Urban Rivers as Dispersal Corridors: Which Factors Are Important for the Spread of Alien Woody Species along the Danube?
Abstract
1. Introduction
2. Materials and Methods
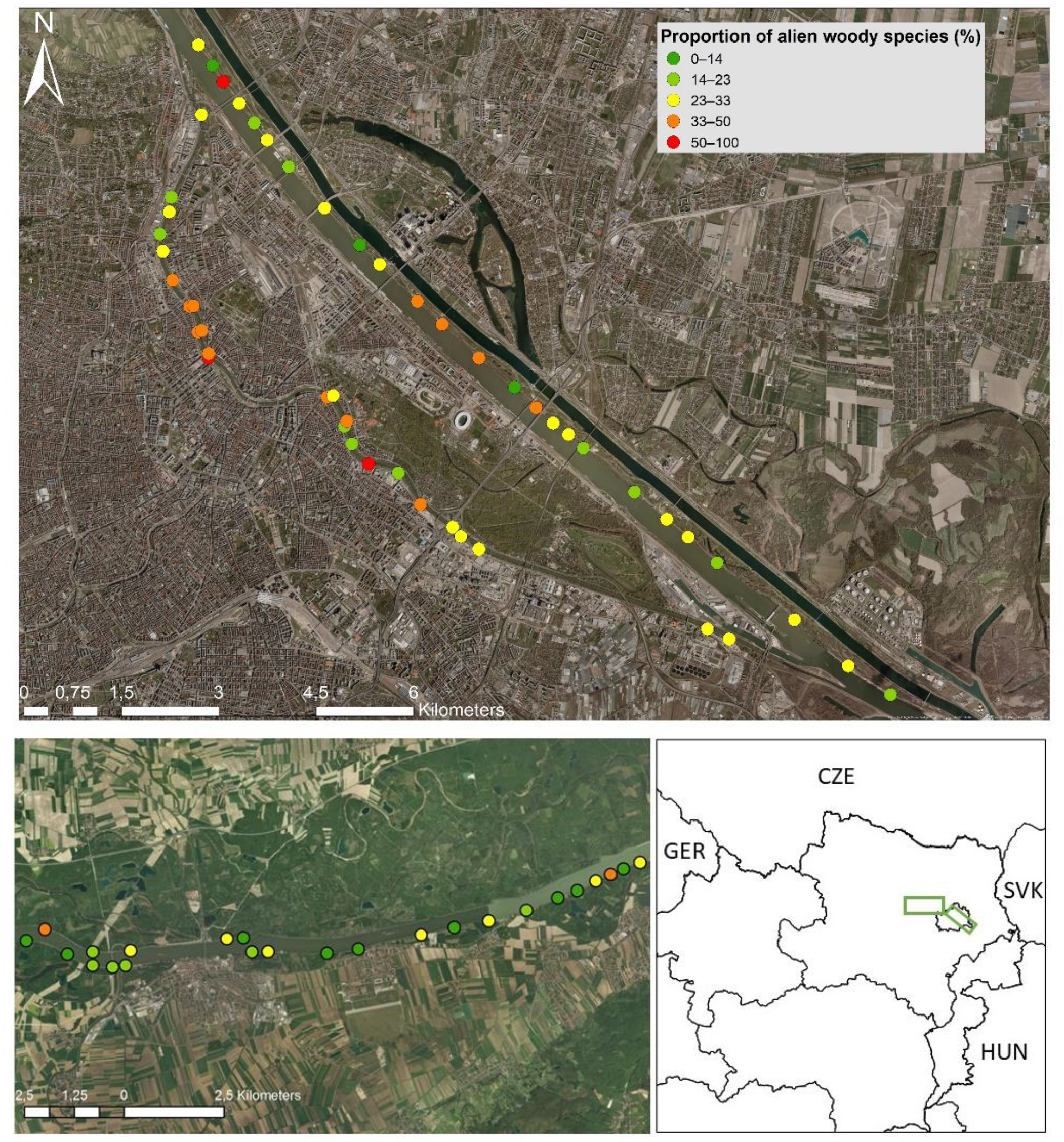
2.1. Study Region and Sites
2.2. Plot Selection, Species and Environmental Data Compilation
2.3. Statistical Analyses
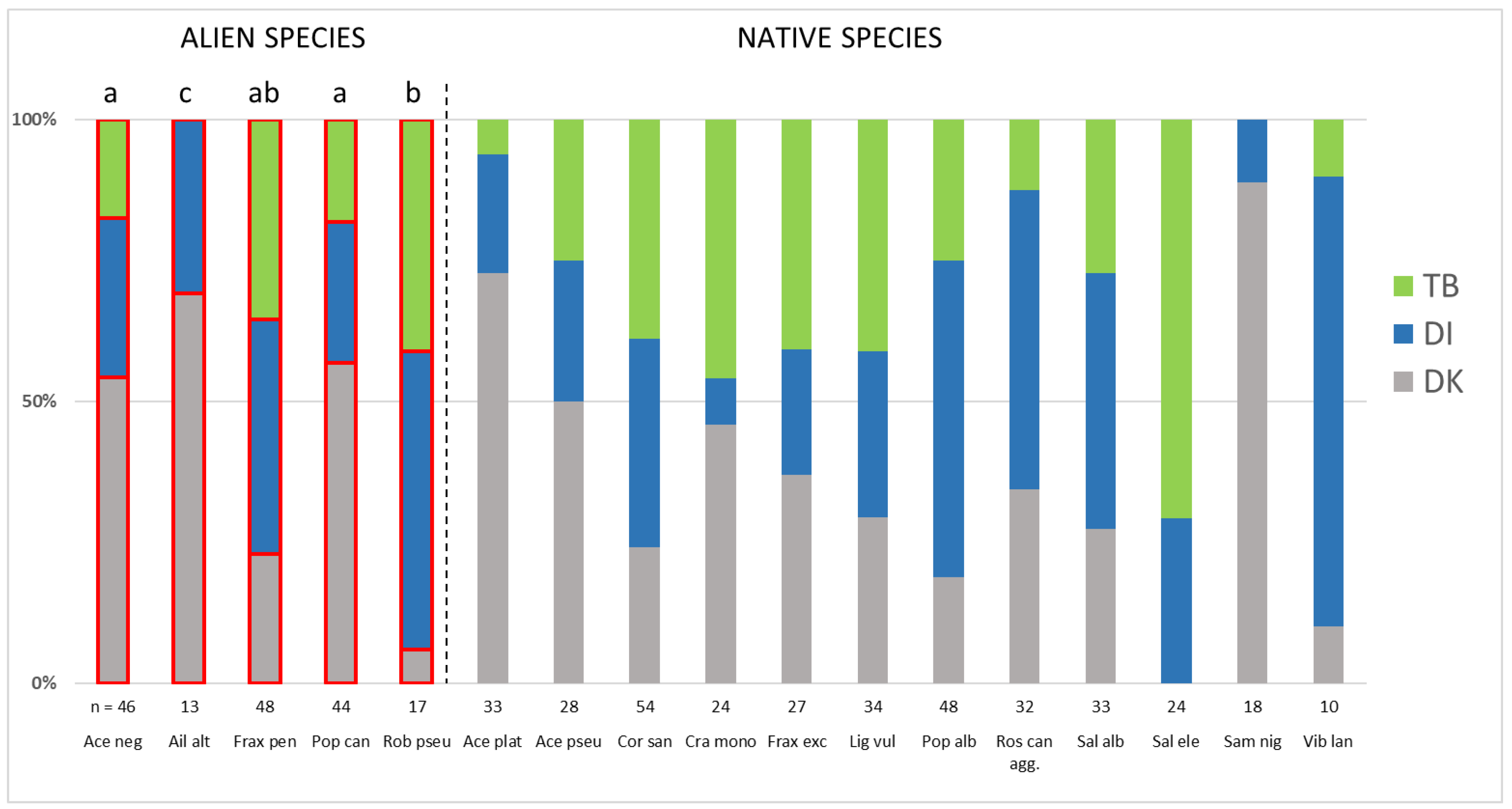
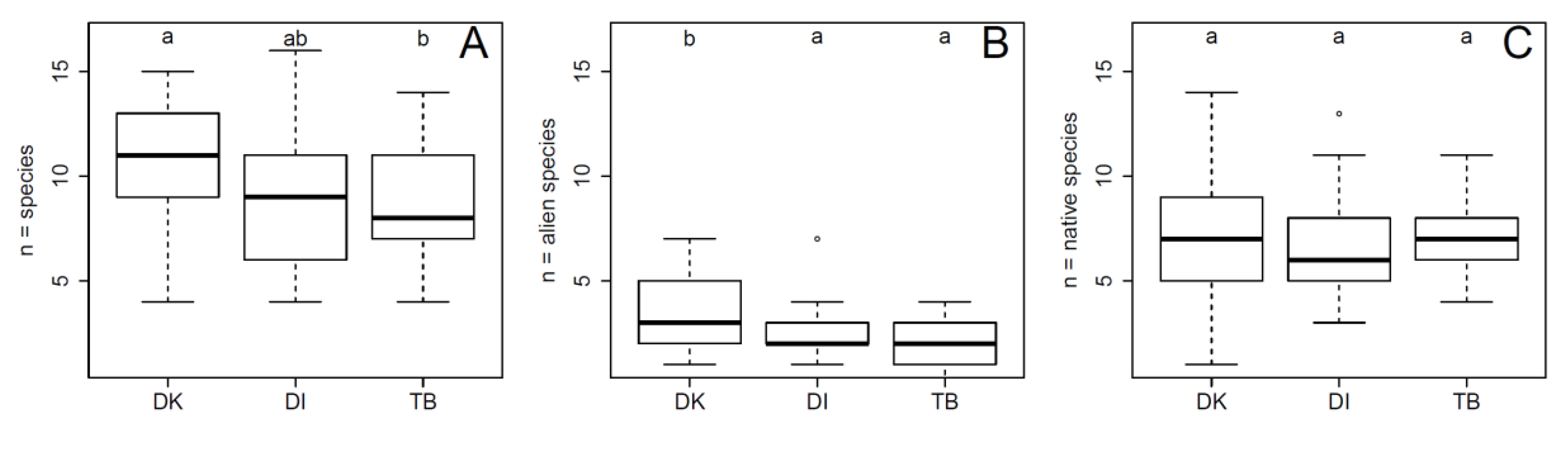
3. Results
4. Discussion
4.1. The Distribution of Alien Woody Species in River Corridors along an Urban–Peri-Urban–Rural Gradient
4.2. The Distribution of Widespread Alien Woody Species across Study Regions and the Underlying Factors
4.3. Implications for Management and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Winter, M.; Schweiger, O.; Klotz, S.; Nentwig, W.; Andriopoulos, P.; Arianoutsou, M.; Basnou, C.; Delipetrou, P.; Didžiulis, V.; Hejda, M.; et al. Plant extinctions and introductions lead to phylogenetic and taxonomic homogenization of the European flora. Proc. Natl. Acad. Sci. USA 2009, 106, 21721–21725. [Google Scholar] [CrossRef]
- Chytrý, M.; Maskell, L.C.; Pino, J.; Pyšek, P.; Vilà, M.; Font, X.; Smart, S.M. Habitat invasions by alien plants: A quantitative comparison among Mediterranean, subcontinental and oceanic regions of Europe. J. Appl. Ecol. 2008, 45, 448–458. [Google Scholar] [CrossRef]
- Chytrý, M.; Pyšek, P.; Tichý, L.; Knollová, I.; Danihelka, J. Invasions by alien plants in the Czech Republic: A quantitative assessment across habitats. Preslia 2005, 77, 339–354. [Google Scholar]
- Pyšek, P. Alien and native species in Central European urban floras: A quantitative comparison. J. Biogeogr. 1998, 25, 155–163. [Google Scholar] [CrossRef]
- Aronson, M.F.J.; La Sorte, F.A.; Nilon, C.H.; Katti, M.; Goddard, M.A.; Lepczyk, C.A.; Warren, P.S.; Williams, N.S.G.; Cilliers, S.; Clarkson, B.; et al. A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc. R. Soc. B Biol. Sci. 2014, 281, 20133330. [Google Scholar] [CrossRef]
- Onandia, G.; Schittko, C.; Ryo, M.; Bernard-Verdier, M.; Heger, T.; Joshi, J.; Kowarik, I.; Gessler, A. Ecosystem functioning in urban grasslands: The role of biodiversity, plant invasions and urbanization. PLoS ONE 2019, 14, e0225438. [Google Scholar] [CrossRef]
- Kowarik, I. On the role of alien species in urban flora and vegetation. In Urban Ecology; Marzloff, J.M., Shulenberger, E., Endlicher, W., Alberti, M., Bradley, G., Ryan, C., Simon, U., ZumBrunnen, C., Eds.; Springer: Boston, MA, USA, 2008; pp. 321–338. [Google Scholar]
- Kühn, I.; Brandl, R.; Klotz, S. The flora of German cities is naturally species rich. Evol. Ecol. Res. 2004, 6, 749–764. [Google Scholar]
- Mayer, K.; Haeuser, E.; Dawson, W.; Essl, F.; Kreft, H.; Pergl, J.; Pyšek, P.; Weigelt, P.; Winter, M.; Lenzner, B.; et al. Naturalization of ornamental plant species in public green spaces and private gardens. Biol. Invasions 2017, 19, 3613–3627. [Google Scholar] [CrossRef]
- Pilsl, P.; Schröck, C.; Kaiser, R.; Gewolf, S.; Nowotny, G.; Stöhr, O. Neophytenflora der Stadt Salzburg. Sauteria. Verlag Alexander Just Dorfbeuern/Salzburg 2008, 17, 1–608. [Google Scholar]
- von der Lippe, M.; Kowarik, I. Do cities export biodiversity? Traffic as dispersal vector across urban-rural gradients. Divers. Distrib. 2008, 14, 18–25. [Google Scholar] [CrossRef]
- Williams, N.S.G.; Hahs, A.K.; Vesk, P.A. Urbanisation, plant traits and the composition of urban floras. Perspect. Plant. Ecol. Evol. Syst. 2015, 17, 78–86. [Google Scholar] [CrossRef]
- Säumel, I.; Kowarik, I. Propagule morphology and river characteristics shape secondary water dispersal in tree species. Plant. Ecol. 2013, 214, 1257–1272. [Google Scholar] [CrossRef]
- Schmiedel, D.; Tackenberg, O. Hydrochory and water induced germination enhance invasion of Fraxinus pennsylvanica. For. Ecol. Manag. 2013, 304, 437–443. [Google Scholar] [CrossRef]
- Säumel, I.; Kowarik, I. Urban rivers as dispersal corridors for primarily wind-dispersed invasive tree species. Landsc. Urban. Plan. 2010, 94, 244–249. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D. Traits associated with invasiveness in alien plants: Where do we stand? In Biological Invasions; Nentwig, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 97–125. [Google Scholar]
- Krumm, F.; Vitkova, L. Introduced Tree Species in European Forests: Opportunities and Challenges; European Forest Institute: Joensuu, Finland, 2016; pp. 1–426. [Google Scholar]
- Richardson, D.M.; Rejmánek, M. Trees and shrubs as invasive alien species—A global review. Divers. Distrib. 2011, 17, 788–809. [Google Scholar] [CrossRef]
- Botham, M.S.; Rothery, P.; Hulme, P.E.; Hill, M.O.; Preston, C.D.; Roy, D.B. Do urban areas act as foci for the spread of alien plant species? An assessment of temporal trends in the UK. Divers. Distrib. 2009, 15, 338–345. [Google Scholar] [CrossRef]
- Muhar, S.; Muhar, A.; Siegrist, D.; Egger, G. Rivers of the Alps: Diversity in Nature and Culture; Haupt Verlag: Bern, Switzerland, 2019; pp. 1–480. [Google Scholar]
- ZAMG. Digitaler Klimaatlas Österreichs; Zentralanstalt für Meteorologie und Geodynamik: Vienna, Austria, 2010. [Google Scholar]
- Ließ, N.; Drescher, A. Ailanthus altissima spreading in the Danube National Park-possibilities of control. Neobiota 2008, 7, 84–95. [Google Scholar]
- Buchmann, B.; Sterk, H.; Schickl, R. Der Donaukanal: Geschichte-Planung-Ausführung. Available online: https://www.wien.gv.at/stadtentwicklung/studien/pdf/b003547.pdf (accessed on 29 February 2020).
- Michlmayr, F. Die Grundzüge des Donauinsel-Projekts. Denisia 2002, 3, 11–25. [Google Scholar]
- Essl, F.; Rabitsch, W. Neobiota in Österreich; Umweltbundesamt: Dessau-Roßlau, Germany, 2002; pp. 1–432. [Google Scholar]
- Fischer, M.A.; Oswald, K.; Adler, W. Exkursionsflora für Österreich, Liechtenstein, Südtirol; Biologiezentrum der oö: Linz, Austria, 2008; pp. 1–1392. [Google Scholar]
- Statistik Austria. Regionalstatistische Rastereinheiten. Available online: http://www.statistik.at/web_de/klassifikationen/regionale_gliederungen/regionalstatistische_rastereinheiten/index.html (accessed on 12 December 2019).
- Kuttner, M.; Essl, F.; Peterseil, J.; Dullinger, S.; Rabitsch, W.; Schindler, S.; Hülber, K.; Gattringer, A.; Moser, D. A new high-resolution habitat distribution map for Austria, Liechtenstein, southern Germany, South Tyrol and Switzerland. Eco.mont 2015, 7, 18–29. [Google Scholar] [CrossRef]
- Brian, A.; Venables, B.; Bates, D.M.; Firth, D.; Ripley, M.B. Package ‘MASS’. Available online: https://cran.r-project.org/web/packages/MASS/MASS.pdf (accessed on 12 December 2019).
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 14 January 2020).
- Rinaldo, A.; Gatto, M.; Rodriguez-Iturbe, I. River networks as ecological corridors: A coherent ecohydrological perspective. Adv. Water Resour. 2018, 112, 27–58. [Google Scholar] [CrossRef] [PubMed]
- Höfle, R.; Dullinger, S.; Essl, F. Different factors affect the local distribution, persistence and spread of alien tree species in floodplain forests. Basic Appl. Ecol. 2014, 15, 426–434. [Google Scholar] [CrossRef]
- Catford, J.A.; Daehler, C.C.; Murphy, H.T.; Sheppard, A.W.; Hardesty, B.D.; Westcott, D.A.; Rejmánek, M.; Bellingham, P.J.; Pergl, J.; Horvitz, C.C.; et al. The intermediate disturbance hypothesis and plant invasions: Implications for species richness and management. Perspect. Plant. Ecol. Evol. Syst. 2012, 14, 231–241. [Google Scholar] [CrossRef]
- Essl, F.; Mang, T.; Moser, D. Ancient and recent alien species in temperate forests: Steady state and time lags. Biol. Invasions 2012, 14, 1331–1342. [Google Scholar] [CrossRef]
- Morimoto, J.; Kominami, R.; Koike, T. Distribution and characteristics of the soil seed bank of the black locust (Robinia pseudoacacia) in a headwater basin in northern Japan. Landscape. Ecol. Engen. 2010, 6, 193–199. [Google Scholar] [CrossRef]
- Prots, B.; Drescher, A.; Vykhor, B. Invasion ecology of Green ash Fraxinus pennsylvanica Marsh. in the Transcarpathia (Ukraine). Biol. Syst. 2011, 3, 269–276. [Google Scholar]
- Walter, J.; Essl, F.; Englisch, T.; Kiehn, M. Neophytes in Austria: Habitat preferences and ecological effects. Neobiota 2005, 6, 13–25. [Google Scholar]
- Smulders, M.J.M.; Beringen, R.; Volosyanchuk, R.; Vanden Broeck, A.; van der Schoot, J.; Arens, P.; Vosman, B. Natural hybridisation between Populus nigra L. and P. x canadensis Moench. Hybrid offspring competes for niches along the Rhine river in the Netherlands. Tree Genet. Genom. 2008, 4, 663–675. [Google Scholar] [CrossRef]
- Wickert, K.L.; O’Neal, E.S.; Davis, D.D.; Kasson, M.T. Seed production, viability, and reproductive limits of the invasive Ailanthus altissima (tree-of-heaven) within invaded environments. Forests 2017, 8, 226. [Google Scholar] [CrossRef]
- Neilreich, A. Flora von Wien. Eine Aufzählung der in den Umgebungen Wiens Wild Wachsenden oder im Grossen Gebauten Gefäßpflanzen, Nebst einer Pflanzengeografischen Übersicht; F. Beck Universitäts-Buchhandlung: Vienna, Austria, 1849. [Google Scholar]
- Wessely, J. Wiener Alleebäume. Oesterr Monatsschr. Forstwes. 1871, 21, 417–420. [Google Scholar]
- Kowarik, I.; Säumel, I. Biological Flora of Central Europe: Ailanthus altissima (Mill.) Swingle. Perspect. Plant. Ecol. Evol. Syst. 2007, 8, 207–237. [Google Scholar] [CrossRef]
- Drescher, A.; Magnes, M. Anthropochoren im Nationalpark Donau-Auen-Ziel von Bekämpfungsmaßnahmen oder Bereicherung der Biodiversität? Wallner, R., Ed.; Aliens. Neobiota in Österreich: Vienna, Austria, 2002; pp. 141–144. [Google Scholar]
- Cierjacks, A.; Kowarik, I.; Joshi, J.; Hempel, S.; Ristow, M.; von der Lippe, M.; Weber, E. Biological Flora of the British Isles: Robinia pseudoacacia. J. Ecol. 2013, 101, 1623–1640. [Google Scholar] [CrossRef]
- Gaertner, M.; Novoa, A.; Fried, J.; Richardson, D.M. Managing invasive species in cities: A decision support framework applied to Cape Town. Biol. Invasions 2017, 19, 3707–3723. [Google Scholar] [CrossRef]
- Lapin, K.; Oettel, J.; Steiner, H.; Langmaier, M.; Sustic, D.; Starlinger, F.; Kindermann, G.; Frank, G. Invasive alien plant species in unmanaged forest reserves, Austria. Neobiota 2019, 96, 71–96. [Google Scholar] [CrossRef]
| Species | Donaukanal | Donauinsel | Tullner Becken | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| h | s | t | h | s | t | h | s | t | h | s | t | |
| Acer negundo | 8 | 11 | 6 | 5 | 6 | 2 | 1 | 3 | 4 | 14 | 20 | 12 |
| Ailanthus altissima | 3 | 2 | 4 | 1 | 1 | 2 | 0 | 0 | 0 | 4 | 3 | 6 |
| Fraxinus pennsylvanica | 5 | 2 | 4 | 3 | 8 | 9 | 7 | 8 | 2 | 15 | 18 | 15 |
| Populus x canadensis | 4 | 1 | 20 | 2 | 2 | 7 | 2 | 0 | 6 | 8 | 3 | 33 |
| Robinia pseudoacacia | 0 | 0 | 1 | 5 | 4 | 0 | 1 | 2 | 4 | 6 | 6 | 5 |
| Alien Woody Species | Predictor | Intercept | Std. Error | p-Value |
|---|---|---|---|---|
| Acer negundo | population | 0.61 | 0.25 | 0.02 |
| urban | 0.61 | 0.25 | 0.02 | |
| disturbance | 0.23 | 0.38 | 0.54 | |
| Ailanthus altissima | population | 2.40 | 0.62 | <0.01 |
| urban | 1.49 | 0.47 | <0.01 | |
| disturbance | 0.54 | 0.53 | 0.31 | |
| Fraxinus pennsylvanica | population | 0.02 | 0.26 | 0.93 |
| urban | −0.18 | 0.27 | 0.51 | |
| disturbance | −0.34 | 0.39 | 0.38 | |
| Populus x canadensis | population | −0.52 | 0.43 | 0.23 |
| urban | −1.51 | 0.83 | 0.07 | |
| disturbance | −0.65 | 0.47 | 0.18 | |
| Robinia pseudoacacia | population | −1.58 | 0.32 | 0.07 |
| urban | −0.79 | 0.38 | 0.04 | |
| disturbance | −0.01 | 0.49 | 0.98 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagner, S.; Moser, D.; Essl, F. Urban Rivers as Dispersal Corridors: Which Factors Are Important for the Spread of Alien Woody Species along the Danube? Sustainability 2020, 12, 2185. https://doi.org/10.3390/su12062185
Wagner S, Moser D, Essl F. Urban Rivers as Dispersal Corridors: Which Factors Are Important for the Spread of Alien Woody Species along the Danube? Sustainability. 2020; 12(6):2185. https://doi.org/10.3390/su12062185
Chicago/Turabian StyleWagner, Sabrina, Dietmar Moser, and Franz Essl. 2020. "Urban Rivers as Dispersal Corridors: Which Factors Are Important for the Spread of Alien Woody Species along the Danube?" Sustainability 12, no. 6: 2185. https://doi.org/10.3390/su12062185
APA StyleWagner, S., Moser, D., & Essl, F. (2020). Urban Rivers as Dispersal Corridors: Which Factors Are Important for the Spread of Alien Woody Species along the Danube? Sustainability, 12(6), 2185. https://doi.org/10.3390/su12062185




