Research and Application of Biochar in Soil CO2 Emission, Fertility, and Microorganisms: A Sustainable Solution to Solve China’s Agricultural Straw Burning Problem
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochar Production
2.2. Equipment and Reagents
2.3. Soil Incubation
2.4. Pot Experiment
2.5. Physical and Chemical Properties
2.6. Determination of Microbial Diversity
2.6.1. Total DNA Extraction
2.6.2. Polymerase Chain Reaction (PCR) Extension of ITS2 Region and 16S rDNA-V4 Region
2.6.3. Quality Control and Analysis of Lower Machine Data
2.7. Statistical Analysis
3. Results and Analysis
3.1. Comparative Analysis of Basic Physical and Chemical Properties of Different Biochars and Biomass
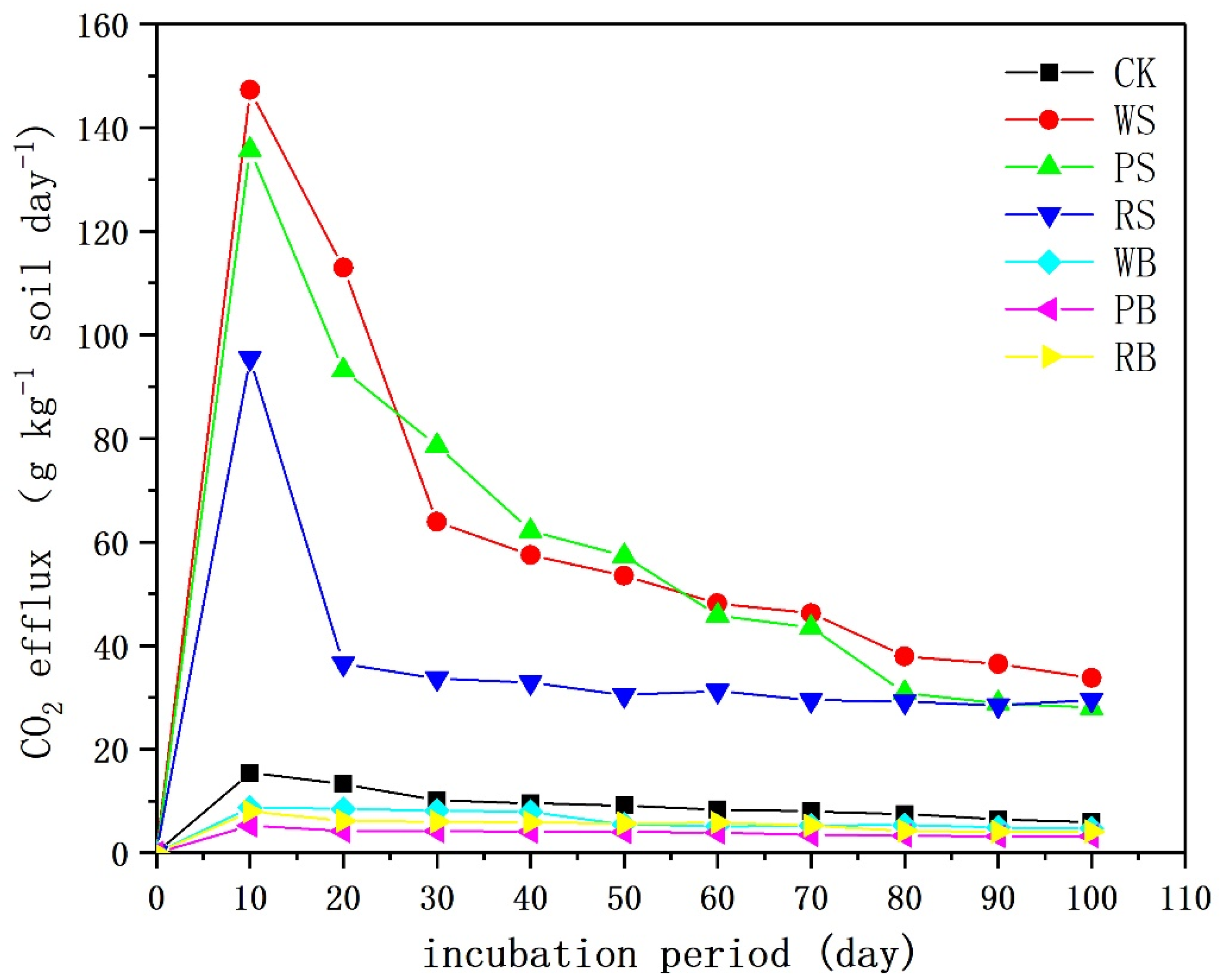
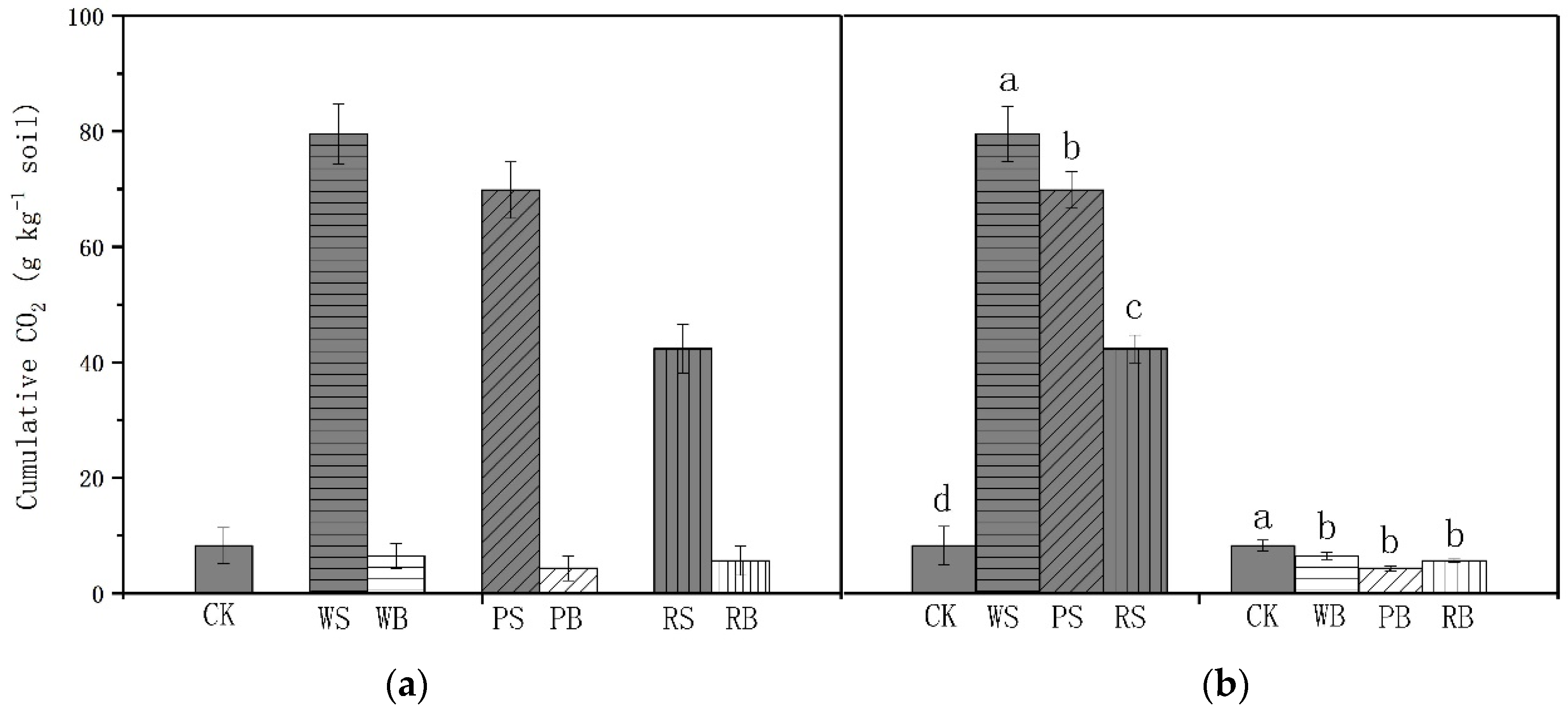
3.2. Soil CO2 Emissions
3.3. Soil Fertility
3.3.1. pH and Conductivity (EC)
3.3.2. Soil Bulk Density, Water Holding Capacity, Temperature
3.3.3. Soil Organic Matter
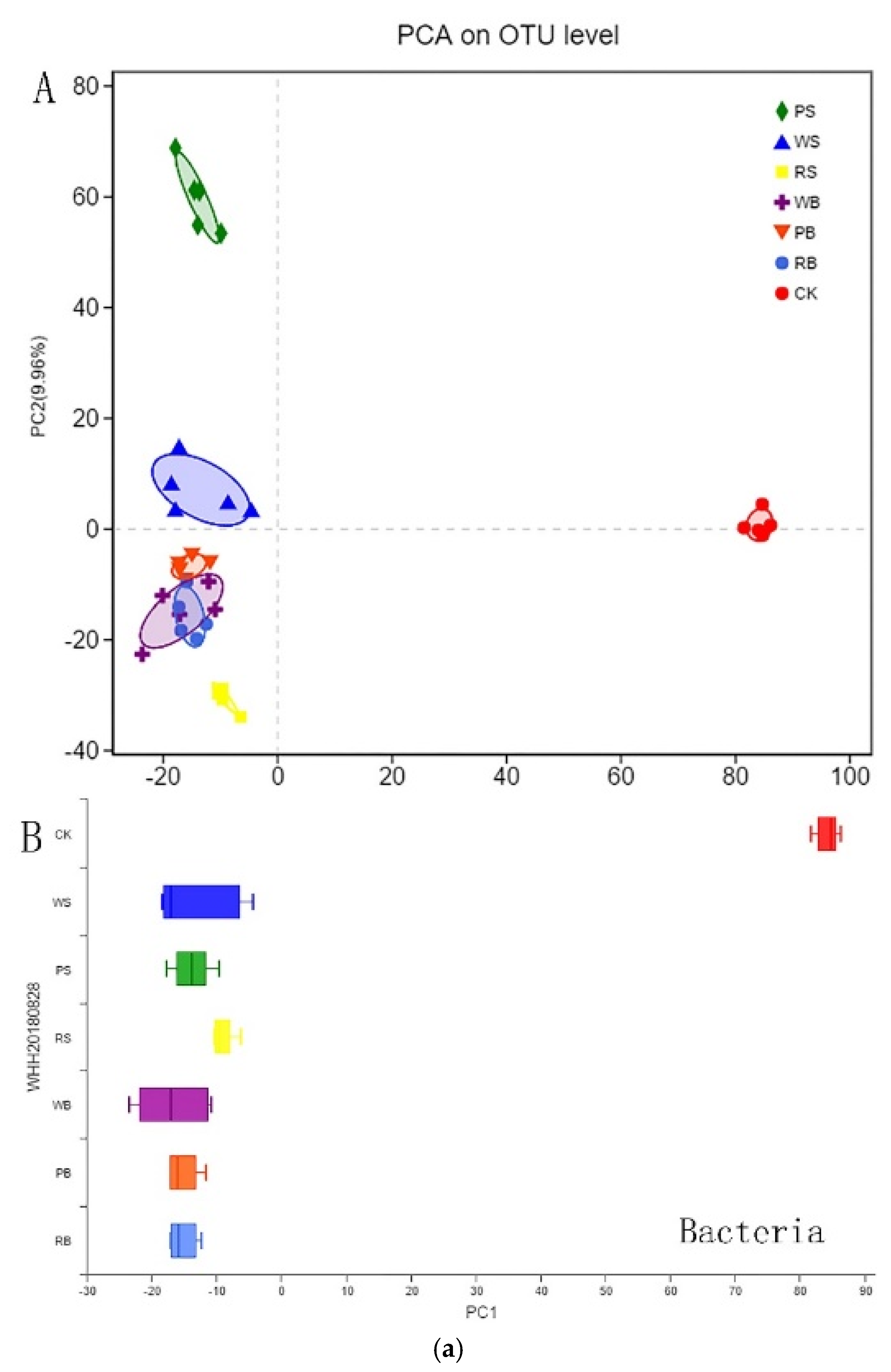
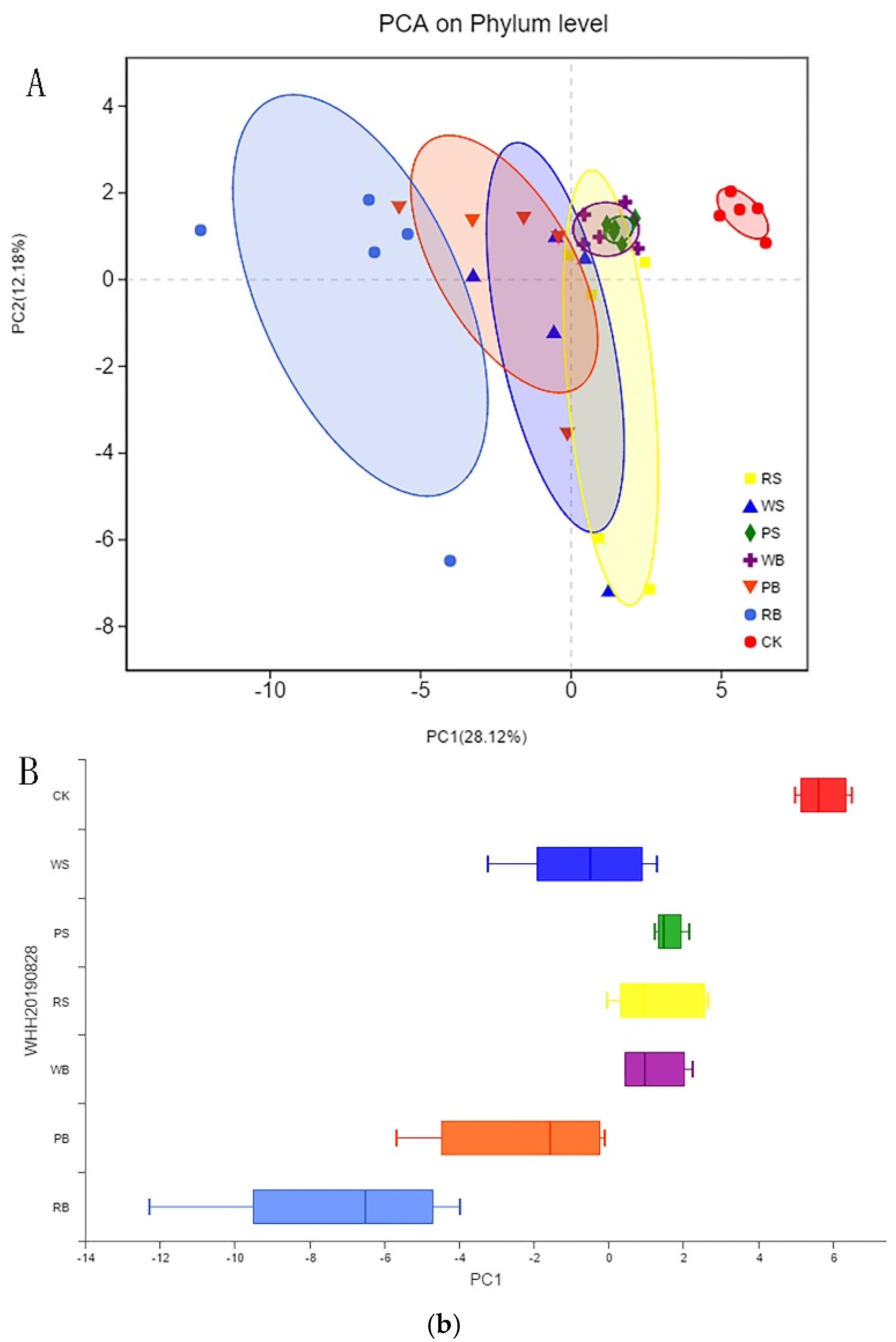
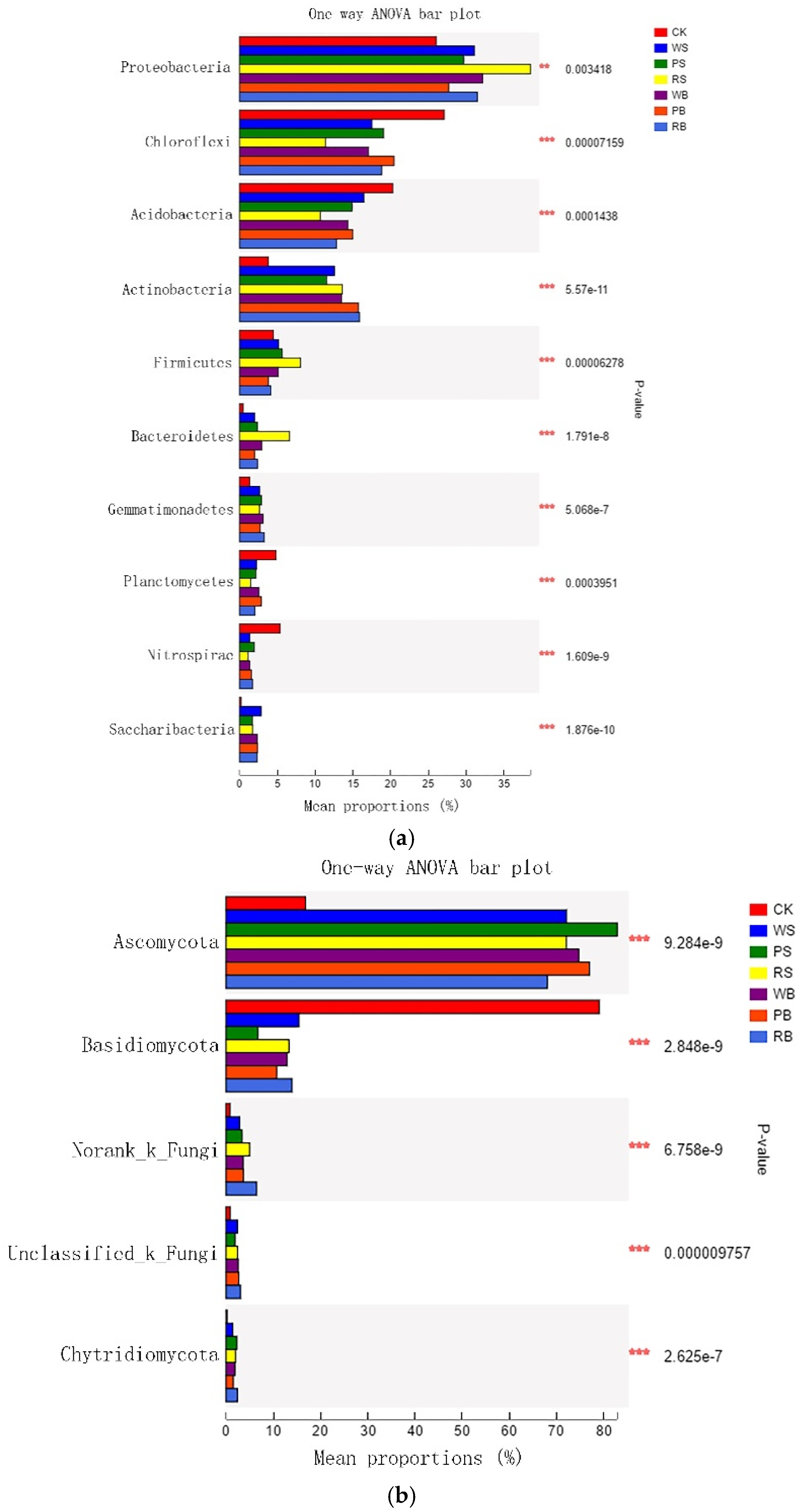
3.4. Microbial Diversity
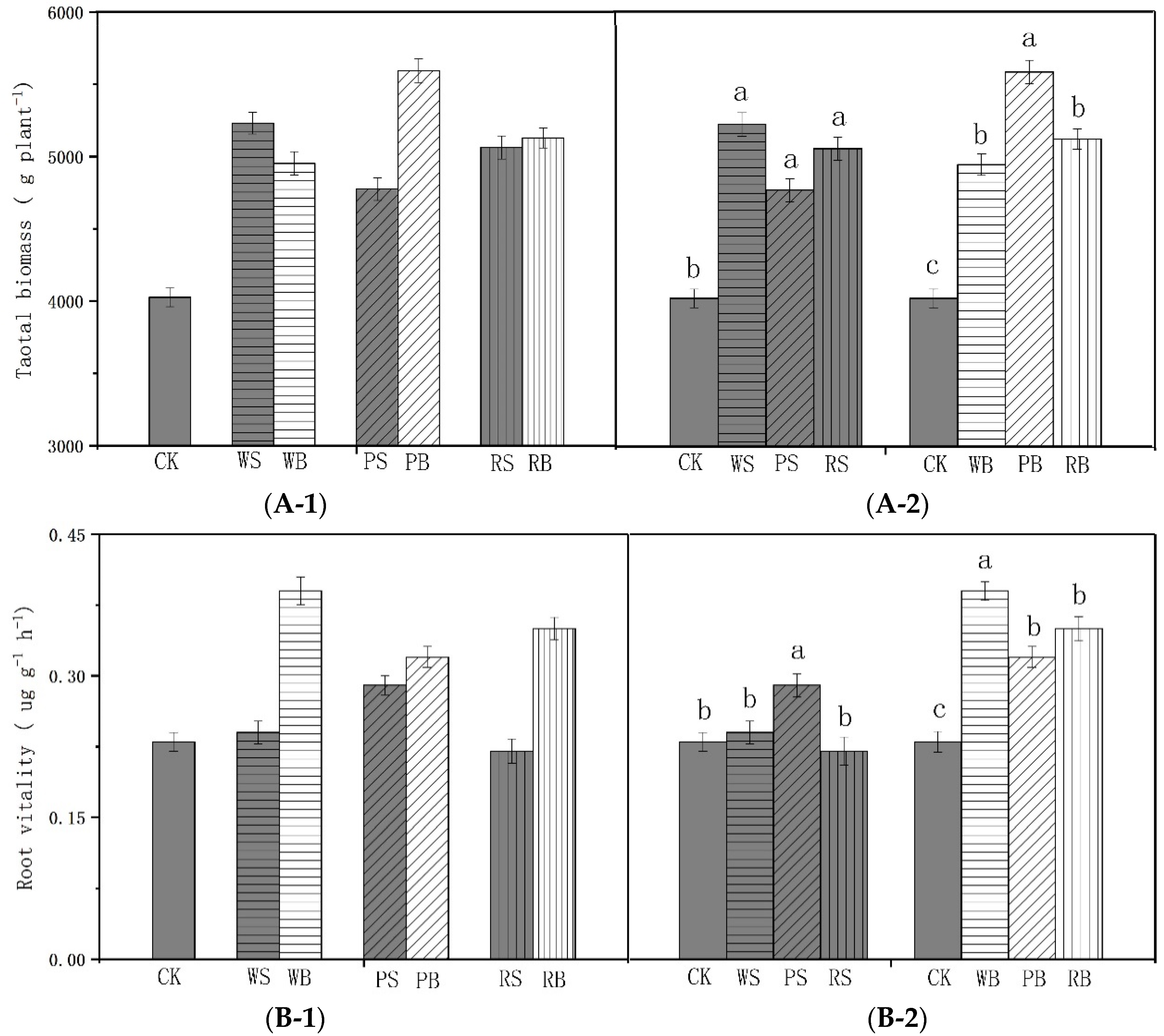
3.5. Tobacco Biomass and Root Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Graber, E.R.; Harel, Y.M.; Kolton, M.; Cytryn, E.; Silber, A.; David, D.R.; Tsechansky, L.; Borenshtein, M.; Elad, Y. Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil 2010, 337, 481–496. [Google Scholar] [CrossRef]
- Steinbeiss, S.; Gleixner, G.; Antonietti, M. Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil Boil. Biochem. 2009, 41, 1301–1310. [Google Scholar] [CrossRef]
- Mohan, D.; Abhishek, K.; Sarswat, A.; Patel, M.; Singh, P.; Pittman, C.U. Biochar production and applications in soil fertility and carbon sequestration—A sustainable solution to crop-residue burning in India. RSC Adv. 2018, 8, 508–520. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon Dioxide Capture: Prospects for New Materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef]
- Gadalla, M.; Olujić, Ž.; Jobson, M.; Smith, R. Estimation and reduction of CO2 emissions from crude oil distillation units. Energy 2006, 31, 2398–2408. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.; Crowley, D. Biochar effects on soil biota—A review. Soil Boil. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.; Van Der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Spokas, K. Impact of biochar field aging on laboratory greenhouse gas production potentials. GCB Bioenergy 2012, 5, 165–176. [Google Scholar] [CrossRef]
- Lehmann, J.; da Silva, J.P., Jr.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Boil. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Ghani, W.A.W.A.K.; Mohd, A.; Da Silva, G.; Bachmann, R.; Taufiq-Yap, Y.H.; Rashid, U.; Al-Muhtaseb, A.H. Biochar production from waste rubber-wood-sawdust and its potential use in C sequestration: Chemical and physical characterization. Ind. Crop. Prod. 2013, 44, 18–24. [Google Scholar] [CrossRef]
- Busch, D.; Kammann, C.I.; Grünhage, L.; Muller, C. Simple Biotoxicity Tests for Evaluation of Carbonaceous Soil Additives: Establishment and Reproducibility of Four Test Procedures. J. Environ. Qual. 2012, 41, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Kammann, C.; Ratering, S.; Eckhard, C.; Muller, C. Biochar and Hydrochar Effects on Greenhouse Gas (Carbon Dioxide, Nitrous Oxide, and Methane) Fluxes from Soils. J. Environ. Qual. 2012, 41, 1052–1066. [Google Scholar] [CrossRef] [PubMed]
- Karhu, K.; Mattila, T.; Bergström, I.; Regina, K. Biochar addition to agricultural soil increased CH4 uptake and water holding capacity—Results from a short-term pilot field study. Agric. Ecosyst. Environ. 2011, 140, 309–313. [Google Scholar] [CrossRef]
- Baronti, S.; Vaccari, F.P.; Miglietta, F.; Calzolari, C.; Lugato, E.; Orlandini, S.; Pini, R.; Zulian, C.; Genesio, L. Impact of biochar application on plant water relations in Vitis vinifera (L.). Eur. J. Agron. 2014, 53, 38–44. [Google Scholar] [CrossRef]
- Yanai, Y.; Toyota, K.; Okazaki, M. Effects of charcoal addition on N2O emissions from soil resulting from rewetting air-dried soil in short-term laboratory experiments. Soil Sci. Plant Nutr. 2007, 53, 181–188. [Google Scholar] [CrossRef]
- Marchetti, C. On geoengineering and the CO2 problem. Clim. Chang. 1977, 1, 59–68. [Google Scholar] [CrossRef]
- Sissoko, A.; Kpomblekou-A, K. Carbon decomposition in broiler litter-amended soils. Soil Boil. Biochem. 2010, 42, 543–550. [Google Scholar] [CrossRef]
- Rillig, M.; Wagner, M.; Salem, M.; Antunes, P.; George, C.; Ramke, H.-G.; Titirici, M.; Antonietti, M. Material derived from hydrothermal carbonization: Effects on plant growth and Arbuscular mycorrhiza. Appl. Soil Ecol. 2010, 45, 238–242. [Google Scholar] [CrossRef]
- Li, H.; Dong, T.; Wang, M. Effects of Biochar on Microbial Communities and Metabolic Activity in Rhizospheric Soil of Banana Seedlings. J. Microbiol. 2016, 36, 42–48. [Google Scholar]
- Chen, Z.B.; Gao, X.; Wang, D.B.; Guo, L.H.; Wang, D.K.; Xu, S.G. Effects of Different Biochar Application Rates on Rhizosphere Soil Microbial Diversity of Tobacco. Acta Agric. Boreali.-Sin. 2018, 33, 224–232. [Google Scholar]
- Sheng, Y.; Zhu, L. Biochar alters microbial community and carbon sequestration potential across different soil pH. Sci. Total Environ. 2018, 622–623, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Clemensson-Lindell, A. Triphenyltetrazolium chloride as an indicator of fine-root vitality and environmental stress in coniferous forest stands: Applications and limitations. Plant Soil 1994, 159, 297–300. [Google Scholar] [CrossRef]
- Maiti, S.; Dey, S.; Purakayastha, S.; Ghosh, B. Physical and thermochemical characterization of rice husk char as a potential biomass energy source. Bioresour. Technol. 2006, 97, 2065–2070. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Hess, M.; Sczyrba, A.; Egan, R.; Kim, T.-W.; Chokhawala, H.; Schroth, G.; Luo, S.; Clark, D.S.; Chen, F.; Zhang, T.; et al. Metagenomic Discovery of Biomass-Degrading Genes and Genomes from Cow Rumen. Science 2011, 331, 463–467. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef]
- Kuhlbusch, T. Method for Determining Black Carbon in Residues of Vegetation Fires. Environ. Sci. Technol. 1995, 29, 2695–2702. [Google Scholar] [CrossRef] [PubMed]
- Mukome, F.N.D.; Parikh, S.J. Chemical, physical, and surface characterization of biochar. In Biochar: Production, Characterization and Applications; Ok, Y.S., Uchimiya, S.M., Chang, S.X., Bolan, N., Eds.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Zhang, M.; Liu, X.; Wang, W.; Zhou, M. Decomposition analysis of CO2 emissions from electricity generation in China. Energy Policy 2013, 52, 159–165. [Google Scholar] [CrossRef]
- Toyama, A.; Matsumoto, I.; Izumichi, T. Method of Producing the Gaseous and Liquefied Nitrogen and an Apparatus Used Therefor. U.S. Patent No. 3,736,762, 5 June 1973. [Google Scholar]
- Martin, S.L.; Clarke, M.L.; Othman, M.; Ramsden, S.; West, H.M. Biochar-mediated reductions in greenhouse gas emissions from soil amended with anaerobic digestates. Biomass Bioenergy 2015, 79, 39–49. [Google Scholar] [CrossRef]
- Singla, A.; Iwasa, H.; Inubushi, K. Effect of biogas digested slurry based-biochar and digested liquid on N2O, CO2 flux and crop yield for three continuous cropping cycles of komatsuna (Brassica rapa var. perviridis). Boil. Fertil. Soils 2014, 50, 1201–1209. [Google Scholar] [CrossRef]
- Lim, S.-S.; Choi, W.-J.; Lee, K.-S.; Ro, H.-M. Reduction in CO2 emission from normal and saline soils amended with coal fly ash. J. Soils Sediments 2012, 12, 1299–1308. [Google Scholar] [CrossRef]
- Ohlsson, K.E.A. Carbonation of Wood Ash Recycled to a Forest Soil as Measured by Isotope Ratio Mass Spectrometry. Soil Sci. Soc. Am. J. 2000, 64, 2155–2161. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, M.; Wu, Y.; Wang, H.; Chen, Y.; Wu, W. Reducing CH4 and CO2 emissions from waterlogged paddy soil with biochar. J. Soils Sediments 2011, 11, 930–939. [Google Scholar] [CrossRef]
- Libra, J.; Ro, K.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Kimber, S.; Morris, S.; Downie, A.; Berger, E.; Rust, J.; Scheer, C. Influence of biochars on flux of N2O and CO2 from Ferrosol. Soil Res. 2010, 48, 555–568. [Google Scholar] [CrossRef]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of greenwaste biochar as a soil amendment. Soil Res. 2007, 45, 629. [Google Scholar] [CrossRef]
- Herath, H.; Arbestain, M.C.; Hedley, M. Effect of biochar on soil physical properties in two contrasting soils: An Alfisol and an Andisol. Geoderma 2013, 209, 188–197. [Google Scholar] [CrossRef]
- Soinne, H.; Hovi, J.; Tammeorg, P.; Turtola, E. Effect of biochar on phosphorus sorption and clay soil aggregate stability. Geoderma 2014, 219, 162–167. [Google Scholar] [CrossRef]
- Steiner, C.; Das, K.; Garcia, M.; Förster, B.; Zech, W. Charcoal and smoke extract stimulate the soil microbial community in a highly weathered xanthic Ferralsol. Pedobiologia 2008, 51, 359–366. [Google Scholar] [CrossRef]
- Devereux, R.C.; Sturrock, C.; Mooney, S.J. The effects of biochar on soil physical properties and winter wheat growth. Earth Environ. Sci. Trans. R. Soc. Edinb. 2012, 103, 13–18. [Google Scholar] [CrossRef]
- Tryon, E.H. Effect of Charcoal on Certain Physical, Chemical, and Biological Properties of Forest Soils. Ecol. Monogr. 1948, 18, 81–115. [Google Scholar] [CrossRef]
- Wang, H.; Ren, T.; Zhang, Z.; Yuan, Y.; Wang, B.; Kuang, G.; Liu, D.; Liu, G. Effects of Biochar on Tobacco-planting Soil Improvement and Tobacco Quality in Mudanjiang Area. Agric. Sci. Technol. 2017, 18, 820–826. [Google Scholar]
- Kolb, S.E.; Fermanich, K.J.; Dornbush, M.E. Effect of charcoal quantity on microbial biomass and activity in temperate soils. Soil Sci. Soc. Am. J. 2009, 73, 1173–1181. [Google Scholar] [CrossRef]
- Palumbo, A.V.; Porat, I.; Phillips, J.R.; Amonette, J.E.; Drake, M.M.; Brown, S.D.; Schadt, C.W. Leaching of mixtures of biochar and fly ash. In Proceedings of the Report of World of Coal Ash (WOCA) Conference, Lexington, KY, USA, 4–7 May 2009. [Google Scholar]
- Kimetu, J.M.; Lehmann, J. Stability and stabilisation of biochar and green manure in soil with different organic carbon contents. Soil Res. 2010, 48, 577. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.-X.; Wu, W.; Shi, D.; Yang, M.; Zhong, Z.-K. Evaluation of Biochar Effects on Nitrogen Retention and Leaching in Multi-Layered Soil Columns. Water Air Soil Pollut. 2010, 213, 47–55. [Google Scholar] [CrossRef]
- Rondon, M.A.; Lehmann, J.; Ramírez, J.; Hurtado, M. Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Boil. Fertil. Soils 2006, 43, 699–708. [Google Scholar] [CrossRef]
- Prendergast-Miller, M.; Duvall, M.; Sohi, S.; Prendergast-Miller, M.T. Biochar-root interactions are mediated by biochar nutrient content and impacts on soil nutrient availability. Eur. J. Soil Sci. 2013, 65, 173–185. [Google Scholar] [CrossRef]
- Whitman, T.; Singh, B.P.; Zimmerman, A.R.; Lehmann, J.; Joseph, S. Priming effects in biochar-amended soils: Implications of biochar-soil organic matter interactions for carbon storage. Biochar Environ. Manag. Sci., Technol. Implement. 2015, 2, 455–488. [Google Scholar]
- Verheijen, F.; Jeffery, S.; Bastos, A.C.; Van der Velde, M.; Diafas, I. Biochar Application to Soils–A Critical Scientific Review of Effects on Soil Properties; European Commission: Brussels, Belgium, 2010. [Google Scholar]
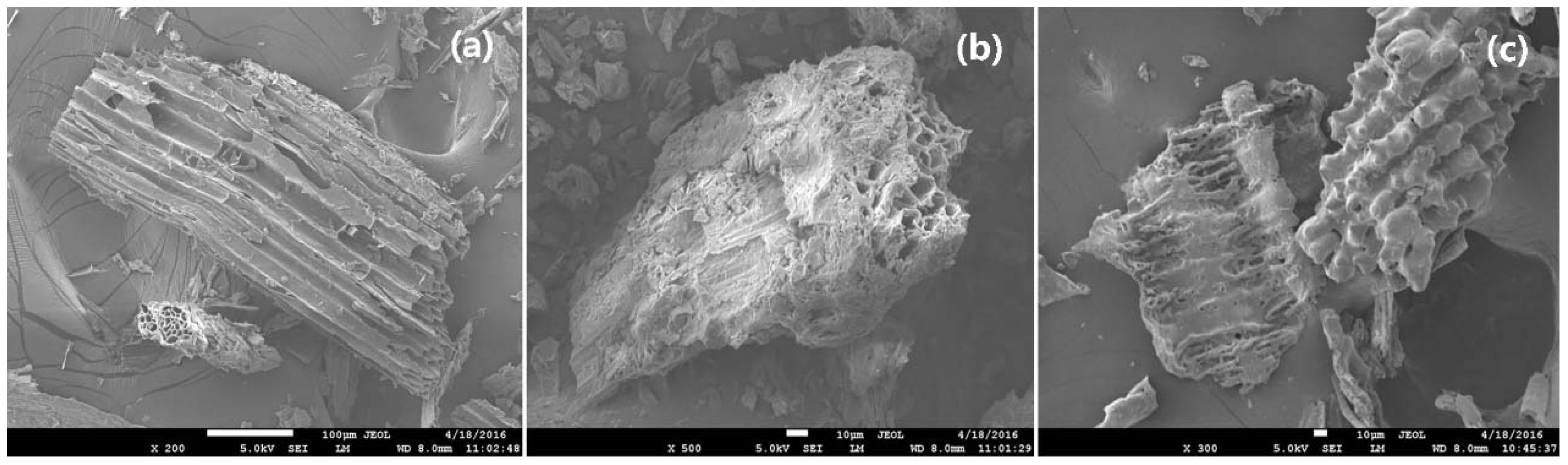
| Sample | pH | N % | P % | K % | C % | H % | C/N | H/C | Moisture Content (%) | Ash (%) | Volatile Matter (%) | Fixed Carbon (%) | Biochar Yield (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WS | 7.67 | 0.65 | 0.18 | 1.26 | 42.58 | 5.92 | 65.51 | 1.11 | 5.28 | 2.03 | 42.93 | 11.97 | - |
| PS | 8.02 | 1.58 | 0.38 | 1.33 | 65.18 | 5.11 | 41.25 | 0.81 | 5.01 | 21.34 | 75.94 | 12.67 | - |
| RS | 7.95 | 0.97 | 0.30 | 2.28 | 59.62 | 4.82 | 61.46 | 1.28 | 6.34 | 16.75 | 64.96 | 10.05 | - |
| WB | 9.83 | 0.37 | 0.97 | 5.94 | 74.89 | 2.36 | 202.41 | 21.73 | 0.49 | 5.17 | 8.49 | 68.29 | 24.6 |
| PB | 9.36 | 0.76 | 1.82 | 6.27 | 87.65 | 1.72 | 115.33 | 18.46 | 0.81 | 10.45 | 15.64 | 96.48 | 32.5 |
| RB | 8.65 | 0.47 | 1.35 | 10.56 | 81.27 | 1.54 | 172.91 | 28.84 | 0.64 | 2.39 | 13.94 | 83.07 | 30.7 |
| Soil | 6.09 | 0.05 | 0.02 | 0.03 | 0.68 | 0.85 | 11.6 | 0.81 | 0.45 | - | - | - |
| Product | Unit | Electricity | N2 | Total CO2 Emissions | ||
|---|---|---|---|---|---|---|
| Consumed Electricity | CO2 from Electricity Production | Consumed N2 | CO2 from N2 Production | |||
| Biochar | 1 kg | 0.027 kWh | 23.22 g | 15.7 L | 1.88 g | 25.02 g |
| Parameters | pH | pb (g/m3) | Water Capacity (%) | EC (uS/cm) | Temperature (°C) | Available Nitrogen (mg/kg) | Available Phosphorus (mg/kg) | Available Potassium (g/kg) | Soil Organic Matter (g/kg) |
|---|---|---|---|---|---|---|---|---|---|
| CK | 6.15 ± 0.47aB | 1.34 ± 0.26aA | 54.32 ± 2.14cC | 35.97 ± 2.42bC | 27.55 ± 0.26aA | 82.95 ± 1.69cD | 21.91 ± 0.87cC | 157.49 ± 0.37cC | 7.54 ± 0.22eE |
| WS | 6.2 ± 0.36aAB | 1.12 ± 0.15aA | 56.48 ± 2.05cC | 40.15 ± 1.64aAB | 27.64 ± 0.39aA | 141.54 ± 0.24abAB | 37.21 ± 0.64aA | 368.59 ± 2.39aA | 15.55 ± 0.15dD |
| PS | 6.25 ± 0.15aAB | 1.28 ± 0.19aA | 55.82 ± 1.38cC | 39.87 ± 1.25aAB | 27.98 ± 0.84aA | 144.62 ± 2.31aA | 37.94 ± 0.59aA | 277.21 ± 0.57bB | 17.69 ± 0.09cCD |
| RS | 6.19 ± 0.32aAB | 1.06 ± 0.11abA | 55.35 ± 0.89cC | 41.20 ± 2.39aA | 28.01 ± 1.21aA | 139.01 ± 075aAB | 38.45 ± 0.96aA | 264.61 ± 1.33bB | 19.97 ± 0.05cC |
| WB | 6.44 ± 0.37aA | 0.82 ± 0.23bb | 60.89 ± 1.23bB | 41.65 ± 0.86aA | 28.45 ± 0.77aA | 132.59 ± 0.29bC | 35.21 ± 0.33abB | 288.61 ± 1.54bB | 24.18 ± 0.21bB |
| PB | 6.48 ± 0.45aA | 0.98 ± 0.10bAB | 66.02 ± 1.82aA | 42.56 ± 0.97aA | 28.11 ± 0.68aA | 134.81 ± 0.03bBC | 35.79 ± 0.08abB | 276.82 ± 0.21bB | 32.57 ± 0.29aA |
| RB | 6.39 ± 0.28aA | 0.95 ± 0.09bAB | 65.28 ± 2.17aA | 42.11 ± 2.33aA | 28.95 ± 1.54aA | 136.59 ± 0.18abB | 34.88 ± 0.19bB | 270.52 ± 1.09bB | 30.09 ± 0.13aA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Ren, T.; Yang, H.; Feng, Y.; Feng, H.; Liu, G.; Yin, Q.; Shi, H. Research and Application of Biochar in Soil CO2 Emission, Fertility, and Microorganisms: A Sustainable Solution to Solve China’s Agricultural Straw Burning Problem. Sustainability 2020, 12, 1922. https://doi.org/10.3390/su12051922
Wang H, Ren T, Yang H, Feng Y, Feng H, Liu G, Yin Q, Shi H. Research and Application of Biochar in Soil CO2 Emission, Fertility, and Microorganisms: A Sustainable Solution to Solve China’s Agricultural Straw Burning Problem. Sustainability. 2020; 12(5):1922. https://doi.org/10.3390/su12051922
Chicago/Turabian StyleWang, Huanhuan, Tianbao Ren, Huijuan Yang, Yuqing Feng, Huilin Feng, Guoshun Liu, Quanyu Yin, and Hongzhi Shi. 2020. "Research and Application of Biochar in Soil CO2 Emission, Fertility, and Microorganisms: A Sustainable Solution to Solve China’s Agricultural Straw Burning Problem" Sustainability 12, no. 5: 1922. https://doi.org/10.3390/su12051922
APA StyleWang, H., Ren, T., Yang, H., Feng, Y., Feng, H., Liu, G., Yin, Q., & Shi, H. (2020). Research and Application of Biochar in Soil CO2 Emission, Fertility, and Microorganisms: A Sustainable Solution to Solve China’s Agricultural Straw Burning Problem. Sustainability, 12(5), 1922. https://doi.org/10.3390/su12051922




