1. Introduction
The fast global discharge of potentially toxic elements (PTEs) into the hydrosphere has attracted serious focus due to their high bioavailability, bioaccumulation, and biomagnification potentials [
1,
2,
3]. The consumption of aquatic products is the main pathway of PTEs accumulation in the human body [
4]. Some of PTEs such as cadmium (Cd), lead (Pb), arsenic (As), mercury (Hg) and chromium (Cr) have several toxic effects even at low concentrations including series of cancers, anemia, heart attacks, embryotoxicity, neurotoxicity and weakness in the immune system [
5]. Therefore, the maximum permissible limits of these elements have been set by the World Health Organization (WHO) to ensure that zero or only the threshold limits of trace levels (part per billion, ppb) are allowed in a water resource [
6].
On the other hand, PTEs have several phytotoxic effects on plants by targeting central molecules and vital processes in cells [
7]. In this regard, biochemical disorders are the early responses associated with these phytotoxic effects, including enzymes deactivation and several oxidative stress responses linked to the over-production of reactive oxygen species (ROS) [
8,
9,
10]. According to Jalmi et al. [
11], the responses of plant against these early phytotoxic effects are shown in intricate signaling networks functioning in plant cells (e.g., calcium, hormone, and mitogen-activated protein kinase signaling). Furthermore, phytotoxicity of PTEs may also cause severe damage to DNA either through modifying the protein structure of enzymes and/or constraining the production of enzymes at the transcription level, which could also lead to chromosomal aberrations in mitotic cells [
12,
13,
14]. In addition, PTEs phytotoxicity induced several disorders in biochemical and physiological plant systems including disturbance in cell division, cell cycle impairment, disorders in mitochondrial respiration, dysfunctions in synthesis of photosynthetic pigments, alterations in plasma membrane permeability and defects in water status and nutrients uptake [
15]. These biochemical and physiological disorders are associated with several malformations in the anatomical and ultrastructural aspects of plant cells and tissues. The electron-microscopic investigation on reed plants (
Phragmites australis Cav.) grown in heavy metals-polluted soil illustrated substantial ultrastructural alterations in cell membranes and cytoplasmic organelles of root and stem cells [
16]. Additionally, ultrastructural investigation of reed plants showed a distortion in epidermis and mesoderm, which inhibited the radial migration of fluids from roots into peripheral parts and constrained the uptake and translocation of nutrients from roots into shoots. Following these biochemical, physiological and anatomical deteriorations, the common morphological symptoms of PTEs phytotoxicity are chlorosis, withering, falling of leaves as well as defects in shoot and root development [
17].
Over the classical techniques of water effluent decontamination (e.g., adsorption, ion exchange, precipitation, coagulation-flocculation, and membrane separation), rhizofiltration could be considered as the most efficient and affordable one at relatively low concentrations of PTEs and large quantities of contaminated water resources. This eco-friendly and cost-effective technique involves using aquatic plans to sequester PTEs from contaminated water effluents into their biomass [
18]. Water hyacinth (
Eichhornia crassipes) has received significant attention to clean-up contaminated water effluents due to its hyperaccumulating potentials of PTEs, rapid proliferation, extensive rooting network and high resistance against biotic and abiotic stress conditions [
19,
20]. In addition, the potential reuse of water hyacinth biomass in several applications (e.g. biofuels production and biosorbents engineering) provides a circular win-win approach for recycling phytoremediation disposals into value-added products [
21]. The bioaccumulation factors of Fe, Mn, Zn, Pb, Cu into water hyacinth biomass can reach respectively 8081, 1540, 3663, 3083, and 538, suggesting its hyperaccumulating potentials to PTEs [
22]. Smolyakov et al. [
23] evaluated the phytoremediation efficiency of Zn (500 µg L
−1), Cu (250 µg L
−1), Pb (250 µg L
−1) and Cd (50 µg L
−1) by water hyacinth plants. After 8 days, the removal efficiencies of Cu, Pb, Zn, and Cd were respectively 92, 89, 82 and 76%. Another investigation revealed that water hyacinth plant could remove about 99.5% of Cr (VI) from industrial mines wastewater as well as reducing total dissolved solids (~19%), biological oxygen demand (~50%) and chemical oxygen demand (~34%) [
24].
Despite intensive research undertaken on wastewater purification using water hyacinth plants, research trials undertaken to improve its adaptability and bioaccumulation potentials to PTEs are still very few. In this regard, studying the genotoxicity of PTEs based on protein profile and DNA variability has advantageous due to their sensitivity and rapid responses. PTEs-induced phytotoxicity caused significant alterations in leaf protein profiles including reduction of photosynthetic protein and cellular damage at the DNA level and organelles such as mitochondria or lysosomes [
25]. In this regard, the behavior of PTEs on biochemical parameters of water hyacinth is contradictory. For instance, the interference of Cd and Mn ions with protein synthesis caused inhibition in RNA and DNA content. However, Zn ions caused the opposite effect through increasing RNA and DNA content and protein synthesis [
26]. To the best of the authors’ knowledge, molecular and anatomical investigations on water hyacinth plants grown under toxic elements stress are still insufficient. Therefore, the main objectives of this study are to investigate the effect of PTEs contamination on DNA pattern, protein profile characters, and anatomical structure of water hyacinth populations grown in two drainage water resources compared to another population grown in freshwater source (Nile River).
3. Discussion
Large-scale utilization of water hyacinth for contaminated effluent purification is of great importance due to its high tolerance against biotic and abiotic stress conditions [
29]. Although PTEs concentration in drainage water resources was relatively low, their mutual effects caused several deteriorations on molecular and anatomical characters of water hyacinth plants. These results are in agreement with [
30] as water hyacinth plants can survive under a mixture of PTEs (Cd, Co, Cr, Cu, Mn, Ni, Pb, and Zn) up to 3 mg L
−1 and under Pb
2+ stress up to 100 mg L
−1. Concentrations of Ba and Sr were the highest among other PTEs (0.053–0.1016 mg L
−1) and (0.3437–1.107 mg L
−1), respectively. The translocation factor (TF) of the PTEs was > 1 suggesting the higher rhizofiltration efficiency of these metals by water hyacinth plants. According to Ma et al. [
28], hyperaccumulating plants with TF > 1 are classified as high-efficient plants for PTEs translocation from roots into shoots. PTEs showed higher accumulation in roots compared to leaves. PTEs are mainly localized in vascular tissues and epidermal cells to mediate their translocation to other plant tissues [
15]. Additionally, it may be localized as precipitates into metal binding compounds existed in cell walls (carbohydrates, cellulose, hemicellulose and lignin)
Results of PCA were performed by applying Varimax rotation with Kaiser normalization to assist the interpretation of PTEs concentration (
Table A1 and
Table A2, and
Figure A1, supporting information). PCA is commonly used in such studies to investigate the relationship between elements and their potential origins. Data as the different groups of elements that correlate together might have a similar common origin and similar behavior. The initial principal components (PC1 and PC2) explained about 87.5% of the variation (72.17 and 15.32%, respectively). In addition, PC1 and PC2 had the highest e eigenvalues (11.55 and 2.45, respectively) as indicated in
Table A1 in
Appendix A, supporting information. Principle components 1 (PC1) showed high positive correlation and was loaded with Ba, Sr, Cu, Mn, Zn, Sb, and Ni, and it is suggested that these elements are derived from natural and anthropogenic origins. However, the rotated component matrix revealed that Fe, Cd, Cr, As, Al, Co, Ti, and Mo showed negative correlation and strongly correlated with principal component 2 (PC2), and these elements might be derived from industrial origins.
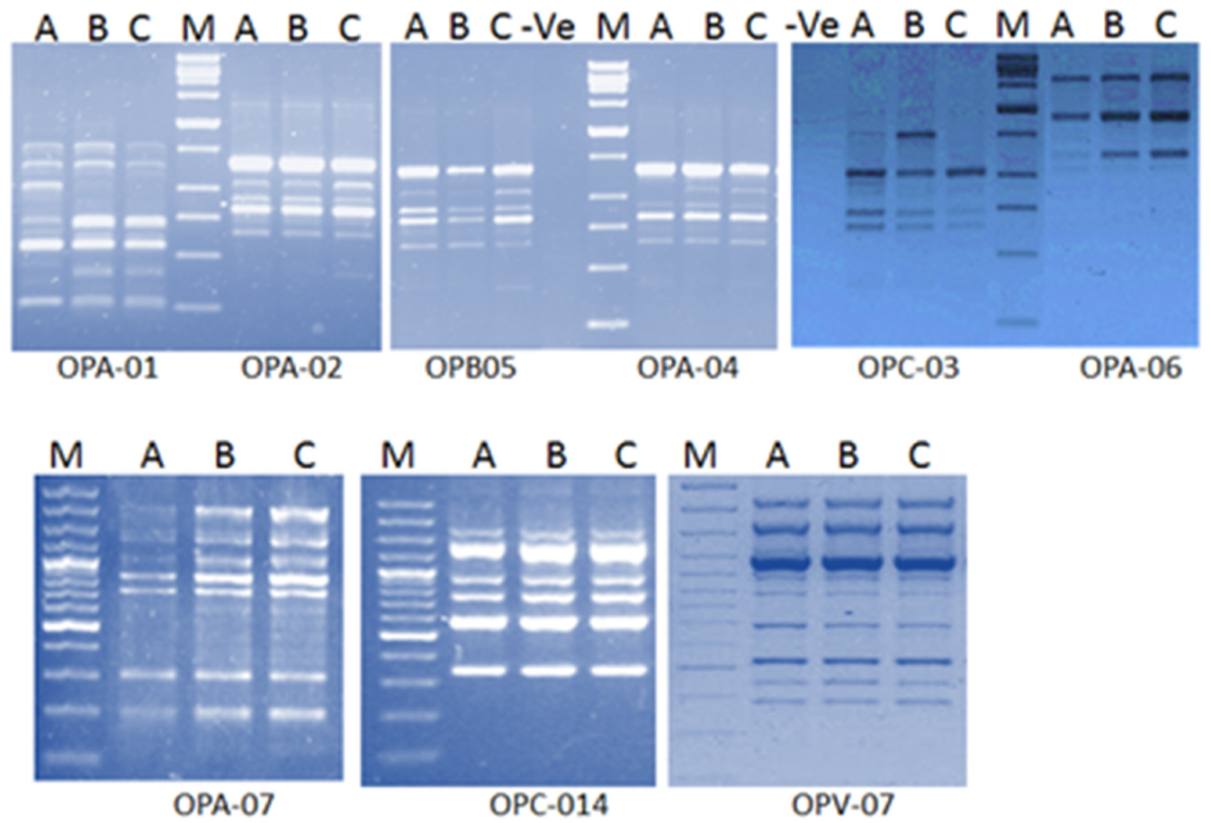
The obtained results illustrated the high tolerance of water hyacinth against PTEs stress. Genotoxicity of toxic elements can be assessed by molecular techniques such as Randomly Amplified Polymorphic DNA (RAPD). RAPD analysis showed generation of new DNA bands in plants grown in drainage water resources. These bands have not existed in plants grown in freshwater. Some of these bands have only existed in plants grown in the higher contaminated drainage water (
Figure 1). [
31] illustrated that DNA alterations detected by RAPD analysis offered a useful biomarker assay for the evaluation of genotoxic effects of PTEs in
Capsicum annum. It was also reported that Cd has the capacity not only to cause morbidity to the exposed organisms but also has the potential to induce genotoxic adverse effects [
32,
33]. RAPD assay indicated damages and mutations in DNA induced directly and/or indirectly by the phytotoxic effect of copper [
34]. RAPD assay also showed variations in band intensity, loss of typical bands and appearance of new bands suggesting several damages in DNA of barley seedling treated with Cd (30–120 mg/L
−1) [
35].
The present study demonstrated the presence of 21 protein bands separated from leaves of water hyacinth plants grown in freshwater. Meanwhile, other plants grown in Elamom and Nashrat drains showed the presence of 22 and 23 protein bands, respectively. The protein profile of roots revealed detection of three protein bands of control plants. However, 16 protein bands were detected in roots of water hyacinth plants grown in drainage water resources. These findings are consistent with several reports confirming that PTEs exposure might cause complete elimination of some protein bands and creation of new ones [
36,
37]. Proteins are directly responsible for most biological processes in living cells. Therefore, it is necessary to conduct proteomic studies, which elucidate protein presence and role under certain environmental conditions [
38]. It is well known that PTEs stress can activate a range of potential cellular mechanisms in plants, some of which being the mobilization of specific molecules such as stress proteins that play a very significant role in Cd detoxification and tolerance in plants [
39,
40]. The changes in protein banding patterns have been attributed to the occurrence of either gene mutation or induction of cytological aberrations. The absence of some bands might be due to the deletion of their corresponding genes.
Anatomically, water hyacinth has an epidermis layer, which is not covered by a cuticle or only covered with thin cuticle layer to support gas and nutrients absorption from surrounding water. The adverse effects of PTEs on cell growth and sizing caused a noticeable reduction in root diameter as well as thickness in cortex and vascular cylinder diameter in plants grown in drainage water. So far, no sufficient details are available regarding the anatomical responses of water hyacinth under PTEs stress. According to [
41] no injurious effects on root anatomy of water hyacinth grown under the presence of As. Contrariwise, [
42] reported that arsenic (As) stress caused several deteriorations in anatomical characters of water hyacinth leaves due to the reduction of cell size. The harmful effect of PTEs on the anatomical structure of water hyacinth may be attributed to the adverse effects on cell organelles and the nutrients imbalance in plant tissues [
43,
44]. The same harmful effects on anatomical structure were recorded under differs biotic and abiotic stresses in numerous plants [
45,
46,
47,
48,
49]
4. Materials and Methods
4.1. Plant Material
A single clone from a population of water hyacinth covering large distribution area of Nile River (freshwater) was selected and surrounded by an opened cage to ensure the similar genetic background of experimented plants. Three groups of two weeks-old clones were transplanted in three different water resources: (i) freshwater source (Nile River) located at Desouk District, Kafr El-Sheikh Governorate (31°08′07.0″ N 30°38′01.6″ E), (ii) Elamom drain, Shubrakhit District, Beheira Governorate (31°01′40.2″ N 30°40′33.7″ E) and (iii) Nashart drain, Kafr El-Sheikh Governorate (31°05′51.3″ N 30°57′45.9″ E). The three homogenous clones were surrounded by a cages and left for three months until sexual reproduction and formation of homogenous clones comprising stolons, rhizomes, long pendant roots, and fruit clusters.
Samples were taken from each population for elemental, molecular, biochemical and anatomical analyses. Experimental procedures of all analyses were conducted at Genetics, Soil Sciences and Plant Pathology & Biotechnology Laboratory (PPBL) as well as EPCRS Excellence Center (certified according to ISO 17025, ISO 9001, ISO 14001 and OHSAS 18001), Department of Agricultural Botany, Faculty of Agriculture, Kafrelsheikh University, Kafr El-Sheikh, Egypt.
4.2. Elemental Analysis
Uniform-sized bottles were rinsed with the representative resources before water sampling. Water samples were taken at a depth of 10 cm from the surface of fresh and drainage water. Representative water samples were inserted into bottles leaving an appropriate head-space, and the bottles were tightly closed by caps to avoid potential contamination. Bottles were placed directly in the fridge until elemental analysis.
Water hyacinth plants were divided into roots and leaves in order to distinguish between the accumulation potentials of different plant organs. Plant samples (roots and leaves) were air-dried for a week, oven-dried at 70 °C until weight constant and mechanically ground to a fine powder using a stainless steel grinder. Plant samples were acid-digested for elemental determination of Ag, Al, As, Ba, Ca, Cd, Co, Cr, Cu, Fe, K, Mg, Mn, Mo, Na, Ni, P, Sb, Sr, Ti, V and Zn. Subsamples (1.0 g) of roots and leaves were digested using the mixture of concentrated nitric acid (HNO
3), hydrochloric acid (HCl) and 30% hydrogen peroxide (H
2O
2) [
50,
51].
Total elements concentration in water and plant samples was determined using Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES, model 5100, Agilent, Santa Clara, CA 95051, United States) at the Institute of Graduate Studies & Research, Alexandria University (accredited according to ISO/IEC 17025-2005). In the current study, bioaccumulation factor (BAF) and translocation factor (TF) for the most detected PTEs in water resources (Al, Ba, Cu, Mn, Sr, Ti, V, Zn) are given by:
where C
water, C
root, and C
leaves are PTEs concentrations in water (mg L
−1), leaves (mg kg
−1), and roots (mg kg
−1), respectively [
28]. Quality control of data was performed with the use of repeat measurements for all obtained data. The mean relative standard deviation (RSD) was less than 5%. Principal component analysis (PCA) was performed using MVSP software ver 3.13 (Kovach Computing Services, Pentraeth, UK) [
52] to assist the interpretation of PTEs concentration in water and plant samples.
4.3. Molecular Analysis
4.3.1. Total DNA Extraction
Total DNA was extracted from young leaves using the modified cetyl trimethylammonium bromide (CTAB) method [
53] with some modifications. Leaves were wrapped in filter papers under hand pressure for five min to remove moisture, and 150 mg of samples were ground using pistil and mortar. Thereafter, 600 μL of preheated (65 °C) extraction buffer (2% CTAB, 20 mM EDTA, 100 mM Tris-HCl, 1.4 M NaCl, 2% polyvinylpyrrolidone, and 0.2% mercaptoethanol) were added. The mixture was transferred to a centrifuge tube (2 mL), incubated for 30 min in a 65 °C water bath, and samples were inverted every 5 min. 600 μL of chloroform-isoamyl alcohol (24:1) was added and mixed by inverting the tubes carefully for 8 times, and the mixture was centrifuged at 12,000 rpm for 10 min at room temperature. The supernatant was collected, carefully mixed with a two-third volume of ice-cold isopropanol, and the DNA samples were collected by centrifuging for 10 min. RNase (10 μg/mL) was added to the 50 μL of TE buffer (10 mM Tris and 0.1 mM EDTA) prior to dissolving the DNA to remove any RNA, and the mixture was incubated at 37 °C for 30 min. After incubation, 100 μL and 750 μL volumes of 3.0 M sodium acetate and ice-cold absolute ethanol were added, respectively. The DNA was collected by high-speed centrifugation for 10 min, carefully washed with ice-cold absolute and 70% ethanol and centrifuged at 120000 rpm for 10 min. Finally, samples were dried at room temperature and dissolved in 50–100 μL of TE buffer. The quality and concentration of
DNA were determined by a P330 photometer (EMPLEN, Munich, Germany).
4.3.2. RAPD—PCR
For DNA amplification, nine decamer RAPD primers (Operon technologies, CA, USA) were used (
Table 1). PCR was performed as follows: initial denaturation at 94 °C for 5 min; followed by 35 cycles at 94 °C for 1 min, specific annealing temperature (Ta) for 30 s according the primer sequence and 72 °C for 3 min and the final extension step at 72 °C for 10 min. Amplification was carried out in MJ Mini Bio RAD, thermal cycler in 25 µL reaction volume containing the following reagents: 1.0 µL of dNTPs (10 mM), 1.0 µL of MgCl2 (25 mM), 5 µL of 10 × buffer, 1.0 µL of primer (10 pmol), 1.0 µL of DNA (25 ng/µL), 0.3 µl of Taq polymerase (5u/µL) and 15.7 d.d. H
2O. The amplification products were resolved electrophoretically on 1.5% agarose gels in TAE buffer (40 mM Tris-acetate, 20 mM glacial acetic acid, 1 mM EDTA, pH 7) at 75 V. The gel was stained with ethidium bromide and then destained with tap water. Bands were detected on UV-trans-illuminator and photographed by a gel documentation system (UVITEC, city, UK).
4.3.3. Total Protein Extraction
Total proteins were extracted from water hyacinth leaves and roots. Briefly, approximately 0.5 g powder of fresh leaves and roots were homogenized by mechanical grinding and mixed well with 500 μl of the protein extraction buffer (62.5 mM Tris-Hcl, pH 6.8, 2% SDS, 10% glycerol, 5% β-mercaptoethanol, 5 M Urea and 0.01% bromophenol blue) by vortexing. Protein extracts were centrifuged at 14,000 rpm for 10 min at 4 °C and separated by 12% (SDS-PAGE) according to [
54]. Molecular weights of different bands were calibrated with a mixture of standard protein markers (Molecular Weight Marker, M. W. 14.000–66.000; Catalog No. SDS7, Sigma-Aldrich, Munich, Germany). The banding profile was stained by Coomasie blue dye then photographed and scored.
4.4. Anatomical Studies
Roots, leaves, and petioles of water hyacinth plants were sampled for anatomical characterization. Samples (0.5 cm length) were placed in FAA solution (killing and fixing), washed in 50% ethyl alcohol, and dehydrated in butyl alcohol series. Samples were impeded in paraffin wax (56–58 °C). Transverse sections (15 microns thick) were done with rotary microtome model 820, fixed with albumin, stained with a combination of safranin and light green, and finally fixed in Canada balsam [
55]. The sections were investigated microscopically and photomicrographed (Leica, Wetzlar, Germany) [
48,
56,
57].
5. Conclusions
Water hyacinth (Eichhornia crassipes (Mart.) Solms) is one of the most critical aquatic plants in Egypt and worldwide. Due to its hyper accumulating potentials of PTEs and its high resistance against biotic and abiotic stress conditions, water hyacinth has received significant attention to clean-up contaminated water effluents. However, research trials undertaken to improve adaptability of water hyacinth against PTEs stress are still very few. The novelty aspects of this research are to study molecular, biochemical and anatomical characters of water hyacinth subjected to PTEs stress. Although PTE’s concentration in drainage water resources was relatively low, the hyperaccumulating potentials of water hyacinth maximized their concentration in plant tissues. Immobile PTEs (e.g., Al, As, Cd, Co, Cr, Cu, Fe, Mn, Mo, Ni, Sb, Ti, V, and Zn) showed higher accumulation in roots; however, mobile PTEs (e.g., Ba and Sr) exhibited higher accumulation in leaves. DNA alterations detected by RAPD analysis confirmed the genotoxic effects of PTEs. Protein profile alterations in electrophoretic profiles and banding patterns were observed on plants grown in drainage water resources, especially protein isolated from roots. The ultrastructural analysis also showed several deteriorations on the anatomical structure of plants grown under PTEs stress. Further investigations should be undertaken to explore molecular and biochemical characters of plants grown under higher PTEs concentrations.