Energy Utilization of Algae Biomass Waste Enteromorpha Resulting in Green Tide in China: Pyrolysis Kinetic Parameters Estimation Based on Shuffled Complex Evolution
Abstract
1. Introduction
2. Materials and Methods
2.1. Thermogravimetric Measurements
2.2. Pyrolysis Kinetics
2.2.1. Kissinger Method
2.2.2. SCE Optimization Algorithm
3. Results and Discussion
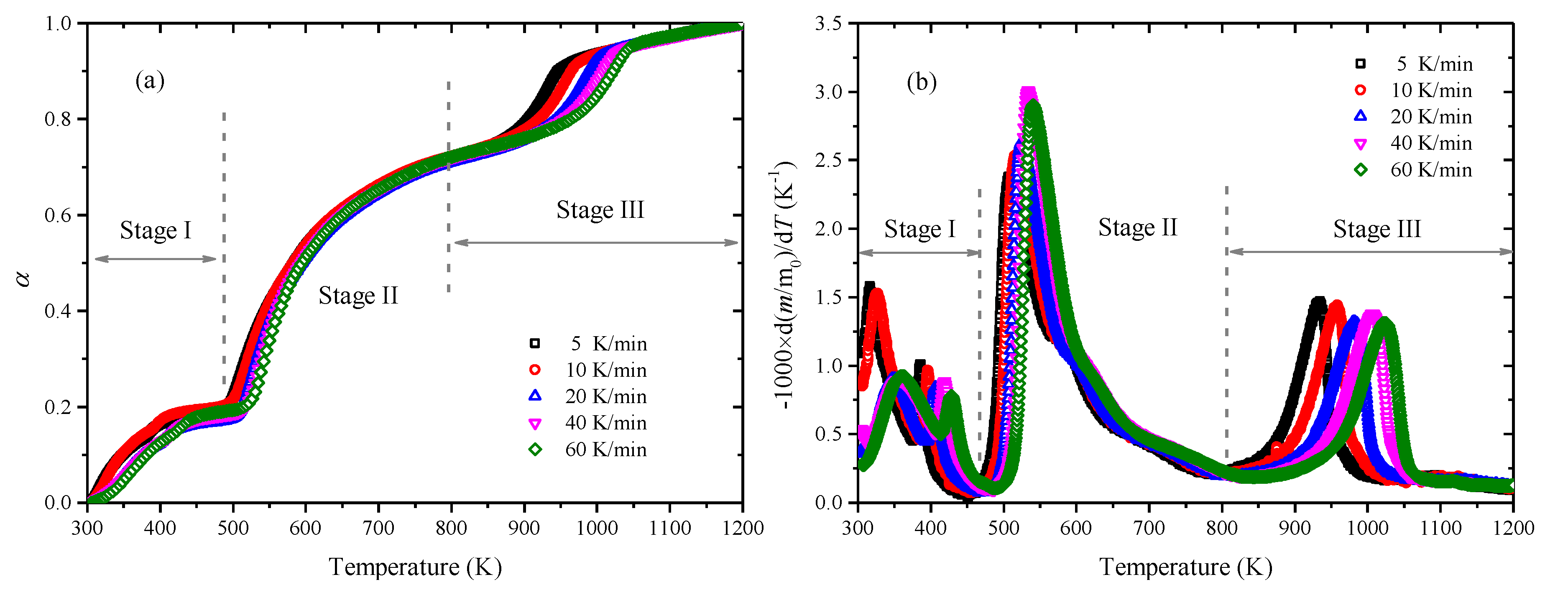
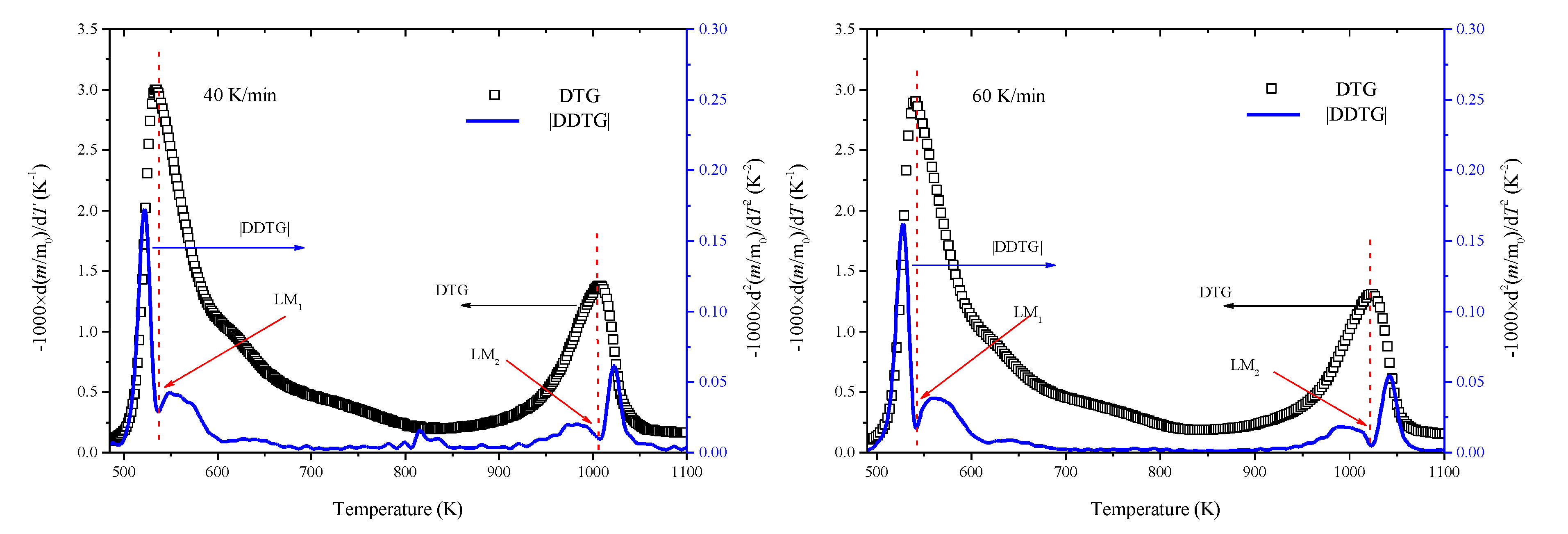
3.1. Thermogravimetric Analysis
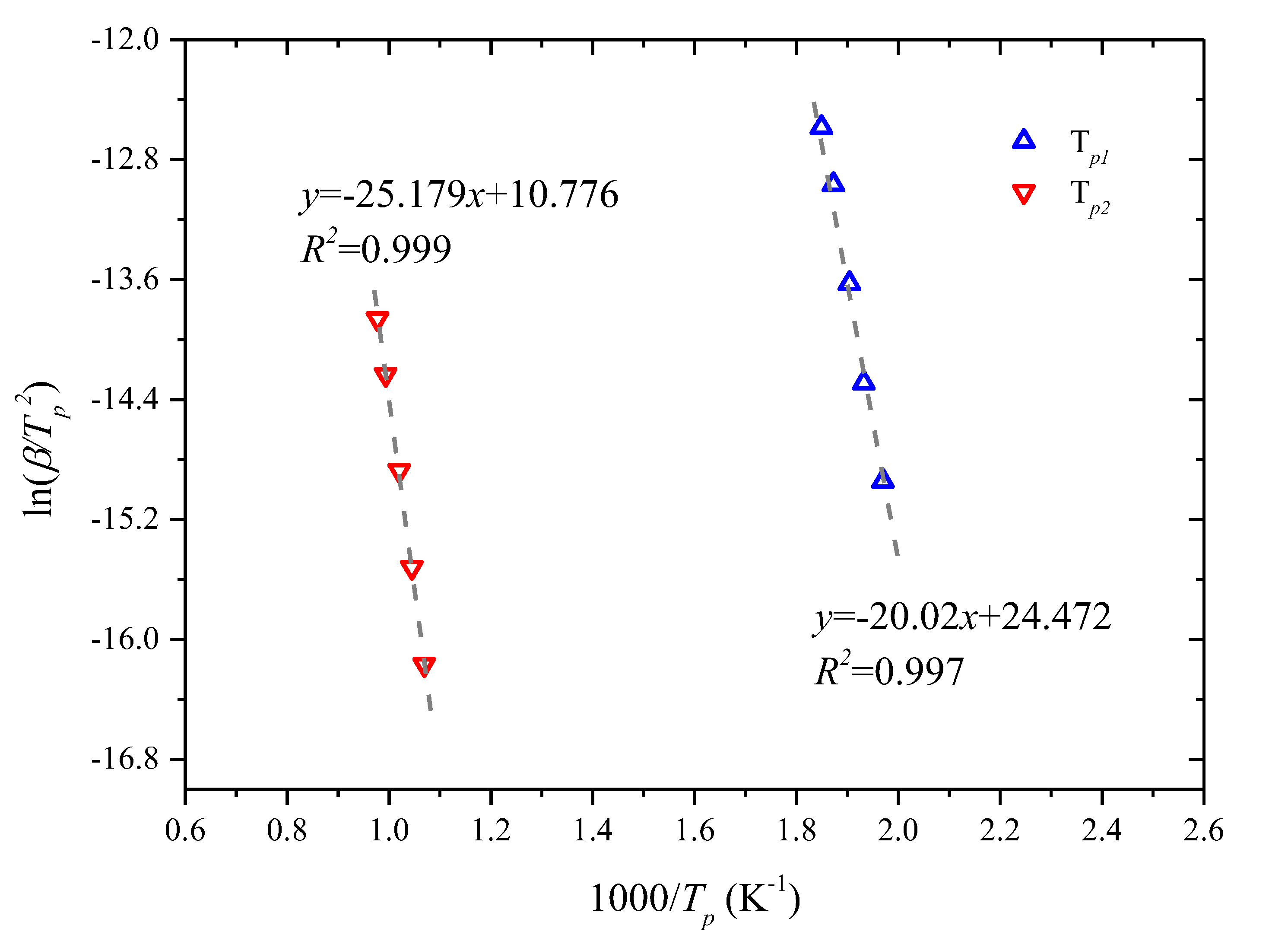
3.2. Kinetic Analysis Based on the Kissinger Method
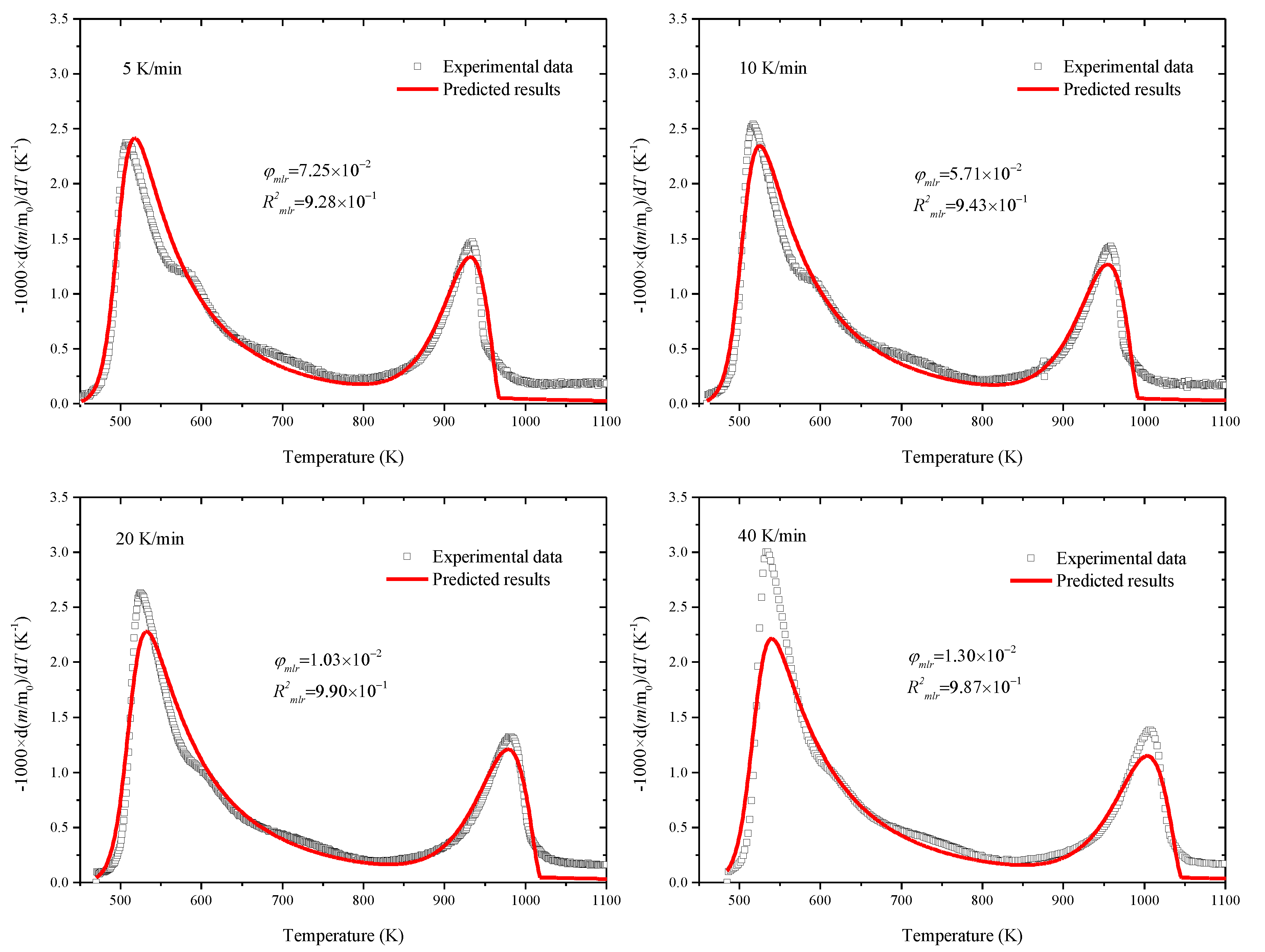
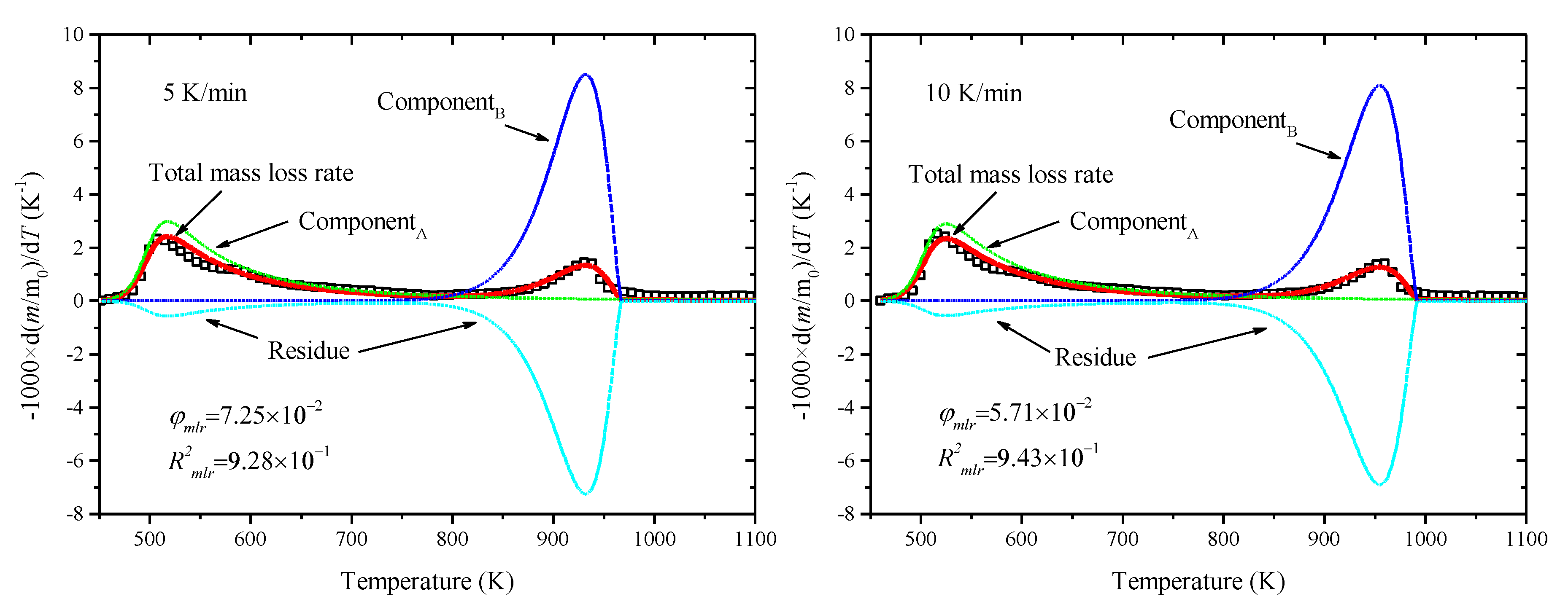
3.3. Estimation of Kinetic Parameters by SCE
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, S.; Jiang, X.; Wang, N.; Yu, L.; Li, Z.; He, P. Research on pyrolysis characteristics of seaweed. Energy Fuels 2007, 21, 3723–3729. [Google Scholar] [CrossRef]
- Zhao, B.; Fang, Y.; Wu, K.; Zhang, F.; Wang, J. A Method of Large-Scale Resource Utilization of Algae—Eutrophic Waste from Lake Chao, China: Preparation and Performance Optimization of Composite Packaging Materials. Sustainability 2019, 11, 6462. [Google Scholar] [CrossRef]
- Gao, X.; Xing, W.; Zhou, J.; Wang, G.; Zhuo, S.; Liu, Z.; Xue, Q.; Yan, Z. Superior capacitive performance of active carbons derived from Enteromorpha prolifera. Electrochim. Acta 2014, 133, 459–466. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, X.; Han, X.; Liu, J. Combustion characteristics of seaweed biomass. 1. Combustion characteristics of Enteromorpha clathrata and Sargassum natans. Energy Fuels 2009, 23, 5173–5178. [Google Scholar] [CrossRef]
- Li, D.; Chen, L.; Zhao, J.; Zhang, X.; Wang, Q.; Wang, H.; Ye, N. Evaluation of the pyrolytic and kinetic characteristics of Enteromorpha prolifera as a source of renewable bio-fuel from the Yellow Sea of China. Chem. Eng. Res. Des. 2010, 88, 647–652. [Google Scholar] [CrossRef]
- Hua, M.-Y.; Li, B.-X. Co-pyrolysis characteristics of the sugarcane bagasse and Enteromorpha prolifera. Energy Convers. Manag. 2016, 120, 238–246. [Google Scholar] [CrossRef]
- Ceylan, S.; Goldfarb, J.L. Green tide to green fuels: TG–FTIR analysis and kinetic study of Ulva prolifera pyrolysis. Energy Convers. Manag. 2015, 101, 263–270. [Google Scholar] [CrossRef]
- Guo, Y.; Tan, C.; Wang, P.; Sun, J.; Li, W.; Zhao, C.; Lu, P. Structure-performance relationships of magnesium-based CO2 adsorbents prepared with different methods. Chem. Eng. J. 2020, 379, 122277. [Google Scholar] [CrossRef]
- Pal, P.; Chew, K.W.; Yen, H.-W.; Lim, J.W.; Lam, M.K.; Show, P.L. Cultivation of Oily Microalgae for the Production of Third-Generation Biofuels. Sustainability 2019, 11, 5424. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, D.; Cao, B.; Qian, L.; Hu, Y.; Liu, L.; Yuan, C.; Abomohra, A.E.-F.; He, Z.; Wang, Q. Bio-char and bio-oil characteristics produced from the interaction of Enteromorpha clathrate volatiles and rice husk bio-char during co-pyrolysis in a sectional pyrolysis furnace: A complementary study. J. Anal. Appl. Pyrolysis 2018, 135, 219–230. [Google Scholar] [CrossRef]
- Wang, S.; Cao, B.; Liu, X.; Xu, L.; Hu, Y.; Afonaa-Mensah, S.; Abomohra, A.E.-F.; He, Z.; Wang, Q.; Xu, S. A comparative study on the quality of bio-oil derived from green macroalga Enteromorpha clathrata over metal modified ZSM-5 catalysts. Bioresour. Technol. 2018, 256, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Perrot, J.-F.; Subiantoro, A. Municipal waste management strategy review and waste-to-energy potentials in New Zealand. Sustainability 2018, 10, 3114. [Google Scholar] [CrossRef]
- Xu, Z.; Xiao, X.; Fang, P.; Ye, L.; Huang, J.; Wu, H.; Tang, Z.; Chen, D. Comparison of Combustion and Pyrolysis Behavior of the Peanut Shells in Air and N2: Kinetics, Thermodynamics and Gas Emissions. Sustainability 2020, 12, 464. [Google Scholar] [CrossRef]
- Chen, R.; Li, Q.; Zhang, Y.; Xu, X.; Zhang, D. Pyrolysis kinetics and mechanism of typical industrial non-tyre rubber wastes by peak-differentiating analysis and multi kinetics methods. Fuel 2019, 235, 1224–1237. [Google Scholar] [CrossRef]
- Kong, D.; Peng, R.; Sun, X.; Zhang, J.; Ping, P.; Du, J. Study of the influence of crude oil on the spontaneous combustion risk of sulfurized rust in crude oil tanks. Fuel 2019, 255, 115816. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, D.; Li, M.; He, J.-J.; Gao, Z.-H.; Zhou, Y.; Sun, J.-H. Pyrolytic behavior of waste extruded polystyrene and rigid polyurethane by multi kinetics methods and Py-GC/MS. Fuel 2018, 222, 11–20. [Google Scholar] [CrossRef]
- Zhou, Y.; Bu, R.; Gong, J.; Yan, W.; Fan, C. Experimental investigation on downward flame spread over rigid polyurethane and extruded polystyrene foams. Exp. Therm. Fluid Sci. 2018, 92, 346–352. [Google Scholar] [CrossRef]
- Kong, B.; Wang, E.; Lu, W.; Li, Z. Application of electromagnetic radiation detection in high-temperature anomalous areas experiencing coalfield fires. Energy 2019, 189, 116144. [Google Scholar] [CrossRef]
- Duan, Q.; Gupta, V.K.; Sorooshian, S. Shuffled complex evolution approach for effective and efficient global minimization. J. Optim. Theory Appl. 1993, 76, 501–521. [Google Scholar] [CrossRef]
- Duan, Q.; Sorooshian, S.; Gupta, V.K. Optimal use of the SCE-UA global optimization method for calibrating watershed models. J. Hydrol. 1994, 158, 265–284. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, C.; Chaos, M.; Chen, R.; Lu, S. Estimation of beech pyrolysis kinetic parameters by shuffled complex evolution. Bioresour. Technol. 2016, 200, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhang, J.; He, Q.; Huang, B.; Mao, S. The application and validity of various reaction kinetic models on woody biomass pyrolysis. Energy 2019, 179, 784–791. [Google Scholar] [CrossRef]
- Ding, Y.; Huang, B.; Li, K.; Du, W.; Lu, K.; Zhang, Y. Thermal interaction analysis of isolated hemicellulose and cellulose by kinetic parameters during biomass pyrolysis. Energy 2020, 195, 117010. [Google Scholar] [CrossRef]
- Chaos, M. Spectral aspects of bench-scale flammability testing: Application to hardwood pyrolysis. Fire Saf. Sci. 2014, 11, 165–178. [Google Scholar] [CrossRef]
- Ross, A.; Jones, J.; Kubacki, M.; Bridgeman, T. Classification of macroalgae as fuel and its thermochemical behaviour. Bioresour. Technol. 2008, 99, 6494–6504. [Google Scholar] [CrossRef]
- Ding, Y.; Ezekoye, O.A.; Lu, S.; Wang, C.; Zhou, R. Comparative pyrolysis behaviors and reaction mechanisms of hardwood and softwood. Energy Convers. Manag. 2017, 132, 102–109. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, W.; Yu, L.; Lu, K. The accuracy and efficiency of GA and PSO optimization schemes on estimating reaction kinetic parameters of biomass pyrolysis. Energy 2019, 176, 582–588. [Google Scholar] [CrossRef]
- Kim, S.-S.; Ly, H.V.; Kim, J.; Choi, J.H.; Woo, H.C. Thermogravimetric characteristics and pyrolysis kinetics of Alga Sagarssum sp. biomass. Bioresour. Technol. 2013, 139, 242–248. [Google Scholar] [CrossRef]
- Zhao, H.; Yan, H.; Dong, S.; Zhang, Y.; Sun, B.; Zhang, C.; Ai, Y.; Chen, B.; Liu, Q.; Sui, T. Thermogravimetry study of the pyrolytic characteristics and kinetics of macro-algae Macrocystis pyrifera residue. J. Therm. Anal. Calorim. 2013, 111, 1685–1690. [Google Scholar] [CrossRef]
- Li, K.-Y.; Huang, X.; Fleischmann, C.; Rein, G.; Ji, J. Pyrolysis of medium-density fiberboard: Optimized search for kinetics scheme and parameters via a genetic algorithm driven by Kissinger’s method. Energy Fuels 2014, 28, 6130–6139. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, Y.; Zhang, J.; Zhou, R.; Ren, Z.; Guo, H. Kinetic parameters estimation of pinus sylvestris pyrolysis by Kissinger-Kai method coupled with Particle Swarm Optimization and global sensitivity analysis. Bioresour. Technol. 2019, 293, 122079. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Ezekoye, O.A.; Zhang, J.; Wang, C.; Lu, S. The effect of chemical reaction kinetic parameters on the bench-scale pyrolysis of lignocellulosic biomass. Fuel 2018, 232, 147–153. [Google Scholar] [CrossRef]
- Ding, Y.; Fukumoto, K.; Ezekoye, O.A.; Lu, S.; Wang, C.; Li, C. Experimental and numerical simulation of multi-component combustion of typical charring material. Combust. Flame 2020, 211, 417–429. [Google Scholar] [CrossRef]
| Ultimate Analysis | Value | Biochemical Composition | Value |
|---|---|---|---|
| C | 22.74 | Lipids | 1.28 |
| H | 6.27 | Proteins | 23.99 |
| N | 3.14 | Carbohydrates | 40.00 |
| S | 1.27 | ||
| O | 7.89 |
| Temperature (K) | Heating Rate (K/min) | ||||
|---|---|---|---|---|---|
| 5 | 10 | 20 | 40 | 60 | |
| Tp1 | 507.59 | 517.46 | 525.09 | 533.97 | 540.79 |
| Tp2 | 935.46 | 957.10 | 980.27 | 1006.57 | 1023.11 |
| Parameters | Initial Values | Search Range | Optimized Values |
|---|---|---|---|
| YA,0 | 0.50 | [0.25, 0.75] | 0.38 |
| ln(AA/s-1) | 27.47 | [13.73, 41.20] | 41.19 |
| EA (kJ/mol) | 166.45 | [83.22, 249.67] | 199.85 |
| nA | 1.00 | [0.00, 10.00] | 9.14 |
| vA | 0.50 | [0.00, 1.00] | 0.19 |
| YB,0 | 1- YA,0 | [0.00, 1.00] | 0.62 |
| ln(AB/s-1) | 14.00 | [7.00, 21.00] | 20.95 |
| EB (kJ/mol) | 209.34 | [104.67, 314.01] | 208.91 |
| nB | 1.00 | [0.00, 10.00] | 0.66 |
| vB | 0.50 | [0.00, 1.00] | 0.85 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, L.; Zhang, J.; Ding, Y. Energy Utilization of Algae Biomass Waste Enteromorpha Resulting in Green Tide in China: Pyrolysis Kinetic Parameters Estimation Based on Shuffled Complex Evolution. Sustainability 2020, 12, 2086. https://doi.org/10.3390/su12052086
Zhong L, Zhang J, Ding Y. Energy Utilization of Algae Biomass Waste Enteromorpha Resulting in Green Tide in China: Pyrolysis Kinetic Parameters Estimation Based on Shuffled Complex Evolution. Sustainability. 2020; 12(5):2086. https://doi.org/10.3390/su12052086
Chicago/Turabian StyleZhong, Lingna, Juan Zhang, and Yanming Ding. 2020. "Energy Utilization of Algae Biomass Waste Enteromorpha Resulting in Green Tide in China: Pyrolysis Kinetic Parameters Estimation Based on Shuffled Complex Evolution" Sustainability 12, no. 5: 2086. https://doi.org/10.3390/su12052086
APA StyleZhong, L., Zhang, J., & Ding, Y. (2020). Energy Utilization of Algae Biomass Waste Enteromorpha Resulting in Green Tide in China: Pyrolysis Kinetic Parameters Estimation Based on Shuffled Complex Evolution. Sustainability, 12(5), 2086. https://doi.org/10.3390/su12052086






