Mitigation of Heat Stress in Solanum lycopersicum L. by ACC-deaminase and Exopolysaccharide Producing Bacillus cereus: Effects on Biochemical Profiling
Abstract
1. Introduction
2. Materials and Methods
2.1. Source and Growth Conditions of Bacteria
2.2. Heat Stress Tolerance Assay
2.3. Biochemical Characterization of Bacteria
In Vitro Screening of Bacillus cereus for Plant Growth Promoting Traits
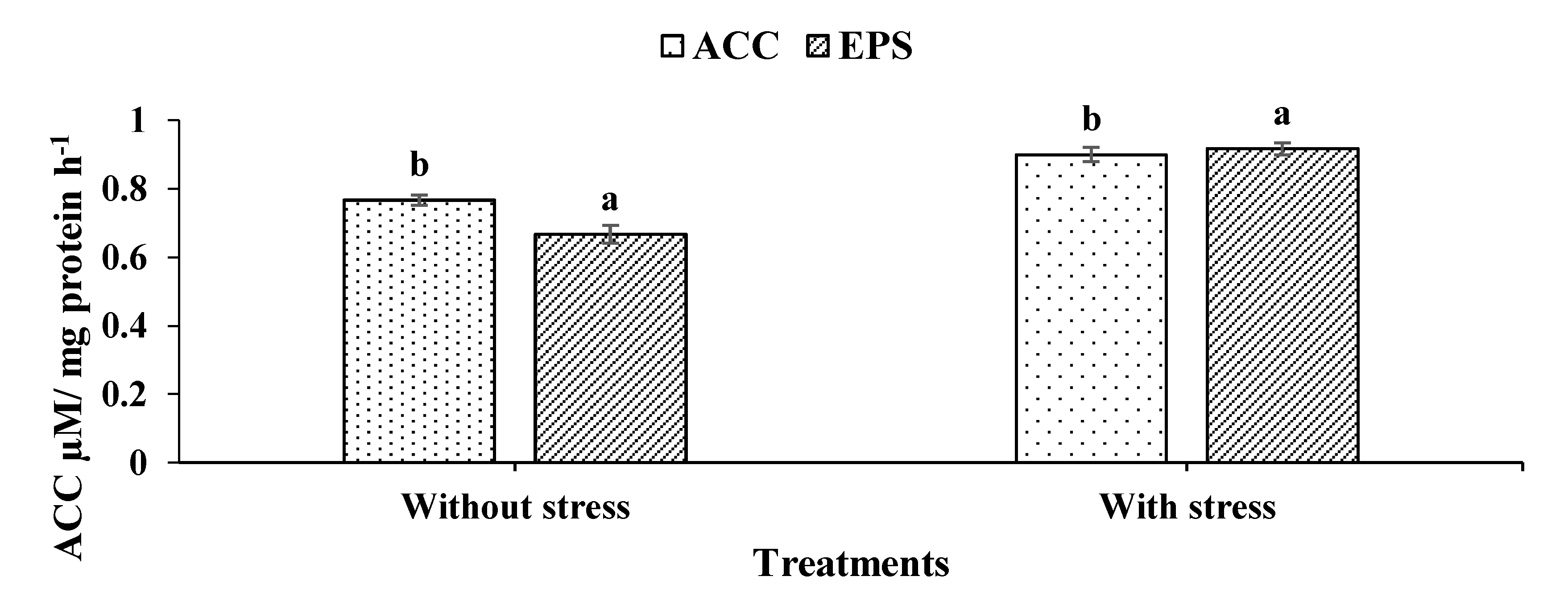
2.4. Screening of Bacillus cereus for ACC Deaminase Activity
Qualitative and Quantitative Assays
2.5. Exopolysaccharide Production (EPS)
2.6. Extracellular Enzyme Assays
2.7. Biochemical Characterization
2.8. Screening for Antibiotic Resistance
2.9. Quantification of Plant Growth Regulators
2.10. Phylogenetic Characterization of Bacterial Isolate
2.11. Greenhouse Experiment
2.11.1. Seed Sterilization and Inoculation with PGPR
2.11.2. Experimental Design and Setup
2.11.3. Plant Growth Traits
2.11.4. Relative Water Content (RWC)
2.11.5. Effects of Bacillus cereus on Photosynthetic Pigments under Normal and Heat Stress Conditions
2.11.6. Antioxidant Enzyme Assays
2.12. Statistical Analysis
3. Results
3.1. Bacterial Isolation and Screening for Heat Tolerance
3.2. Morphological-Physiological and Biochemical Traits of Selected Bacterial Strain
3.3. Strain Identification and Accession Number
3.4. Response of Plant Biomass to the Inoculation of Bacillus cereus
3.5. Relative Water Content
3.6. Chlorophyll Contents
3.7. Protein and Proline Contents
3.8. Antioxidant Activities
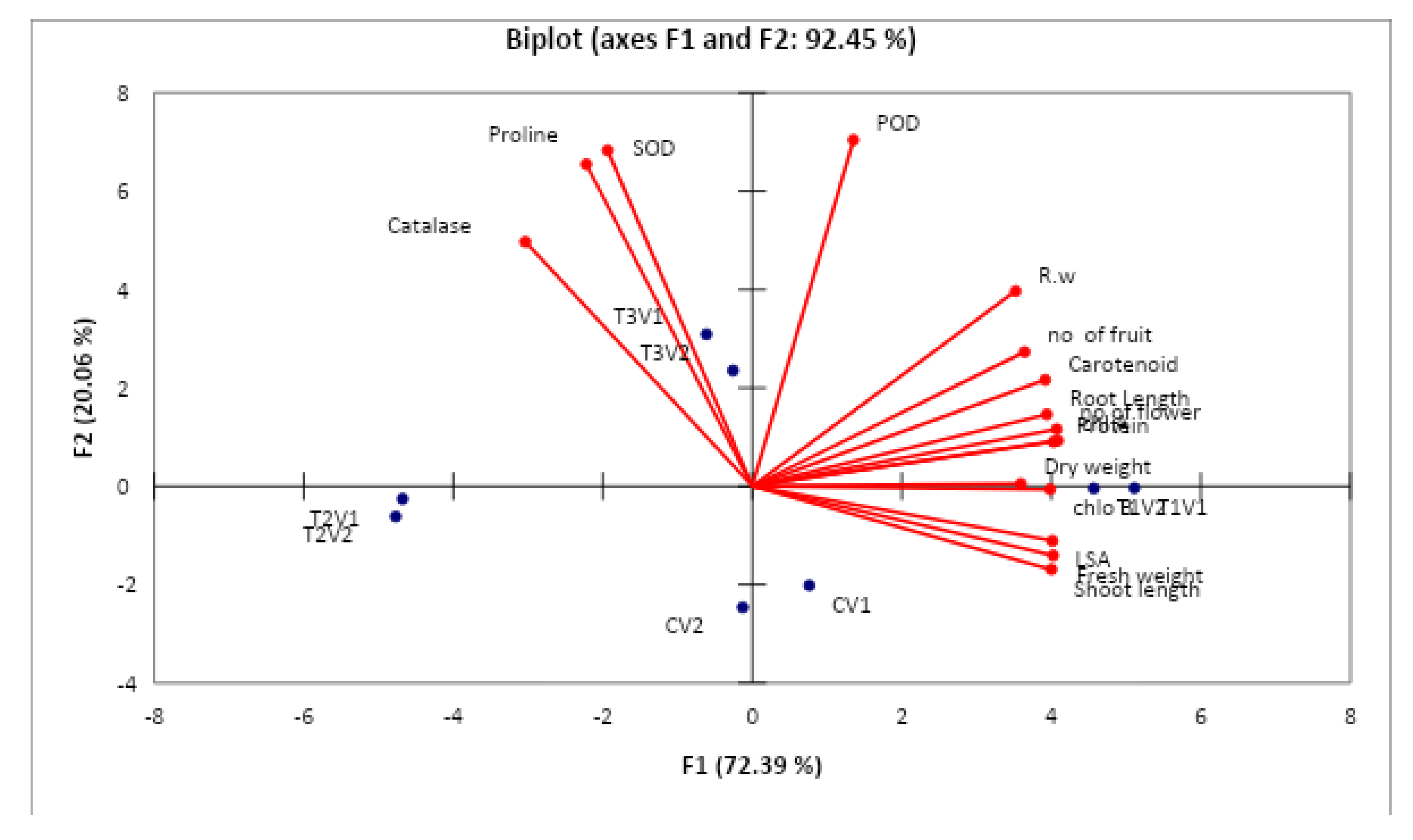
3.9. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grover, M.; Ali, S.Z.; Sandhya, V.; Rasul, A.; Venkateswarlu, B. Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J. Micro Biotech. 2011, 27, 1231–1240. [Google Scholar] [CrossRef]
- Carbonell-Bojollo, R.; Veroz-Gonzalez, O.; Ordoñez-Fernandez, R.; Moreno-Garcia, M.; Basch, G.; Kassam, A.; Repullo-Ruiberriz de Torres, M.A.; Gonzalez-Sanchez, E.J. The Effect of Conservation Agriculture and Environmental Factors on CO2 Emissions in a Rainfed Crop Rotation. Sustainability 2019, 11, 3955. [Google Scholar] [CrossRef]
- Zhang, X.; Rong, X.; Cai, M.; Meng, Q. Collaborative Optimization of Emissions and Abatement Costs for Air Pollutants and Greenhouse Gases from the Perspective of Energy Structure: An Empirical Analysis in Tianjin. Sustainability. 2019, 11, 3872. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Ihsan, M.Z. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Ullah, I.; Ali, S.; Kang, S.M.; Lee, I.J. Alleviation of salt stress response in soybean plants with the endophytic bacterial isolate Curtobacterium sp. SAK1. Ann. Microbiol. 2019, 69, 797–808. [Google Scholar] [CrossRef]
- Barnabas, B.; Katalin, J.; Feher, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef]
- Hedhly, A.; Hormaza, J.I.; Herrero, M.A. Global warming and sexual plant reproduction. Trends Plant Sci. 2009, 14, 30–36. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Djanaguiraman, M. Response of floret fertility and individual grain weight of wheat to high temperature stress: Sensitive stages and thresholds for temperature and duration. Funct. Plant Biol. 2014, 41, 1261–1269. [Google Scholar] [CrossRef]
- Devasirvatham, V.; Gaur, P.M.; Mallikarjuna, N.; Tokachichu, R.N.; Trethowan, R.M.; Tan, D.K. Effect of high temperature on the reproductive development of chickpea genotypes under controlled environments. Funct. Plant Biol. 2012, 39, 1009–1018. [Google Scholar] [CrossRef]
- Sita, K.; Sehgal, A.; Kumar, J.; Kumar, S.; Singh, S.; Siddique, K.H.; Nayyar, H. Identification of high-temperature tolerant lentil (Lens culinaris Medik.) genotypes through leaf and pollen traits. Front. Plant Sci. 2017, 8, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Bains, T.S.; Bindumadhava, H.; Nayyar, H. Responses of mungbean (Vigna radiata L.) genotypes to heat stress: Effects on reproductive biology, leaf function and yield traits. Sci. Hortic. 2015, 197, 527–541. [Google Scholar] [CrossRef]
- Farooq, M.; Bramley, H.; Palta, J.A.; Siddique, K.H.M. Heat stress in wheat during reproductive and grain-filling phases. CRC Crit. Rev. Plant Sci. 2011, 30, 491–507. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Djanaguiraman, M.; Perumal, R.; Ciampitti, I.A. Impact of high temperature stress on floret fertility and individual grain weight of grain sorghum: Sensitive stages and thresholds for temperature and duration. Front. Plant Sci. 2015, 6, 1–11. [Google Scholar] [CrossRef]
- Mattioli, R.; Marchese, D.; D’Angeli, S.; Altamura, M.M.; Costantino, P.; Trovato, M. Modulation of intracellular proline levels affects flowering time and inflorescence architecture in Arabidopsis. Plant Mol. Biol. 2008, 66, 277–288. [Google Scholar] [CrossRef]
- Siddique, A.; Kandpal, G.; Kumar, P.J. Proline accumulation and its defensive role under diverse stress condition in pants: An overview. Pure Appl. Microbiol. 2018, 12, 1655–1659. [Google Scholar] [CrossRef]
- Sato, S.; Kamiyama, M.; Iwata, T.; Makita, N.; Furukawa, H.; Ikeda, H. Moderate increase of mean daily temperature adversely affects fruit set of Lycopersicon esculentum by disrupting specific physiological processes in male reproductive development. Ann. Bot. 2006, 97, 731–738. [Google Scholar] [CrossRef]
- Gill, S.S.; Gill, R.; Trivedi, D.K.; Anjum, N.A.; Sharma, K.K.; Ansari, M.W.; Ansar, A.A.; Johri, A.K.; Prasad, R.; Pereira, E.; et al. Piriformospora indica: Potential and significance in plant stress tolerance. Front. Microbiol. 2016, 7, 332. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC- deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Wang, C.J.; Yang, W.; Wang, C.; Gu, C.; Niu, D.D.; Liu, H.X.; Wang, Y.P.; Guo, J.H. Induction of drought tolerance in cucumber plants by a consortium of three plant growth-promoting rhizobacterium strains. PLoS ONE 2012, 7, e52565. [Google Scholar] [CrossRef]
- Vardharajula, S.; Ali, S.Z.; Grover, M.; Reddy, G.; Venkateswaralu, B. Effect of plant growth promoting Pseudomonas spp. on compatible solutes antioxidant status and plant growth of maize under drought stress. Plant Growth Regul. 2010, 62, 21–30. [Google Scholar]
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant growth promoting bacteria that confer resistance to water stress in tomato and pepper. Plant Sci. 2004, 166, 525–530. [Google Scholar] [CrossRef]
- Sarma, R.K.; Saikia, R. Alleviation of drought stress in mung bean by strain Pseudomonas aeruginosa GGRJ21. Plant Soil. 2014, 377, 111–126. [Google Scholar] [CrossRef]
- Han, Q.Q.; Lü, X.P.; Bai, J.P.; Qiao, Y.; Paré, P.W.; Wang, S.M.; Wang, Z.L. Beneficial soil bacterium Bacillus subtilis (GB03) augments salt tolerance of white clover. Front. Plant Sci. 2014, 5, 525. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Singh, P.; Tiwari, R.; Meena, K.K.; Yandigeri, M.; Singh, D.P.; Arora, D.K. Salt-tolerant rhizobacteria-mediated induced tolerance in wheat (Triticum aestivum) and chemical diversity in rhizosphere enhance plant growth. Biol. Fertil. Soil. 2011, 47, 907–916. [Google Scholar] [CrossRef]
- Rafique, M.; Ortas, I.; Ahmed, I.A.; Rizwan, M.; Afridi, M.S.; Sultan, T.; Chaudhary, H.J. Potential impact of biochar types and microbial inoculants on growth of onion plant in differently textured and phosphorus limited soils. J. Environ. Manag. 2019, 247, 672–680. [Google Scholar] [CrossRef]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of plant growth promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef]
- Warnita, W.; Ardi, A.; Zulfa, Y. Effect of mulch and indigenous rhizobacteria isolate on growth and yield of potato (Solanum tuberosum L.). Asian J. Agric. Biol. 2019, 239–245. [Google Scholar]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Sandhya, V.; Ali, S.K.Z.; Grover, M.; Reddy, G.; Venkateswarlu, B. Alleviation of drought stress effects in sunflower seedlings by exopolysaccharides producing Pseudomonas putida strain P45. Biol. Fertil. Soil. 2009, 46, 17–26. [Google Scholar] [CrossRef]
- Bramhachari, P.V.; Dubey, S. Isolation and characterization of exopolysaccharide produced by Vibrio harveyi strain VB23. Lett. App. Microbiol. 2006, 43, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R.; Penrose, D.M.; Li, J. A model for the lowering of plant ethylene concentrations by plant growth promoting bacteria. J. Theor. Biol. 1998, 190, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Zahir, Z.A.; Ghani, U.; Naveed, M.; Nadeem, S.M.; Asghar, H.N. Comparative effectiveness of Pseudomonas and Serratia sp. containing ACC-deaminase for improving growth and yield of wheat (Triticum aestivum L.) under salt-stressed conditions. Arch. Microbiol. 2009, 191, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Dell’Amico, J.; Torrecilla, A.; Rodríguez, P.; Morte, A.; Sánchez-Blanco, M.J. Responses of tomato plants associated with the arbuscular mycorrhizal fungus Glomus clarum during drought and recovery. J. Agric. Sci. 2002, 138, 387–393. [Google Scholar] [CrossRef]
- Ronga, D.; Gallingani, T.; Zaccardelli, M.; Perrone, D.; Francia, E.; Milc, J.; Pecchioni, N. Carbon footprint and energetic analysis of tomato production in the organic vs. the conventional cropping systems in Southern Italy. J. Clean. Prod. 2019, 220, 836–845. [Google Scholar] [CrossRef]
- World Processing Tomato Council (WPTC). Available online: www.wptc.to/pdf/releases/WPTC%20crop%20update%20as%20of%2025%20October%202018.pdf (accessed on 24 July 2019).
- Caradonia, F.; Ronga, D.; Catellani, M.; Azevedo, C.V.G.; Terrazas, R.A.; Robertson-Albertyn, S.; Francia, E.; Bulgarell, D. Nitrogen Fertilizers Shape the Composition and Predicted Functions of the Microbiota of Field-Grown Tomato Plants. Phytobiomes J. 2019, 3, 315–325. [Google Scholar] [CrossRef]
- Babu, A.N.; Jogaiah, S.S.I.I.; Nagaraj, A.K.; Tran, L.S.P. Improvement of growth, fruit weight and early blight disease protection of tomato plants by rhizosphere bacteria is correlated with their beneficial traits and induced biosynthesis of antioxidant peroxidase and polyphenol oxidase. Plant Sci. 2015, 231, 62–73. [Google Scholar] [CrossRef]
- Yasmin, S.; Hafeez, F.Y.; Mirza, M.S.; Rasul, M.; Arshad, H.M.; Zubair, M.; Iqbal, M. Biocontrol of bacterial leaf blight of rice and profiling of secondary metabolites produced by rhizospheric Pseudomonas aeruginosa BRp3. Front. Microbiol. 2017, 8, 1895. [Google Scholar] [CrossRef]
- Gupta, R.R.; Singal, R.; Shanker, A.; Kuhad, R.C.; Saxena, R.K. A modified plate assay for screening phosphate solubilizing microorganisms. J. Gen. Appl. Microbiol. 1994, 40, 255–260. [Google Scholar] [CrossRef]
- Loper, J.; Schroth, M. Influence of bacterial sources of indole-3-acetic acid on root elongation of sugar beet. Phytopathology 1986, 76, 386–389. [Google Scholar] [CrossRef]
- Ahmad, F.; Ahmad, I.; Khan, M.S. Indole acetic acid production by the indigenous isolates of Azotobacter and fluorescent Pseudomonas in the presence and absence of tryptophan. Tur. J. Biol. 2005, 29, 29–34. [Google Scholar]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Castric, P.A. Hydrogen cyanide, a secondary metabolite of Pseudomonas aeruginosa. Can. J. Microbiol. 1975, 21, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R.; Karaturovic, D.M.; Newell, P.C. A novel procedure for rapid isolation of plant growth promoting pseudomonas. Can. J. Microbiol. 1995, 41, 533–536. [Google Scholar] [CrossRef]
- Dworkin, M.; Foster, J. Experiments with some microorganisms which utilize ethane and hydrogen. J. Bacteriol. 1958, 75, 592. [Google Scholar] [CrossRef]
- Penrose, D.M.; Glick, B.R. Methods for isolating and characterizing ACC-deaminase containing plant growth-promoting rhizobacteria. Physiol. Plantarum. 2003, 118, 10–15. [Google Scholar] [CrossRef]
- Mu’minah, B.; Subair, H.F. Isolation and screening Bacterial Exopolysaccharide (EPS) from potato rhizosphere in highland and the potential as a producer Indole Acetic Acid (IAA). Procedia Food Sci. 2015, 3, 74–81. [Google Scholar] [CrossRef]
- Kazempour, M.N. Biological control of Rhizoctonia solani, the causal agent of rice sheath blight by antagonistic bacteria in greenhouse and field conditions. Plant Pathol. J. 2004, 3, 88–96. [Google Scholar]
- Adebiyi, C.A.B.; Akinyaanju, J.A. Thermophillic amylase producers from the soil. Niger. J. Sci. Technol. 1998, 11, 30–38. [Google Scholar]
- Namasivayam, E.; John, R.D.; Mariappan, K.; Jiji, A.; Kumar, M.; Jayaraj, R.L. Production of extracellular pectinase by bacillus cereus isolated from market solid waste. J. Bioanal. Biomed. 2011, 3, 70–75. [Google Scholar] [CrossRef]
- Hussain, A.; Kamran, M.A.; Javed, M.T.; Hayat, K.; Farooq, M.A.; Ali, N.; Chaudhary, H.J. Individual and combinatorial application of Kocuria rhizophila and citric acid on phytoextraction of multi-metal contaminated soils by Glycine max L. Environ. Exp. Bot. 2019, 159, 23–33. [Google Scholar] [CrossRef]
- Yasmin, H.; Bano, A. Isolation and characterization of phosphate solubilizing bacteria from rhizosphere soil of weeds of khewra salt range. Pak. J. Bot. 2011, 43, 1663–1668. [Google Scholar]
- Ma, Y.; Oliveira, R.S.; Nai, F.; Rajkumar, M.; Luo, Y.; Rocha, I.; Freitas, H. The hyper accumulator Sedum plumbizincicola harbors metal- resistant endophytic bacteria that improve its phytoextraction capacity in multi-metal contaminated soil. J. Environ. Manag. 2015, 156, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Saber, M.A.F.; Abdelhafez, A.A.; Hassan, E.A.; Ramadan, E.M. Characterization of fluorescent pseudomonads isolates and their efficiency on the growth promotion of tomato plant. Ann. Agric. Sci. 2015, 60, 131–140. [Google Scholar] [CrossRef][Green Version]
- Batool, R.; Rehman, S.U.; Rafique, M.; Amna; Ali, J.; Mukhtar, T.; Mahmood, S.; Sultan, T.; Munis, F.H.; Chaudhary, H.J. Biocontrol potential of Bacillus gibsonii and Brevibacterium frigoritolerans in suppression of Fusarium stalk rot of maize: A sustainable approach. Asian J. Agric. Biol. 2019, 7, 320–333. [Google Scholar]
- Balestri, M.; Bottega, S.; Spano, C. Response of Pteris vittata to different cadmium treatments. Acta Physiol. Plant 2014, 36, 767–775. [Google Scholar] [CrossRef]
- Afridi, M.S.; Amna, S.; Mahmood, T.; Salam, A.; Mukhtar, T.; Mehmood, S.; Ali, J.; Khatoon, Z.; Bibi, M.; Javed, M.T.; et al. Induction of tolerance to salinity in wheat genotypes by plant growth promoting endophytes: Involvement of ACC deaminase and antioxidant enzymes. Plant Physiol. Biochem. 2019, 139, 569–577. [Google Scholar] [CrossRef]
- Lu, T.; Yu, H.; Li, Q.; Chai, L.; Jiang, W. Improving plant growth and alleviating photosynthetic inhibition and oxidative stress from low-light stress with exogenous GR24 in tomato (Solanum lycopersicum L.) seedlings. Fronts. Plant. Sci. 2019, 10, 490. [Google Scholar] [CrossRef]
- Niñerola, A.; Ferrer-Rullan, R.; Vidal-Suñé, A. Climate Change Mitigation: Application of Management Production Philosophies for Energy Saving in Industrial Processes. Sustainability 2020, 12, 717. [Google Scholar] [CrossRef]
- Colantoni, A.; Monarca, D.; Marucci, A.; Cecchini, M.; Zambon, I.; Di Battista, F.; Maccario, D.; Grazia Saporito, M.; Beruto, M. Solar Radiation Distribution inside a Greenhouse Prototypal with Photovoltaic Mobile Plant and Effects on Flower Growth. Sustainability 2018, 10, 855. [Google Scholar] [CrossRef]
- Fikfak, A.; Kosanovic, S.; Konjar, M.P.; Grom, J.; Zbašnik-Senegačnik, M. The Impact of Morphological Features on Summer Temperature Variations on the Example of Two Residential Neighborhoods in Ljubljana, Slovenia. Sustainability 2017, 9, 122. [Google Scholar] [CrossRef]
- Spain, A.; Alm, E. Implications of microbial heavy metal tolerance in the environment. Rev. Undergrad. Res. 2003, 2, 1–6. [Google Scholar]
- Ali, S.; Charles, T.C.; Glick., B.R. Amelioration of high salinity stress damage by plant growth-promoting bacterial endophytes that contain ACC- deaminase. Plant Phys. Biochem. 2014, 80, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Barnawal, D.; Bharti, N.; Maji, D.; Chanotiya, C.S.; Kalra, A. 1-Aminocyclopropane-1-carboxylic acid (ACC) deaminase-containing rhizobacteria protect Ocimum sanctum plants during waterlogging stress via reduced ethylene generation. Plant Phys. Biochem. 2012, 58, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xin, K.; Liu, H.; Cheng, J.; Shen, X.; Wang, Y.; Zhang, L. Pantoea alhagi, a novel endophytic bacterium with ability to improve growth and drought tolerance in wheat. Sci. Rep. 2017, 7, 41564. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, S.M.; Arif, M.S.; Riaz, M.; Iqbal, Z.; Ashraf, M. PGPR with varied ACC- deaminase activity induced different growth and yield response in maize (Zea mays L.) under fertilized conditions. Eur. J. Soil Biol. 2013, 57, 27–34. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J.; Remans, R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 2007, 31, 425–448. [Google Scholar] [CrossRef]
- Iqbal, A.; Hasnain, S. Aeromonas punctata PNS-1: A promising candidate to change the root morphogenesis of Arabidopsis thaliana in MS and sand system. Acta Physiol. Plant. 2013, 35, 657–665. [Google Scholar] [CrossRef]
- Spaepen, S.; Bossuyt, S.; Engelen, K.; Marchal, K.; Vanderleyden, J. Phenotypical and molecular responses of A. thaliana roots as a result of inoculation with the auxin producing bacterium Azospirillum brasilense. N. Phytol. 2014, 201, 850–861. [Google Scholar] [CrossRef]
- Kang, S.M.; Khan, A.L.; You, Y.H.; Kim, J.G.; Kamran, M.; Lee, I.J. Gibberellin production by newly isolated strain Leifsonia soli SE134 and its potential to promote plant growth. J. Microbiol. Biotechnol. 2014, 24, 106–112. [Google Scholar] [CrossRef]
- Barea, J.M.; Brown, M.E. Effects on plant growth produced by Azotobacter paspali related to synthesis of plant growth regulating substances. J. App. Bacteriol. 1974, 37, 583–593. [Google Scholar] [CrossRef]
- Azcon, R.; Barea, J. Synthesis of auxins, gibberellins and cytokinins by Azotobacter vinelandii and Azotobacter bijerinkii related to effects produced on tomato plants. Plant Soil. 1975, 43, 609–619. [Google Scholar] [CrossRef]
- Liu, F.; Xing, S.; Ma, H.; Du, Z.; Ma, B. Cytokinin-producing, plant growth-promoting rhizobacteria that confer resistance to drought stress in Platycladus orientalis container seedlings. Appl. Microbiol. Biotechnol. 2013, 97, 9155–9164. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.Z.; Sandhya, V.; Grover, M.; Linga, V.R.; Bandi, V. Effect of inoculation with a thermo-tolerant plant growth promoting Pseudomonas putida strain AKMP7 on growth of wheat (Triticum spp.) under heat stress. J. Plant. Interact. 2011, 6, 239–246. [Google Scholar] [CrossRef]
- Daim, I.A.A.; Bejai, S.; Meijer, J. Improved heat stress tolerance of wheat seedlings by bacterial seed treatment. Plant Soil. 2014, 379, 337–350. [Google Scholar] [CrossRef]
- Ali, S.Z.; Sandhya, V.; Grover, M.; Kishore, N.; Rao, L.V.; Venkateswarlu, B. Pseudomonas sp. strain AKM-P6 enhances tolerance of sorghum seedlings to elevated temperatures. Biol. Fertil. Soil. 2009, 46, 45–55. [Google Scholar] [CrossRef]
- Chandra, D.; Srivastava, R.; Glick, B.R.; Sharma, A.K. Drought-tolerant Pseudomonas spp. improve the growth performance of finger millet (Eleusine coracana (L.) Gaertn.) under non-stressed and drought-stressed conditions. Pedosphere 2018, 28, 227–240. [Google Scholar] [CrossRef]
- Kinet, J.M.; Peet, M.M. Tomato. In The Physiology of Vegetable Crops; Wien, H.C., Ed.; CAB International: Wallingford, UK, 1997; pp. 207–258. [Google Scholar]
- Marcelis, L.F.M.; Hooijdonk, H.V. Effect of salinity on growth, water use and nutrient use in radish (Raphanus sativus L.). Plant Soil. 1999, 215, 57–64. [Google Scholar] [CrossRef]
- Rehman, S.U.; Afzal, W.; Anjum, T.; Choudhry, H.J.; Ahmad, S.R.; Aslam, M. Screening and evaluation of indigenous halo-tolerant microbes for salt stress alleviation in celery (Apium graveolens). Soil Environ. 2019, 38, 42–47. [Google Scholar] [CrossRef]
- Ali, J.; Mahmood, T.; Hayat, K.; Afridi, M.S.; Ali, F.; Chaudhary, H.J. Phytoextraction of Cr by maize (Zea mays L.). The role of plant growth promoting endophyte and citric acid under polluted soil. Arch. Environ. Protect. 2018, 44, 73–82. [Google Scholar]
- Ansari, F.A.; Ahmad, I. Plant growth promoting attributes and alleviation of salinity stress to wheat by biofilm forming Brevibacterium sp. FAB3 isolated from rhizospheric soil. Saudi J. Biol. Sci 2018. [Google Scholar] [CrossRef]
- Ahamd, M.; Hussain, A.; Akhtar, F.U.Z.M.; Zafar-Ul-Hye, M.; Iqbal, Z.; Naz, T.; Iqbal, M.M. Effectiveness of multi-strain biofertilizer in combination with organic sources for improving the productivity of Chickpea in Drought Ecology. Asian J. Agric. Biol. 2017, 5, 228–237. [Google Scholar]
- Ahmadi, G.; Jaafarinia, M. Evaluating the Effects of Biofertilizers on Growth and Emergence of Barley (HORDEUM VULGARE). Asian J. Agri. Biol. 2015, 3, 125–131. [Google Scholar]
- Xu, H.C.; Tie, C.; Wang, Z.; He, M.R. Physiological basis for the differences of productive capacity among tillers in winter wheat. J. Int. Agr. 2015, 14, 1958–1970. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Ikram, R.; Ali, B. Co-inoculation of auxin producing PGPR and rhizobia enhanced growth of Vigna mungo (L.) under cadmium stress. Asian J. Agric. Biol. 2018, 6, 46–54. [Google Scholar]
| Characteristics | Properties | ||
|---|---|---|---|
| No. of bacterial isolates screened against heat stress 21 | 50°C 12 | 55°C 7 | 60°C 2 |
| Morphological Attributes | Rod shaped, colony colour: off white, form: circular, elevation: convex, margin: lobate, opacity: transparent, gram stain: + (positive), temperature range: 30–C60 °C | ||
| Biochemical attributes | Positive for: IAA, Phosphorous solubilization, ACC-deaminase, EPS production, Ammonia, catalase, amylase, pectinase and protease, CIT (sodium citratrate), Urea (urea hydrolysis), TDA (Tryptophan deaminase), ODC (Ornithine decarboxylase), H2S, IND (indole), GLU (Acid from glucose), MAL (Acid from maltose), SuC (Acid from sucrose), SORB (Acid from sorbitol), INOS (Acid from inositol), MEL (Acid from Melibiose), ADO (Acid from adonitol) and RAF (Acid from Raffinose). Negative for: Hydrogen cyanide, Siderophores, LDC (Lysine decarboxylase), MALO (sodium malonate), ONPG (ortho nitro phenyl β-D-galactopyranoside), ADH (Arginine dihydrolase), VP (Voges proskauer), GEL (Gelatin hydrolysis), MANN (Acid from mannitol), RHAM (Acid from Rhamnose) and ARAB: acid from arabinose. | ||
| Indole Acetic Acid (µg/mL) | Gibberellic Acid (µg/mL) | Kinetin(µg/mL) | |||
|---|---|---|---|---|---|
| NT | HT | NT | HT | NT | HT |
| 0.55 ± 0.026 | 0.44 ± 0.028 | 19.8 ± 1.18 | 14.22 ± 1.01 | 43.6 ± 17.3 | 25.18 ± 4.74 |
| Antibiotics | Diameter (mm) | Resistance Level |
|---|---|---|
| Erythromycin(E15) | 12 | Intermediate |
| Rifampicin (RD5) | 9 | Resistant |
| Ampicilin (AMP) | 3 | Resistant |
| Streptomycin (S10) | 8 | Resistant |
| Chloramphenolicum (C30) | 16 | Susceptible |
| Gentamycin (CN10) | 5 | Resistant |
| Fosomycin (FOS 50) | 12 | Intermediate |
| Spectinomycin (SH25) | 6 | Resistant |
| Neomycin (N10) | 15 | Intermediate |
| Tetracyclin (TE 30) | 2 | Resistant |
| Lincomycin (My15) | 9 | Resistant |
| Clindamycin (DA2) | 8 | Resistant |
| Penicillin (P10) | 10 | Intermediate |
| Kanamycin (K30) | 16 | Susceptible |
| SL (cm) | RL (cm) | FW (g) | DW (g) | LSA (m2) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | Riogrande | Sweetie | Riogrande | Sweetie | Riogrande | Sweetie | Riogrande | Sweetie | Riogrande | Sweetie |
| Control | 50 ± 1.15c | 45.5 ± 0.43d | 15.4 ± 0.46bc | 12.4 ± 0.49d | 39.7 ± 1.07c | 34.3 ± 0.58d | 11.0 ± 1.11c | 11.3 ± 0.38c | 27.2 ±1.58c | 25.5 ± 0.84cd |
| T1 | 65 ± 2.08a | 57.3 ± 0.41b | 22.6 ± 1.21a | 17.1 ± 0.41b | 55.1 ± 1.21a | 47.1 ± 1.02b | 18.0 ± 0.42b | 21.1 ±1.85a | 39.0 ± 0.96a | 31.9 ± 0.94b |
| T2 | 17.1± 0.99g | 15.7 ± 0.437g | 7.6 ± 0.40e | 7.66 ± 0.28e | 11.2 ± 0.34g | 12.8 ± 0.60g | 4.1 ± 0.46e | 8.96 ± 0.26cd | 10.7 ± 0.34f | 9.16 ± 0.31f |
| T3 | 36.5 ± 1.53e | 28.63 ± 0.81f | 15.3 ± 1.09bc | 14.9 ± 0.43c | 29.5 ± 0.52e | 21.8 ± 0.32f | 7.1 ± 0.43d | 15.7 ± 0.89b | 22.6 ± 1.90d | 17.1 ± 0.95e |
| Treatments | Flower Number | Fruit Numbers | ||
|---|---|---|---|---|
| Riogrande | Sweetie | Riogrande | Sweetie | |
| Control | 14.6 ± 1.45b | 13.3 ± 1.45b | 7.66 ± 0.88cd | 8.33 ± 0.88cd |
| T1 | 27.3 ± 2.72a | 28.6 ± 3.28a | 15 ± 4.50 ab | 19.6 ± 0.881a |
| T2 | 7.33 ± 0.88c | 5 ± 1.73c | 3.33 ± 0.33d | 3.66 ± 1.45d |
| T3 | 7.33 ± 0.88b | 15.6 ± 2.02b | 14.3 ± 1.45ab | 9.66 ± 0.33bc |
| Chlorophyll a | Chlorophyll b | Carotenoid | Relative Water Content | |||||
|---|---|---|---|---|---|---|---|---|
| Treatments | Riogrande | Sweetie | Riogrande | Sweetie | Riogrande | Sweetie | Riogrande | Sweetie |
| Control | 1.76 ± 0.03b | 1.38 ± 0.09c | 1.53 ± 0.01c | 1.44 ± 0.02d | 7.83 ± 0.10e | 9.25 ± 0.03d | 53 ± 1.73d | 43.6 ± 1.76e |
| T1 | 2.63 ± 0.06a | 2.84 ± 0.06a | 1.92 ± 0.02a | 1.68 ± 0.024b | 20.3 ± 0.63a | 21.1 ± 0.50a | 74.3 ± 1.20a | 69.6 ± 0.88ab |
| T2 | 0.66 ± 0.02d | 0.48 ± 0.14d | 0.55 ± 0.01f | 0.41 ± 0.05g | 4.19 ± 0.02f | 2.73 ± 0.02g | 44.3 ± 2.60e | 35.3 ± 1.45f |
| T3 | 1.46 ± 0.04c | 1.89 ± 0.11b | 1.36 ± 0.03d | 1.24 ± 0.023e | 11.8 ± 0.28c | 13.3 ± 0.26b | 66.6 ± 0.88bc | 60.3 ± 0.33cd |
| POD (μmolg−1 FW min−1) | SOD (μmolg−1 FW min−1) | CAT (μmolg−1 FW min−1) | Proline (μmolg−1 FW min−1) | Protein (μmolg−1 FW min−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | Riogrande | Sweetie | Riogrande | Sweetie | Riogrande | Sweetie | Riogrande | Sweetie | Riogrande | Sweetie |
| Control | 0.03 ± 0.008g | 0.05 ± 0.008Fg | 2.13 ± 0.03e | 1.64 ± 0.02g | 0.03 ± 0.008e | 0.02 ± 0.002f | 24.4 ± 1.25f | 21.3 ± 1.09g | 3.53 ± 0.03c | 2.23 ± 0.03e |
| T1 | 1.12 ± 0.01d | 1.35 ± 0.02c | 1.87 ± 0.008f | 1.53 ± 0.027g | 0.44 ± 0.01d | 0.34±0.002e | 32.6 ± 0.42d | 28.1 ±0.43e | 5.50 ± 0.05a | 4.85 ± 0.04b |
| T2 | 0.08 ± 0.008ef | 0.12 ± 0.01e | 5.61 ± 0.01c | 4.47 ± 0.026d | 1.71 ± 0.01b | 1.66 ± 0.03b | 46.1 ±0.65c | 48.5 ± 0.82c | 1.14 ± 0.03g | 1.61 ± 0.08f |
| T3 | 2.13 ± 0.0b | 2.82 ± 0.02a | 8.02 ± 0.03a | 7.49 ± 0.19b | 1.93 ± 0.02a | 1.17 ± 0.02c | 68.4 ± 0.55a | 51.6 ± 0.76b | 2.81 ± 0.01c | 3.40 ± 0.04d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukhtar, T.; Rehman, S.u.; Smith, D.; Sultan, T.; Seleiman, M.F.; Alsadon, A.A.; Amna; Ali, S.; Chaudhary, H.J.; Solieman, T.H.I.; et al. Mitigation of Heat Stress in Solanum lycopersicum L. by ACC-deaminase and Exopolysaccharide Producing Bacillus cereus: Effects on Biochemical Profiling. Sustainability 2020, 12, 2159. https://doi.org/10.3390/su12062159
Mukhtar T, Rehman Su, Smith D, Sultan T, Seleiman MF, Alsadon AA, Amna, Ali S, Chaudhary HJ, Solieman THI, et al. Mitigation of Heat Stress in Solanum lycopersicum L. by ACC-deaminase and Exopolysaccharide Producing Bacillus cereus: Effects on Biochemical Profiling. Sustainability. 2020; 12(6):2159. https://doi.org/10.3390/su12062159
Chicago/Turabian StyleMukhtar, Tehmeena, Shafiq ur Rehman, Donald Smith, Tariq Sultan, Mahmoud F. Seleiman, Abdullah A. Alsadon, Amna, Shafaqat Ali, Hassan Javed Chaudhary, Talaat H. I. Solieman, and et al. 2020. "Mitigation of Heat Stress in Solanum lycopersicum L. by ACC-deaminase and Exopolysaccharide Producing Bacillus cereus: Effects on Biochemical Profiling" Sustainability 12, no. 6: 2159. https://doi.org/10.3390/su12062159
APA StyleMukhtar, T., Rehman, S. u., Smith, D., Sultan, T., Seleiman, M. F., Alsadon, A. A., Amna, Ali, S., Chaudhary, H. J., Solieman, T. H. I., Ibrahim, A. A., & Saad, M. A. O. (2020). Mitigation of Heat Stress in Solanum lycopersicum L. by ACC-deaminase and Exopolysaccharide Producing Bacillus cereus: Effects on Biochemical Profiling. Sustainability, 12(6), 2159. https://doi.org/10.3390/su12062159






