Abstract
Barley (Hordeum vulgare L.) is an annual plant cultivated in spring or autumn. Currently, over 70% of the cultivated barley grains are utilized for preparing fodder, while the rest is used for the production of malt and cereals in the food industry. The purpose of the present work was to evaluate the content of bioactive compounds, antioxidant potential, and cholinesterase inhibitory effect of the aqueous extracts of juvenile barley leaves. It was found that the barley cultivars differed in their content of the determined phytochemicals as well as their antioxidant potential and cholinesterase-inhibitory activity. The water extracts of young barley leaves contained phenolic acids as well as quercetin, rutin, and kaempferitrin. The extracts showed a higher inhibitory effect on 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) than on 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals. Based on the aqueous extracts analyzed, we found that winter cultivars were characterized by the highest iron-chelating activity. Furthermore, barley extracts showed a stronger inhibitory effect against acetylcholinesterase compared to butyrylcholinesterase. The results of the present work indicated that barley cultivars differed in their germination process. Among the tested samples, the highest cholinesterase inhibitory activity was shown by the Basic variety.
1. Introduction
Juvenile barley (Hordeum vulgare L.) is typically a spring cultivar plant harvested after 7–10 days of growth, when it reaches a height of up to 20–30 cm. It is a cereal sprout, and is subjected to drying and crumbling and finally extracted [1]. The various forms of juvenile barley that are available on the market are obtained through different processing methods, which influence their nutritional value. The juice is obtained by pressing the must from juvenile barley, which is then dried at a temperature of approximately 40 °C by evaporating water under pressure in less than 2 min [2]. On the other hand, ground powder of barley grass is obtained by harvesting juvenile leaves, which are then dried and ground [3].
Juvenile barley contains mineral components such as calcium, copper, iron, magnesium, potassium, zinc, and vitamins (B1, B2, B3, B6, B7, C, E, K), in addition to chlorophyll, proteins, enzymes, carotenoids, and antioxidants [4,5,6]. It has been further demonstrated that it contains a series of essential amino acids, including tryptophan, glutamic acid, alanine, aspartic acid, methionine, arginine, lysine, cysteine, glycine, histidine, isoleucine, leucine, phenylalanine, proline, serine, threonine, tyrosine, and valine, as well as small polypeptides. It is believed that the nutritional value of barley is determined by numerous factors such as the species, cultivation site and method, and the growth phase in which it is harvested [7]. Various compounds influencing the health-promoting characteristics of barley grass have been identified, which include, among others, 3-O-feruloylquinic acid, isoorientin-7-O-rutinose, luteolin-6, C-arabinoside-8-C-glucoside, ferulic acid, isovitexin-7-O-glucoside, apigenin-6-C-arabinoside-8-C-glucoside, saponarin, isoorientin-7-O-[6-feruloyl]-glucoside-4′-O-glucoside, isovitexin-7-O-rutinose, isoscoparin-7-O-glucoside, and isoorientin-7-O-[6-feruloyl]-glucoside [8,9].
The literature contains a large number of reports on the positive effect of barley on the biological systems. It has been demonstrated that juvenile barley leaves exhibit physiological functions, including hypolipidemic [10,11] and anti-ulcerative activities [12], through their antioxidative effect. In addition, juice pressed from juvenile barley helps to reduce the level of cholesterol in the plasma and supports the renal function [4]. According to Lahouar et al. [1] and Yamaura et al. [11], juvenile barley exhibits pharmacological effects such as anticancer and anti-inflammatory properties. It has also been identified that juvenile barley leaves improve blood flow and digestion and facilitate general detoxification of an organism [1,2]. Recent studies have shown that the phytochemicals contained in barley influence its potential preventive action against neurodegenerative diseases, including Alzheimer’s disease [13]. Changes in the cerebral tissues result from the intensification of inflammatory processes in the central nervous system by oxidative stress [14]. Oxidative stress is generated due to the imbalance between the activity of reactive oxygen species and other free radicals, their scavenging by antioxidants, and enzymatic processes. During the development of neurodegenerative disorders, peroxides and free radicals may be formed, causing damage to cell components. Therefore, for the prevention of these disorders, antioxidants need to be supplied with the daily diet [15].
The antioxidant effects of polyphenols result from the inhibition of the enzymes involved in the production of free radicals. Polyphenols do not allow the formation of reactive oxygen species through the chelation of transition metal ions, which act as reaction catalysts producing reactive oxygen species, mainly hydroxyl radicals. One of the antioxidant properties of polyphenols is radical scavenging [16]. A common feature of polyphenols, especially those with a hydroxyl group in the ortho or para position, is that they can easily participate in redox reactions. Phenolic compounds are capable of carrying protons and electrons, and therefore, they easily undergo oxidization. Polyphenols may also affect the inhibition of enzymes such as cholinesterases. The available literature indicates that the raw materials that serve as the source of polyphenols can exhibit such inhibitory activities, if they also possess other phytochemicals.
Therefore, the present work aimed to evaluate the content of bioactive compounds, antioxidant potential, and cholinesterase inhibitory properties of the selected spring and winter varieties of barley grass.
2. Materials and Methods
2.1. Materials
The following chemicals were purchased from Sigma-Aldrich (Poznań, Poland): 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS, CAS number 30931-67-0), 2,2-diphenyl-1-picrylhydrazyl (DPPH, CAS number 1898-66-4), 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox, CAS number 53188-07-1), 3-(2-pyridyl)-5,6-bis(4-phenylsulfonic acid)-1,2,4-triazine monosodium salt (ferrozine, CAS number 63451-29-6), 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-[α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranosyloxy]-4H-chromen-4-one (rutin, CAS number 153-18-4), 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one (isoquercetin, CAS number 482-35-9), 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl β-D-galactopyranoside (hyperoside, CAS number 482-36-0), 5,7-dihydroxy-2-(4-hydroxyphenyl)-3-[(2S,3R,4S,5S,6R)-3,4, 5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one (astragalin, CAS number 480-10-4), 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one (quercetin, CAS number 117-39-5), 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one (kaempferol, CAS number 520-18-3), acetylthiocholine iodide (ATChI, CAS number 2260-50-6), butyrylthiocholine chloride (BTCh, CAS number 2963-78-2), acetylcholinesterase (AChE) from human erythrocytes (CAS number 9000-81-1), butyrylcholinesterase (BChE) from equine serum (CAS number 9001-08-5), eserine (CAS number 57-47-6), 2-amino-2-(hydroxymethyl)-1,3-propanediol (Tris-base, CAS number 77-86-1), and 3-(3,4-dihydroxyphenyl)-2-propenoic acid (caffeic acid, CAS number 331-39-5).
In addition, the following reagents were used: Folin–Ciocalteu reagent (Chempur, Poland), high-performance liquid chromatography (HPLC)-grade acetonitrile (Romil, United Kingdom), potassium peroxodisulfate (CAS number 7727-21-1; POCh, Poland), pure formic acid (CAS number 64-18-6; POCh, Poland), sodium chloride (POCh, Poland), magnesium chloride hexahydrate (CAS number 7791-18-6; POCh, Poland), 1 M hydrochloric acid (POCh, Poland), 0.5 M hydrochloric acid (POCh, Poland), 1 M standard aqueous NaOH solution (POCh, Poland), and Arnov’s reagent (Chempur, Poland).
The seeds of eight barley cultivars, including two spring and six winter cultivars, were used as raw materials. The spring cultivars used were Nagradowicki and Iron, and the winter cultivars used were Kobuz, Karahan, Holmes, Quadrige, Zenek, and Basic. The seeds were obtained from Poznańska Hodowla Roślin PHR (Poznań, Poland) and DANKO plant breeding facility (Kościan, Poland). The obtained barley seeds were cultivated in a sprouter at a temperature of 22 °C with ad libitum irrigation, and the grass was harvested after 10 days. After harvest, the materials were examined, packed in zip lock bags, frozen at −35 °C, and then subjected to lyophilization. Lyophilization was performed in a CHRIST 1-4 LSC freeze dryer (Martin Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany) under constant conditions. The condensation temperature in the freeze dryer was maintained at −48 °C, the temperature on the freeze dryer shelf at −20 °C, and the product temperature at −4 °C. The entire process was carried out under reduced pressure for 24 h. After lyophilization, the product was ground in a Retsch mill (Haan, Germany) for 15 s at a speed of 500 rpm at 21 °C to obtain a particle size of 0.5–0.9 mm.
2.2. Methods
2.2.1. Extraction
For extraction, 2 g of juvenile barley powder was weighed and added to a flask. To this, 100 mL of water at 90 °C was added according to the method described before [16]. The whole content of the flask was shaken for 15 min at a temperature of 90 °C. Subsequently, the samples were vacuum filtered in a Büchner funnel. After filtration, the obtained extracts were tightly closed and stored in darkness.
2.2.2. Growth Rate
The growth rate of seeds growing in the automatic sprouter (EasyGreen, Danbury, CT, USA) was measured with a ruler. Tap water was used for irrigation.
2.2.3. Reaction with the Folin–Ciocalteu Reagent
The analysis was performed based on the method described by Cheung et al. [17]. This method is based on the spectrophotometric (Metertech SP-830, Taipei, Taiwan) measurement of the absorbance of the colored complex formed by the reaction between the phenolic groups in the given extract and the Folin–Ciocalteu reagent (Chempur, Piekary Śląskie, Poland), at a wavelength of 765 nm. The calibration curve was constructed based on standard quercetin solutions, which showed excellent linearity (r2 = 0.9621). The results are presented as the equivalent of quercetin (QE) content in mg/g d.w. of the extract.
2.2.4. Polyphenolic Acids Determination
The analysis of polyphenolic acids was carried out according to the colorimetric method described by Galwik-Dziki [18] at a wavelength of 490 nm. For this, 1 mL of the test extract, 1 mL of 0.5 M HCl, 1 mL of Arnov’s reagent, and 1 mL of 1 M NaOH solution were added to 5 mL of distilled water. The final volume of this mixture was made up to 10 mL with distilled water. The content of polyphenolic acids was calculated as the equivalent of caffeic acid (CA) in mg/g d.w. based on the model curve y = 45.87x + 12.54 (r2 = 0.9717).
2.2.5. Chelating Activity
Chelating activity of crude barley extracts was measured according to the method of Tang et al. [19]. The amount of unchelated Fe2+ in crude barley extracts remaining after its reaction with ferrozine was determined as a measure of chelating activity. For this colorimetric assay, 1 mL of sample, 0.1 mL of 2 mM FeCl2, and 0.2 mL of the ferrozine reagent were added to each tube. The mixture was vortexed for ~60 s and left for 20 min at room temperature. Absorbance values were recorded (λ = 562 nm) using the Meterech SP 830 apparatus (Taipei, Taiwan). Deionized water was used as the control, and ferrozine was used as a reference (r2 = 0.9702).
2.2.6. Flavonols Composition
Composition of flavonols was determined using a method described by Kobus et al. [20]. The extracted flavonols were separated and identified by an Agilent UPLC equipped with a Nova-Pak C18 reversed-phase column (3.9 × 150 mm, 5-μm particle size; both from Waters, Milford, MA, USA). Solvent A used was 0.3% (v/v) HCOOH in H2O, while solvent B used was acetonitrile of HPLC purity grade. The flow rate of the solvents was maintained at 1 mL/min. The gradient profile was as follows: 85% of A at 0 min and 25% of A at 40 min. Chromatograms were recorded using a UV–Vis detector at λ = 370 nm. The separated compounds were identified based on retention time mapping using a set of standards. The quantity of the following flavonols was determined using standard solutions (0.001–0.01 μg/mL) of individual compounds: rutin, isoquercetin, hyperoside, astragalin, quercetin, and kaempferol (Sigma-Aldrich, Poznań, Poland).
2.2.7. Antiradical Capacity
Antiradical scavenging potential of the extract against ABTS cation radicals and DPPH radicals was analyzed.
An ABTS scavenging test was conducted using the modified method of Re et al. [21]. Briefly, aqueous stock solutions of ABTS (7 mM) and potassium peroxodisulfate (140 mM) were prepared and mixed to form a working solution of 2.45 mM potassium peroxodisulfate. The mixture was left in the dark and allowed to react at room temperature for 12–16 h. On the day of analysis, the ABTS radical solution was diluted with pure ethanol (POCH, Gliwice, Poland) to achieve an absorbance of 0.700 ± 0.02 at a wavelength of 734 nm. The measurements were taken as follows: 100 μL of barley extract was supplemented with 2.0 mL of the ABTS radical solution. Absorbance values were recorded after precisely 6 min against the respective reagent blank of 100 μL ethanol instead of the sample. The results from triplicate analyses, derived from a calibration curve (r2 = 0.9508) determined for Trolox standard solutions (100–1000 μM), were expressed as mg Trolox equivalents (TE)/g d.w. extract.
A methanolic solution of DPPH was used to evaluate the free-radical scavenging potential of the crude extract according to the method described by Amarowicz et al. [22]. The degree of discoloration of the solution indicated the scavenging efficacy of the added substance. For this analysis, 1 mL of the extract solution was supplemented with 2 mL of pure methanol (Honeywell), followed by 0.25 mL of 1 mM DPPH• ethanolic solution. The mixture was vortexed for ~60 s and left for 20 min at room temperature. Absorbance was recorded at λ = 517 nm (Meterech SP 830, Taipei, Taiwan). Methanol was used to prepare a reference sample and the control. To plot a calibration curve, absorbance values were measured simultaneously for samples containing the respective concentrations of Trolox (Sigma-Aldrich, Poznań, Poland) as a standard (0.5, 1.0, 1.5, and 2.0 mg/mL; r2 = 0.9639). Results are expressed as mg TE/g d.w. extracts.
2.2.8. Cholinesterases Inhibition
Cholinesterase inhibitory activity was analyzed using the method of Ellmann et al. [23] with some modifications [16]. A POLARstar Omega Plate Reader (BMG LABTECH, Ortenberg, Germany) with 96-well plates of a maximum capacity of 300 μl was used for measurements. Acetylcholine/butyrylcholine hydrolysis resulted in a change in the color of the enzymes under study, which was observed during absorbance measurements at a wavelength of 412 nm, 10 min after pipetting onto a microplate. All samples were tested four times. The reagent solutions were prepared in Tris–HCl buffer (50 mM, pH 8). The buffer was obtained by dissolving 0.6057 g of Tris base in 90 mL of pure water, with the addition of 1 M HCl to adjust the pH value to 8, and then filling with water to a final volume of 100 mL. The enzyme solutions were prepared by dissolving 4 units of enzyme in 2 mL of 1 M phosphate buffer (pH 8.3). The reaction mixture contained 35 μL of the test extract, 86 μL of Tris–HCl buffer, 35 μL of 1.5 mM ATChI or BTCh, 194 μL of a mixture of 0.3 mM 5,5′-dithiobis-(2-nitrobenzoic acid), 10 mM NaCl, and 2 mM MgCl2 × 6H2O, as well as AChE or BChE solution. The absorbance was measured after 15 min (BChE) or after 30 min (AChE).
At the same time, the positive control sample containing the known cholinesterase inhibitor, eserine (90.7 μM), and the negative control sample without the cholinesterase inhibitor were analyzed. The cholinesterase inhibitory activity was calculated by generating eserine modeling curves within the following concentration ranges: 0.08–6.50 μM (AChE) and 0.08–8.30 μM (BChE). All the samples were analyzed in triplicate. The results are expressed as serine equivalents (Es)/g d.w.
2.3. Statistical Analysis
Statistical analysis of data was carried out using STATISTICA PL 13.0 (StatSoft, Cracow, Poland) software. Pearson correlation coefficients and significance of differences (post hoc test) (p < 0.05) were evaluated.
3. Results
3.1. Yield Size
The tested barley cultivars were found to show a considerable variability in yield size (p < 0.05; Table 1). After 10 days of growth, the highest yield was recorded for Quadrige cultivar (19.98 cm), while the lowest for Holmes cultivar (13.48 cm). The greatest growth was observed after 4 days of cultivation for all cultivars.

Table 1.
Juvenile barley height [cm].
3.2. Active Compounds and Antiradical Properties
The extracts were tested for the content of active compounds, including total polyphenols and phenolic acids, and antiradical activity, and the corresponding results are provided in Table 2. It was determined that the total content of polyphenolic compounds was highest in the barley extracts of Quadrige and Karahan cultivars (37.67 and 37.35 mg/g d.w., respectively) and lowest in that of Zenek cultivar (25.33 mg/g d.w.). Polyphenolic acids were found to be statistically more abundant only in Quadrige cultivar, while the remaining cultivars contained this group of compounds at similar levels. The extracts of all the studied barley cultivars exhibited chelating activity, among which Zenek and Kobuz cultivars showed the statistically highest activity. The highest antioxidative activity was recorded for the extracts of Karahan and Holmes cultivars in the DPPH and ABTS scavenging test.

Table 2.
Content of total polyphenolic compounds, polyphenolic acids, and antioxidant activity of juvenile barley extracts.
3.3. Flavonols Analysis
The analysis of flavonols in the tested extracts demonstrated that all the extracts had a similar qualitative content, but differed quantitatively (Table 3). The content of flavonols was found to increase in the tested extracts of cultivars in the following order: Quadrige > Kobuz > Nagradowicki > Zenek (p < 0.05). The predominant flavonols were identified to be rutin, quercetin, and kaempferitrin. Among all he tested cultivars, the barley extract from Iron and Karahan contained the lowest amounts of flavonols, catechin, and epicatechin.

Table 3.
Content of flavonoids (μg/g d.w.) in the tested aqueous extracts.
3.4. Cholinesterases Inhibition
Among the tested cultivars, the highest inhibitory capacity against AChE was identified for the barley extract of Iron cultivar at 0.71 μM Es/g d.w. and the lowest activity for Holmes cultivar at 0.10 μM Es/g d.w. (Figure 1). The mean inhibitory capacity was estimated to be 0.46 μM Es/g d.w. The highest BChE inhibitory potential was exhibited by Basic cultivar at 1.33 μM Es/g d.w., while Karahan, Zenek, and Quadrige cultivars showed no inhibition against the enzyme. The mean activity was estimated to be 0.23 μM Es/g d.w.
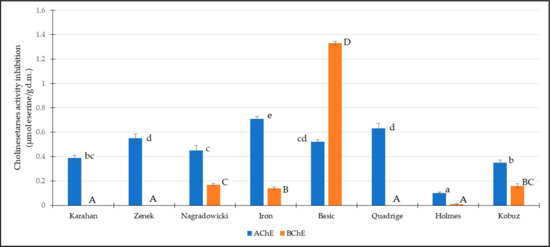
Figure 1.
Inhibitory activity of juvenile barley extracts against acetylcholinesterase (AChE) and butyrylcholinesterase (BChE). Lowercase letters a–c indicate significant statistical differences in AChE inhibition, and uppercase letters show significant differences between samples in BChE inhibition (n = 3).
3.5. Statistical Analysis
Figure 2a shows a dendrogram of cluster analysis performed for the extracts of the tested barley cultivars, including the qualitative and quantitative characteristics of their active compounds. The dendrogram distinguishes three base groups. The first cluster indicates the similarity of extract composition between Karahan and Iron cultivars, followed by the similarity between Zenek and Nagradowicki, and between Quadrige and Holmes, whereas the second cluster indicates similar extract composition of Zenek and Nagradowicki. It was determined that these cultivars were clearly distinguishable from the remaining cultivars in terms of the qualitative and quantitative characteristics of their bioactive compounds. The projection of the results of principal component analysis of the antioxidative and cholinesterase inhibitory activities in the system of two principal components (PC1 and PC2) responsible for close to 60% of the composition variability exhibited the variability of the samples in terms of the activities and the degree of the impact of individual compounds on the characteristics of the extracts (Figure 2b,c).
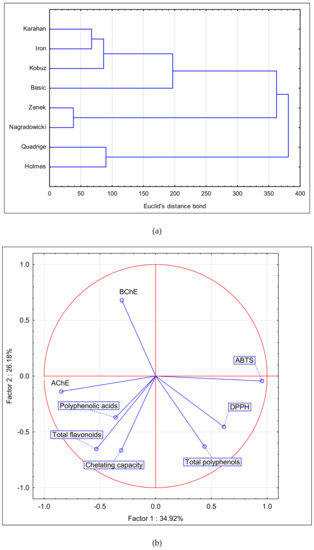
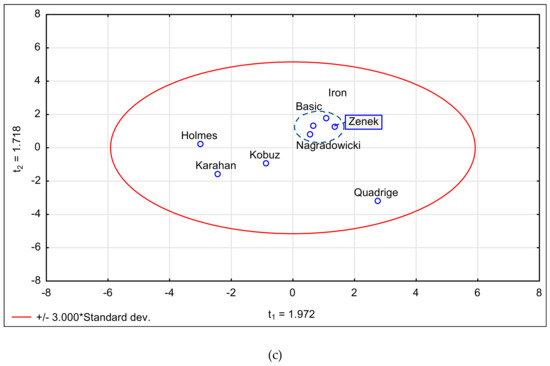
Figure 2.
(a) Euclidean distance projection of the tested Hordeum vulgare L. cultivars, (b) principal scatter diagram of the tested parameters, and (c) principal scatter diagram of the tested cultivars.
PCA projection (biplot) of the factors responsible for nearly 60% of the total variance in barley composition showed that the studied cultivars differed between each other in their composition, antioxidant potential, and cholinesterase inhibitory activity (Figure 2a,c). AChE located adjacent to phenolic acids and flavonoids indicates the poor positive correlation between these factors (Figure 2b, Table 4). The distant position of BChE and total polyphenol vectors may result from the negative correlation between these two factors and the aforementioned factors (Table 4). The cultivars Basic, Zenek, Iron, and Nagradowicki are grouped tightly due to similarity in their content of analyzed compounds and bioactivity (Figure 2c).

Table 4.
Correlation matrix of studied variables in Hordeum samples.
4. Discussion
The stage of raw material selection for a specific use is important for obtaining the best quality products. Similarly, in the case of the extract production from barley grass, the cultivars are selected in such a way as to achieve the highest yield. An important element in the production of barley extract is the method used for extraction. The rate and ease of extraction depend on a range of factors, such as the type of matrix or the subject of extraction, its structure, susceptibility to the penetration of extractant, and contact surface with the solvent, the type of compounds found in the extracted raw material, and the duration and temperature of the process. The present study demonstrated that spring (Nagradowicki and Iron) as well as winter cultivars can produce a satisfactory yield in the form of grass. Similar results were reported earlier in the study of Talbot [24], which showed that the yield size does not determine the extract properties as well as the content of active compounds. The results of the present study confirmed that winter cultivars contained higher amounts of polyphenols and their antioxidative activity was higher. Flavonols are capable of absorbing large amounts of UV radiation, and thus, they are primarily found in the inner layers of plants. It has been demonstrated that they possess good antioxidative and antimicrobial properties [2,25]. Chomchan et al. [26] examined the juice obtained from rice and wheat grass. The authors determined that the free radical scavenging capacity of rice grass is 4.65 ± 0.12 mg TE/g extract and that of wheat grass is 5.51 ± 0.04 mg TE/g extract. These results are comparable with those obtained in the study of Kiewlicz [8], who studied the aqueous extracts of juvenile barley and estimated their DPPH scavenging capacity at 2.43 ± 0.07 IC50 mg/mL. Koga et al. [27] determined the capacity of juvenile barley to scavenge DPPH radical at a level of 49.5%. The study of Gao et al. [3] indicated that the DPPH scavenging capacity of juvenile barley leaves ranged between 44.12 ± 0.75 and 95.81 ± 0.63%, depending on the extraction parameters. Choe et al. [28] demonstrated that the DPPH scavenging capacity of ethanol extracts of barley leaves was 80.3 ± 0.41% at 5 mg/mL and that of methanol extracts was 79.5 ± 0.54% at 5 mg/mL. Khanthapok et al. [29] in their study indicated that the DPPH scavenging capacity of wheat grass was 0.81 ± 0.02 IC50 mg/mL. Rattanapon et al. [30] estimated that the DPPH scavenging capacity of rice grass was 89.32 ± 0.57 mmol TE/g. Anwar et al. [31] showed that the free DPPH scavenging capacity of barley seeds ranged between 90.7 and 168.6 IC50 μg/mL, depending on the cultivar and extractant concentration. Kiewlicz [8] studied the aqueous extracts obtained from juvenile barley leaves and estimated their ABTS scavenging capacity to be 29.37 ± 1.60 μmol Trolox/g d.w. Chomchan et al. [26] stated that the ABTS scavenging capacity of rice grass juice was 38.06 ± 0.38 mg TE/g extract, and that of wheat grass was 39.77 ± 0.27 mg TE/g extract. The above results are in line with the results of the present study. The ABTS scavenging capacity of rice grass determined by Rattanapon et al. [30] was 129.63 ± 1.21 mmol TE/g.
Cholinesterase inhibition is of key significance in the symptom-based treatment of Alzheimer’s disease. Cholinesterases are hydrolases of carboxylic esters, and their main function is to hydrolyze acetylcholine in the nerve–muscle connections, thereby inactivating cholinergic neurotransmission. These are mostly extracellular enzymes, which occur in soluble form or are found to be attached to the external surface of the cells. In humans, the level of cholinesterases, as well as the distribution of their molecular forms, varies in different regions of the brain [13]. Currently, compounds with cholinesterase-inhibiting properties are gaining attention. By blocking these enzymes, cholinesterase inhibitors contribute to an increased amount of acetylcholine in cholinergic synapses, and thus improve neuronal transmission. Hence, numerous ongoing studies focus on specific natural compounds that can act as reversible inhibitors of AChE, which can be used to improve the cholinergic transmission of the central nervous system [32].
Polyphenols can also act as cholinesterase inhibitors [16,33], as indicated by literature data. For instance, the study of Kobus et al. [16] showed that hop extracts exhibited AChE inhibitory activity. The present study showed that barley extracts exhibited higher inhibitory activity against AChE than against BChE, highlighting that certain barley cultivars did not inhibit BChE, while all of them inhibited AChE. The results obtained herein suggest that barley grass serves as a source of antioxidants with antioxidative effect and may act as a cholinesterase inhibitor. Not only phenolic acids and flavonols but also terpenoids as well as alkaloids and vitamins and their chelates are responsible for this action. Lemon balm (Melissa officinalis L.) has been documented to have cholinergic inhibitory properties [14]. It is further believed that cholinesterase inhibitors can be found in the extracts of plants belonging to families including legumes (Fabaceae), heathers (Ericaceae), tamarisks (Tamaricaceae), poppy (Papaveraceae), fumeworts (Fumariaceae), pepper (Piperaceae), and many others, the properties of which remain the subject of numerous studies [34]. Research performed by Szwajgier and Borówiec [35] showed the inhibitory activity of compounds present in Ginkgo biloba, while another study of these authors also confirmed such activity in other edible plants [35,36]. For instance, the highest degrees of cholinesterase inhibition were observed for peach juice (Prunus persica L.), dill aqueous extracts, wild strawberry fruits, potato tubers, and juices from apple cultivars Idared and Champion. Parsley leaves and celery extracts were also shown to exhibit significantly higher inhibitory activity against AChE than against BChE.
The medicinal applicability of plant raw materials is determined by the amount and quality of the active substances they contain. Moreover, the proportion of these compounds is also associated with the intensity of their effects. In the present study, juvenile barley is identified as a new raw material that may be of significance in the food industry due to its antioxidant and cholinesterase inhibitory properties.
5. Conclusions
The results of this work might be considered as an input for further research related to the assessment of the bioactivity of juvenile barley. In order to confirm the pro-health properties of a raw material, it is necessary to perform both in vivo and in vitro experiments. The present study proved that barley can be included as a potential source of bioactive compounds in the diet and the significance of juvenile barley warrants further research.
Author Contributions
Formal analysis, O.S., M.D. and D.S.; Investigation, J.K.-C., O.S., M.D. and K.S.-B.; Methodology, J.K.-C.; Resources, P.S.; Supervision, P.S.; Validation, A.T. and M.L.; Visualization, K.S.-B.; Writing—original draft, J.K.-C., E.G.-G. and M.G.; Writing—review & editing, A.T. and M.L. All authors have read and agreed to the published version of the manuscript.
Funding
The publication was co-financed within the framework of the Ministry of Science and Higher Education program as “Regional Initiative Excellence” in years 2019–2022, project number 005/RID/2018/19 and by statutory funds of the Department of Gastronomy Sciences and Functional Foods of Poznań University of Life Sciences, grant number 506.751.03.00.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lahouar, L.; El Arem, A.; Ghrairi, F.; Chahdoura, H.; Ben Salem, H.; El Felah, M.; Achour, L. Phytochemical content and antioxidant properties of diverse varieties of whole barley (Hordeum vulgare L.) grown in Tunisia. Food Chem. 2014, 145, 578–583. [Google Scholar] [CrossRef]
- Kamiyama, M.; Shibamoto, T. Flavonoids with Potent Antioxidant Activity Found in Young Green Barley Leaves. J. Agric. Food Chem. 2012, 60, 6260–6267. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Zhang, M.; Fang, Z.; Zhong, Q. Optimization of microwave-assisted extraction of flavonoids from young barley leaves. Int. Agrophysics 2017, 31, 45–52. [Google Scholar] [CrossRef]
- Havlíková, L.; Šatínský, D.; Opletal, L.; Solich, P. A Fast Determination of Chlorophylls in Barley Grass Juice Powder Using HPLC Fused-Core Column Technology and HPTLC. Food Anal. Methods 2014, 7, 629–635. [Google Scholar] [CrossRef]
- Paulíčková, I.; Ehrenbergerová, J.; Fiedlerová, V.; Gabrovská, D.; Havlová, P.; Holasová, M.; Kopáček, J.; Ouhrabková, J.; Pinkrová, J.; Rysová, J.; et al. Evaluation of barley grass as a potential source of some nutritional substances. Czech. J. Food Sci. 2007, 25, 65–72. [Google Scholar] [CrossRef]
- Březinová Belcredi, N.; Ehrenbergerová, J.; Fiedlerová, V.; Běláková, S.; Vaculová, K. Antioxidant Vitamins in Barley Green Biomass. J. Agric. Food Chem. 2010, 58, 11755–11761. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zeng, Y.; Yang, X.; Pu, X.; Du, J. Utilization of Barley Functional Foods for Preventing Chronic Diseases in China. Agric. Sci. Technol. 2016, 17, 2195–2204. [Google Scholar]
- Kiewlicz, J. Evaluation of Total Phenolic Content and Antioxidant Properties of the Water Extract of the Powdered Barley Grass (Hordeum vulgare L.). Towaroznawcze Problemy Jakości 2016, 47, 29–37. [Google Scholar] [CrossRef]
- Kowalczewski, P.Ł.; Radzikowska, D.; Ivanišová, E.; Szwengiel, A.; Kačániová, M.; Sawinska, Z. Influence of Abiotic Stress Factors on the Antioxidant Properties and Polyphenols Profile Composition of Green Barley (Hordeum vulgare L.). Int. J. Mol. Sci. 2020, 21, 397. [Google Scholar] [CrossRef]
- Yamaura, K.; Nakayama, N.; Shimada, M.; Bi, Y.; Fukata, H.; Ueno, K. Antidepressant-like effects of young green barley leaf (Hordeum vulgare L.) in the mouse forced swimming test. Pharmacogn. Res. 2012, 4, 22–26. [Google Scholar] [CrossRef]
- Yamaura, K.; Tanaka, R.; Bi, Y.; Fukata, H.; Oishi, N.; Sato, H.; Mori, C.; Ueno, K. Protective effect of young green barley leaf (Hordeum vulgare L.) on restraint stress-induced decrease in hippocampal brain-derived neurotrophic factor in mice. Pharmacogn. Mag. 2015, 11, S86. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, M.; Yokoyama, K.; Nakano, Y.; Nakamura, H. Protective Effects of Barley and Its Hydrolysates on Gastric Stress Ulcer in Rats. Yakugaku Zasshi 2004, 124, 571–575. [Google Scholar] [CrossRef][Green Version]
- Işık, M.; Beydemir, Ş.; Yılmaz, A.; Naldan, M.E.; Aslan, H.E.; Gülçin, İ. Oxidative stress and mRNA expression of acetylcholinesterase in the leukocytes of ischemic patients. Biomed. Pharmacother. 2017, 87, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Akhondzadeh, S.; Noroozian, M.; Mohammadi, M.; Ohadinia, S.; Jamshidi, A.H.; Khani, M. Melissa officinalis extract in the treatment of patients with mild to moderate Alzheimer’s disease: A double blind, randomised, placebo controlled trial. J. Neurol. Neurosurg. Psychiatry 2003, 74, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, B. Zinc homeostasis and neurodegenerative disorders. Front. Aging Neurosci. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Kobus-Cisowska, J.; Szymanowska, D.; Szczepaniak, O.; Kmiecik, D.; Przeor, M.; Gramza-Michałowska, A.; Cielecka-Piontek, J.; Smuga-Kogut, M.; Szulc, P. Humulus lupulus L. hops as a potent antioxidant: Implications for neurodegenerative disorders and antimicrobial effect. Nutrients 2019, 11, 1377. [Google Scholar] [CrossRef]
- Cheung, L.M.; Cheung, P.C.K.; Ooi, V.E.C. Antioxidant activity and total phenolics of edible mushroom extracts. Food Chem. 2003, 81, 249–255. [Google Scholar] [CrossRef]
- Office for Registration of Medicinal Products Medical Devices and Biocidal Products Farmakopea Polska; PT Farm: Warsaw, Poland, 2002.
- Tang, S.; Kerry, J.; Sheehan, D.; Buckley, D. Antioxidative mechanisms of tea catechins in chicken meat systems. Food Chem. 2002, 76, 45–51. [Google Scholar] [CrossRef]
- Kobus, J.; Flaczyk, E.; Siger, A.; Nogala-Kałucka, M.; Korczak, J.; Pegg, R.B. Phenolic compounds and antioxidant activity of extracts of Ginkgo leaves. Eur. J. Lipid Sci. Technol. 2009, 111, 1150–1160. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Amarowicz, R.; Zegarska, Z.; Pegg, R.B.; Karamac, M.; Kosinska, A. Antioxidant and radical scavenging activities of a barley crude extract and its fraction. Czech. J. Food Sci. 2008, 25, 73–80. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Talbot, M. Yield variability of crop varieties in the U.K. J. Agric. Sci. 1984, 102, 315–321. [Google Scholar] [CrossRef]
- Sumińska, P.; Berton, A.; Dietz, W. Subcritical water extraction (SWE) of barley (Hordeum vulgare) straw as method of antimicrobial and antioxidant additives production. World Sci. News 2017, 81, 169–183. [Google Scholar]
- Chomchan, R.; Siripongvutikorn, A.P.D.S.; Puttarak, D.P.; Rattanapon, M.R. Investigation of Phytochemical Constituents, Phenolic Profiles and Antioxidant Activities of Ricegrass Juice compared to Wheatgrass Juice. Funct. Foods Heal. Dis. 2016, 6, 822. [Google Scholar] [CrossRef]
- Koga, R.; Tsubata, M.; Ikeguchi, M.; Takagaki, K.; Irino, N.; Kondo, R. Hypercholesterolemia-reducing Effect of Young Barley Leaf Powder. Nippon Shokuhin Kagaku Kogaku Kaishi 2013, 60, 19–24. [Google Scholar] [CrossRef]
- Choe, J.-H.; Jang, A.; Choi, J.-H.; Choi, Y.-S.; Han, D.-J.; Kim, H.-Y.; Lee, M.-A.; Kim, H.-W.; Kim, C.-J. Antioxidant activities of lotus leaves (Nelumbo nucifera) and barley leaves (Hordeum vulgare) extracts. Food Sci. Biotechnol. 2010, 19, 831–836. [Google Scholar] [CrossRef]
- Khanthapok, P.; Muangprom, A.; Sukrong, S. Antioxidant activity and DNA protective properties of rice grass juices. Sci. Asia 2015, 41, 119. [Google Scholar] [CrossRef]
- Rattanapon, R.; Siripongvutikorn, S.; Usawakesmanee, W.; Thongraung, C. Changes of nutritional value, bioactive compounds and antioxidant activity of primed white rice, Chainat 1, during seedling. Int. Food Res. J. 2017, 24, 2563–2571. [Google Scholar]
- Anwar, F.; Qayyum, H.M.A.; Hussain, A.I.; Iqbal, S. Antioxidant activity of 100% and 80% methanol extracts from barley seeds (Hordeum vulgare L.): Stabilization of sunflower oil. Grasas y Aceites 2010, 61, 237–243. [Google Scholar] [CrossRef]
- Akhtar, M.N.; Lam, K.W.; Abas, F.; Maulidiani Ahmad, S.; Shah, S.A.A.; Atta-ur-Rahman; Choudhary, M.I.; Lajis, N.H. New class of acetylcholinesterase inhibitors from the stem bark of Knema laurina and their structural insights. Bioorg. Med. Chem. Lett. 2011, 21, 4097–4103. [Google Scholar] [CrossRef] [PubMed]
- Kobus-Cisowska, J.; Szymanowska, D.; Maciejewska, P.; Kmiecik, D.; Gramza-Michałowska, A.; Kulczyński, B.; Cielecka-Piontek, J. In vitro screening for acetylcholinesterase and butyrylcholinesterase inhibition and antimicrobial activity of chia seeds (Salvia hispanica). Electron. J. Biotechnol. 2019, 37, 1–10. [Google Scholar] [CrossRef]
- Wszelaki, N. Plants as a source of acetylcholinesterase and butyrylcholinesterase inhibitors. Postępy Fitoterapii 2009, 10, 24–38. [Google Scholar]
- Szwajgier, D.; Borowiec, K. Phenolic acids from malt are efficient acetylcholinesterase and butyrylcholinesterase inhibitors. J. Inst. Brew. 2012, 118, 40–48. [Google Scholar] [CrossRef]
- Szwajgier, D.; Wydrych, M.; Wiecław, E.; Targoński, Z. Anticholinesterase and antioxidant activities of commercial preparations from Ginkgo biloba leaves. Acta Sci. Pol. Hortorum Cultus 2013, 12, 111–125. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).