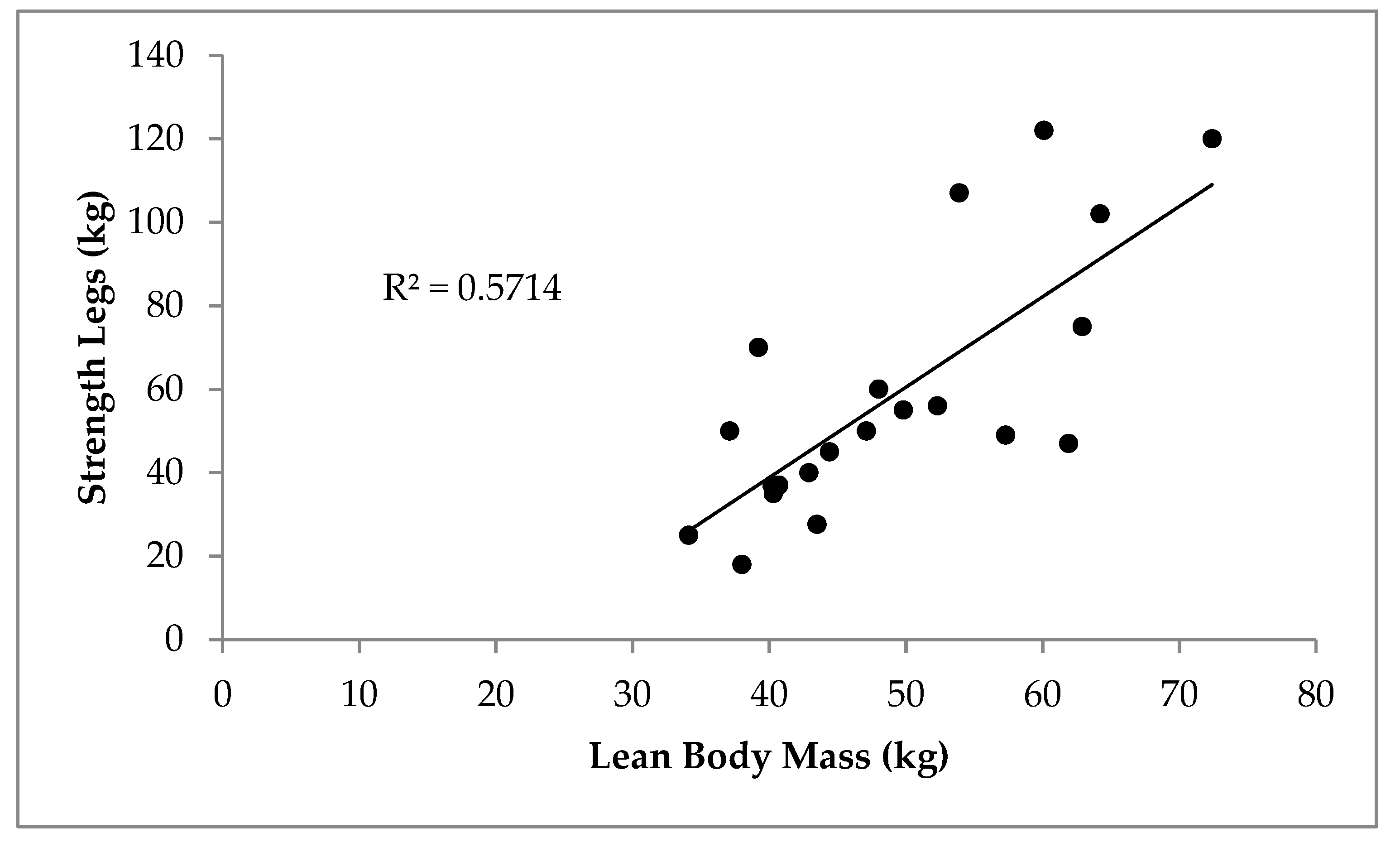
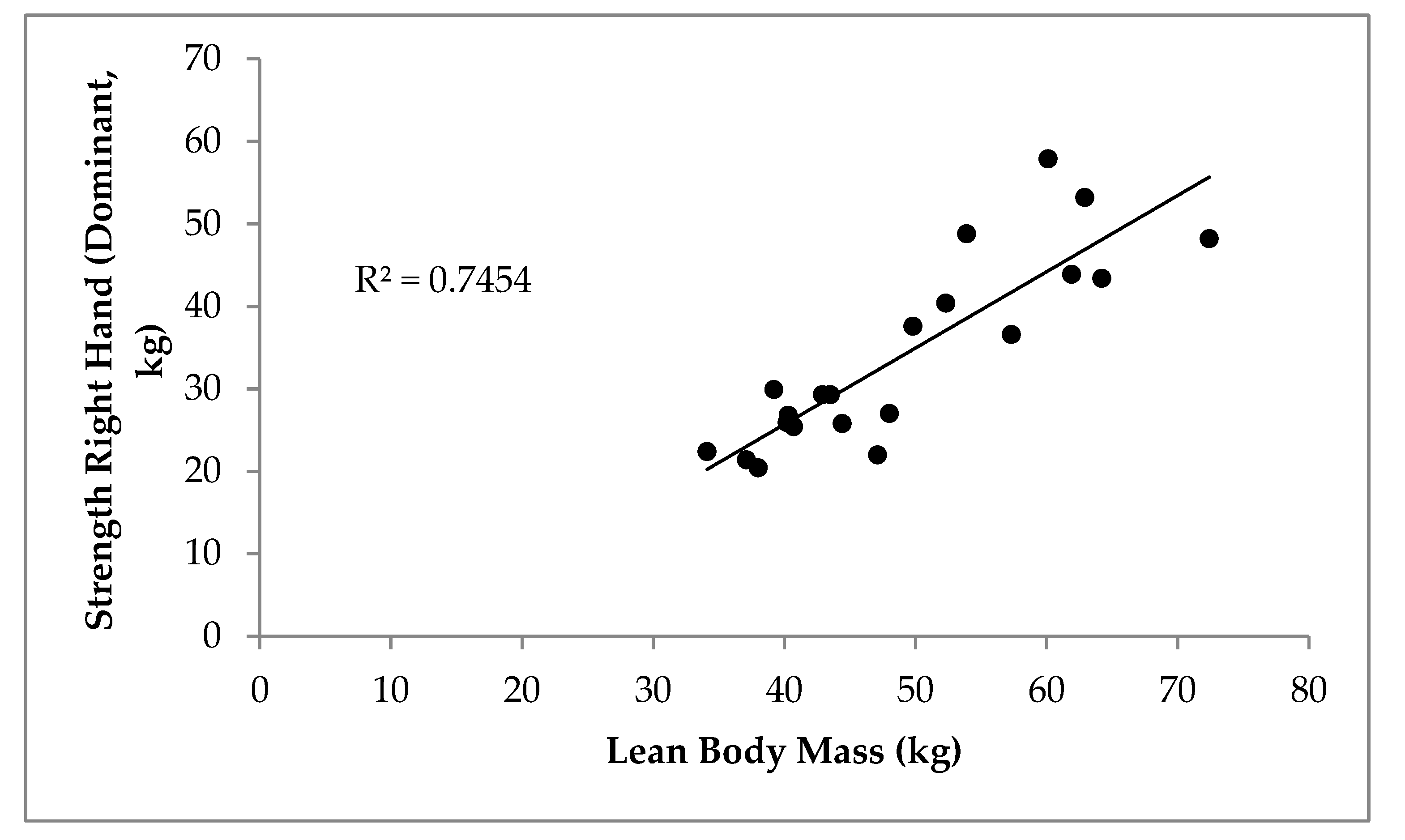
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gasque, P.; Conejo, R.; De Francisco Pascual, J.L.; Lam, A.; Novella, J. Características basales y funcionales de una población que inicia un programa de ejercicio físico. Selección 2005, 14, 108–119. [Google Scholar]
- American College of Sports Medicine. Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med. Sci. Sports Exerc. 1998, 30, 975–991. [Google Scholar]
- Fernández-García, J.C.; Alvero-Cruz, J.R.; Alvarez Carnero, E.; Barrera-Expósito, J.; Carrillo de Albornoz-Gil, M.; Martin-Fernández, M.C. Estimación de la composición corporal en personas mayores. II International Congress of Physical Activity in elderly. In Consejería de Turismo, Comercio y Deporte; Instituto Andaluz del Deporte: Malaga, Spain, 2007. [Google Scholar]
- Regidor, E.; Gutiérrez-Fisac, J.L.; Alfaro, M. Indicadores de Salud 2009. In Evolución de los Indicadores del Estado de Salud en España y su Magnitud en el Contexto de la Unión Europea; Ministerio de Sanidad y Política Social: Madrid, Spain, 2009. [Google Scholar]
- Zhang, J.; Feldblum, P.J.; Fortney, J.A. Moderate physical activity and bone density among perimenopausal women. Am. J. Public Health 1992, 82, 736–738. [Google Scholar]
- Castillo-Rodríguez, A.; Chinchilla-Minguet, J.L. Cardiovascular program to improve physical fitness in those over 60 years old–pilot study. Clin. Interv. Aging 2014, 9, 1269–1275. [Google Scholar] [CrossRef]
- Bae, C.Y.; Kang, Y.G.; Suh, Y.S.; Han, J.H.; Kim, S.S.; Shim, K.W. A model for estimating body shape biological age based on clinical parameters associated with body composition. Clin. Interv. Aging 2013, 8, 11–18. [Google Scholar]
- Poehlman, E.T.; Toth, M.J.; Fishman, P.S.; Vaitkevicius, P.; Gottlieb, S.S.; Fisher, M.L.; Fonong, T. Sarcopenia in aging humans: The impact of menopause and disease. J. Gerontol. A Biol. Sci. Med. Sci. 1995, 50, 73. [Google Scholar]
- He, Q.; Heo, M.; Heshka, S.; Wang, J.; Pierson, R.N., Jr.; Albu, J.; Gallagher, D. Total body potassium differs by sex and race across the adult age span. Am. J. Clin. Nutr. 2003, 78, 72–77. [Google Scholar]
- Katula, J.A.; Sipe, M.; Rejeski, W.J.; Focht, B.C. Strength training in older adults: An empowering intervention. Med. Sci. Sports Exerc. 2006, 38, 106–111. [Google Scholar]
- Gallagher, D.; Visser, M.; De Meersman, R.E.; Sepúlveda, D.; Baumgartner, R.N.; Pierson, R.N.; Heymsfield, S.B. Appendicular skeletal muscle mass: Effects of age, gender, and ethnicity. J. Appl. Physiol. 1997, 83, 229–239. [Google Scholar]
- Peake, J.; Della Gatta, P.; Cameron-Smith, D. Aging and its effects on inflammation in skeletal muscle at rest and following exercise-induced muscle injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, 1485–1495. [Google Scholar]
- Colado, J.C.; Garcia-Masso, X.; Rogers, M.E.; Tella, V.; Benavent, J.; Dantas, E.H. Effects of aquatic and dry land resistance training devices on body composition and physical capacity in postmenopausal women. J. Hum. Kinet. 2012, 32, 185–195. [Google Scholar]
- Sorkin, J.D.; Muller, D.C.; Andres, R. Longitudinal change in height of men and women: Implications for interpretation of the body mass index: The Baltimore Longitudinal Study of Aging. Am. J. Epidemiol. 1999, 150, 969–977. [Google Scholar]
- Short, K.R.; Vittone, J.L.; Bigelow, M.L.; Proctor, D.N.; Nair, K.S. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am. J. Physiol. Endocrinol. Metab. 2004, 286, 92–101. [Google Scholar]
- Idler, N.; Teuner, C.M.; Hunger, M.; Holle, R.; Ortlieb, S.; Schulz, H.; von Berg, A. The association between physical activity and healthcare costs in children–results from the GINIplus and LISAplus cohort studies. BMC Public Health 2015, 15, 437. [Google Scholar]
- Arriscado, D.; Muros, J.J.; Zabala, M.; Dalmau, J.M. Relationship between physical fitness and body composition in primary school children in northern Spain (Logroño). Nutr. Hosp. 2014, 30, 385–394. [Google Scholar]
- Correa Rodríguez, M.; Rueda Medina, B.; González Jiménez, E.; Navarro Pérez, C.F.; Schmidt-RioValle, J. The levels of bone mineralization are influenced by body composition in children and adolescents. Nutr. Hosp. 2014, 30, 763–768. [Google Scholar]
- Kelley, G.A.; Kelley, K.S.; Pate, R.R. Effects of exercise on BMI z-score in overweight and obese children and adolescents: A systematic review with meta-analysis. BMC Pediatr. 2014, 14, 225. [Google Scholar]
- Ekelund, U.; Anderssen, S.A.; Froberg, K.; Sardinha, L.B.; Andersen, L.B.; Brage, S. European Youth Heart Study Group. Independent associations of physical activity and cardiorespiratory fitness with metabolic risk factors in children: The European youth heart study. Diabetologia 2007, 50, 1832–1840. [Google Scholar]
- Morales-Suárez-Varela, M.M.; Clemente-Bosch, E.; Llopis-González, A. Relationship between the level of physical activity and markers of cardiovascular health in Valencian adolescents (Spain). Arch. Argent. Pediatr. 2013, 111, 398–404. [Google Scholar]
- Voss, C.; Sandercock, G.; Higgins, J.W.; Macdonald, H.; Nettlefold, L.; Naylor, P.J.; McKay, H. A cross-cultural comparison of body composition, physical fitness and physical activity between regional samples of Canadian and English children and adolescents. Can. J. Public Health 2014, 105, 245–250. [Google Scholar]
- López Sánchez, G.F.; López Sánchez, L.; Díaz Suárez, A. Efectos de un programa de actividad física en la condición física de escolares con TDAH. Revista Iberoamericana de Ciencias de la Actividad Física y el Deporte 2014, 3, 24–37. [Google Scholar]
- Ortega, F.B.; Ruiz, J.R.; Castillo, M.J. Physical activity, physical fitness, and overweight in children and adolescents: Evidence from epidemiologic studies. Endocrinol. Nutr. 2013, 60, 458–469. [Google Scholar]
- Andreasen, C.H.; Andersen, G. Gene-environment interactions and obesity—Further aspects of genome wide association studies. Nutrition 2009, 25, 998–1003. [Google Scholar]
- Fernández-García, J.C.; Castillo-Rodríguez, A.; Onetti-Onetti, W. Influencia del sobrepeso y la obesidad sobre la fuerza en la infancia. Nutr. Hosp. 2019, 36, 1055–1060. [Google Scholar]
- Onetti, W.; Álvarez-Kurogi, L.; Castillo-Rodríguez, A. Adherencia al patrón de dieta mediterránea y autoconcepto en adolescentes. Nutr. Hosp. 2019, 36, 658–664. [Google Scholar]
- Tercedor Sánchez, P. Actividad Física, Condición Física y Salud; Wanceulen Editorial Deportiva: Sevilla, Spain, 2001. [Google Scholar]
- WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. Available online: http://www.wma.net/en/30publications/10policies/b3/index.html (accessed on 1 July 2016).
- Mohan, V.; Shafiqah, N.; Shamsaimon, B.; Izzat, M.; Japri, B.; Effendi, N. Fore arm circumference and hand length predicts maximal hand grip strength among Malaysian population. Middle East J. Sci. Res. 2014, 21, 634–639. [Google Scholar]
- Johnson, B.L.; Nelson, J.K. Practical Measurements for Evaluation in Physical Education, 4th ed.; Macmillan: Minneapolis, MN, USA, 1979. [Google Scholar]
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.M.; Goodpaster, B.H.; Kritchevsky, S.B. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J. Gerontol. Biol. Sci. 2006, 61, 72–77. [Google Scholar]
- Ruiz, J.; Sui, X.; Lobelo, F.; Morrow, J.; Allen, W.; Jackson, J.A. Association between muscular strength and mortality in men: Prospective cohort study. BMJ J. 2008, 337, 1–9. [Google Scholar]
- Lauretani, F.; Russo, C.R.; Bandinelli, S.; Bartali, B.; Cavazzini, C.; Di Iorio, A.; Ferrucci, L. Age-associated changes in skeletal muscles and their effect on mobility: An operational diagnosis of sarcopenia. J. Appl. Physiol. 2003, 95, 1851–1860. [Google Scholar]
- Iannuzzi-Sucich, M.; Prestwood, K.M.; Kenny, A.M. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J. Gerontol. Biol. Sci. 2002, 57, 772–777. [Google Scholar]
- Rantanen, T.; Guralnik, J.M.; Foley, D.; Masaki, K.; Leveille, S.; Curb, J.D.; White, E. Midlife hand grip strength as a predictor of old age disability. JAMA 1999, 281, 558–560. [Google Scholar]
- Guimarães, B.R.; Pimenta, L.D.; Massini, D.A.; Dos Santos, D.; da Cruz Siqueira, L.O.; Simionato, A.R.; Pessôa Filho, D.M. Muscle strength and regional lean body mass influence on mineral bone health in young male adults. PLoS ONE 2018, 13, 1–13. [Google Scholar]
- Raso, V.; Cassilhas, R.C.; Santana, M.G.D.; Boscolo, R.A.; Viana, V.A.R.; Grassmann, V.; Mello, M.T.D. Predictors of muscle strength in older individuals. Med Express 2016, 3, 1–8. [Google Scholar]
- Castillo-Rodriguez, A.; Onetti-Onetti, W.; Chinchilla-Minguet, J.L. Perceived Quality in Sports Centers in Southern Spain: A Case Study. Sustainability 2019, 11, 3983. [Google Scholar]
- Onetti-Onetti, W.; Castillo-Rodríguez, C.L.; Castillo-Rodríguez, A. Assessment of elderly people characteristics and their relationship with the perceived quality of sport management. Revista Iberoamericana de Ciencias de la Actividad Física y el Deporte 2018, 7, 110–118. [Google Scholar]
- Gijón-Conde, T.; Gorostidi, M.; Camafort, M.; Abad-Cardiel, M.; Martín-Rioboõ, E.; Morales-Olivas, F.; De La Sierra, A. Documento de la Sociedad Española de Hipertensión-Liga Española para la Lucha contra la Hipertensión Arterial (SEH-LELHA) sobre las guías ACC/AHA 2017 de hipertensión arterial. Hipertens Riesgo Vasc. 2018, 35, 119–129. [Google Scholar]
- Bozanic, A.; Toro, P.; Formiga, F. Proyecto DIABDEM: Estudio piloto de la prevalencia de deterioro cognitivo en diabetes mellitus en 2 países hispánicos. Rev. Esp. Geriatr. Gerontol. 2019, 54, 339–345. [Google Scholar]
| Category | Seconds |
|---|---|
| Excellent | >50 |
| Good | 40–50 |
| Mean | 25–39 |
| Regular | 10–24 |
| Poor | <10 |
| Strength: Left Hand (kg) | Strength: Right Hand (kg) | Strength: Legs (kg) | |
|---|---|---|---|
| Impedance (Ohm) | −0.594** | −0.671** | −0.519** |
| Fat Mass (FM) (%) | −0.609** | −0.455** | −0.445** |
| Lean Body Mass (LBM) (kg) | 0.782** | 0.833** | 0.705** |
| Total Water (kg) | 0.787** | 0.842** | 0.699** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo-Rodríguez, A.; Onetti-Onetti, W.; Sousa Mendes, R.; Luis Chinchilla-Minguet, J. Relationship between Leg Strength and Balance and Lean Body Mass. Benefits for Active Aging. Sustainability 2020, 12, 2380. https://doi.org/10.3390/su12062380
Castillo-Rodríguez A, Onetti-Onetti W, Sousa Mendes R, Luis Chinchilla-Minguet J. Relationship between Leg Strength and Balance and Lean Body Mass. Benefits for Active Aging. Sustainability. 2020; 12(6):2380. https://doi.org/10.3390/su12062380
Chicago/Turabian StyleCastillo-Rodríguez, Alfonso, Wanesa Onetti-Onetti, Rui Sousa Mendes, and José Luis Chinchilla-Minguet. 2020. "Relationship between Leg Strength and Balance and Lean Body Mass. Benefits for Active Aging" Sustainability 12, no. 6: 2380. https://doi.org/10.3390/su12062380
APA StyleCastillo-Rodríguez, A., Onetti-Onetti, W., Sousa Mendes, R., & Luis Chinchilla-Minguet, J. (2020). Relationship between Leg Strength and Balance and Lean Body Mass. Benefits for Active Aging. Sustainability, 12(6), 2380. https://doi.org/10.3390/su12062380








