1. Introduction
Recent studies on medical practices have revealed an increase in the lead intoxication phenomenon, with social and economic consequences [
1,
2,
3,
4]. In 2016, the World Health Organization draws attention to the significant number of deaths (540,000) that are caused by the long-term effects of lead exposure, with the largest incidence in developing countries [
5].
There are still scientific dilemmas regarding the maximum lead level accepted as exposure [
6,
7]. This is due to the progressive reduction of norms that are based on scientific evidence that exposure to lead even when blood lead level is below 10 μg/dL interferes with physiological processes [
6]. In fact, the presence of lead in the blood cannot be considered to be safe at any level, in the light of data showing the occurrence of subclinical manifestations at very low blood lead levels [
6]. In this context, many of the current experimental studies address the identification of biomolecular mechanisms of lead toxicity [
8]. These were mainly attributed to the induction of oxidative stress by increasing the reactive oxygen species (superoxide anion, hydrogen peroxide, hydroxyl radicals) or by diminishing the antioxidant defense capacity (by blocking antioxidant enzymes through the depletion of endogenous antioxidants) [
8,
9]. Lead-induced oxidative stress generates lipid peroxidation with severe effects on cell membranes, intracellular components (mitochondria), and genetic material, generating genomic instability [
3,
10].
This paper evaluates the social and economic effects of lead exposure on population. There are several facets of this medical phenomenon. First of all, we can discuss a geographic particularity of Romania that modulates the pathophysiology of this type of poisoning [
11]. Some traditional local habits, such as the distillation of alcohol in households in artisanal equipment, that may contain lead elements, represent a particular feature of the geographic area of Romania. This phenomenon has also been identified in some other areas of Eastern Europe, based on the same ancestral customs, but on a much smaller scale when compared to the situation in Romania [
12,
13]. The lack of clear legislation that is meant to control the production of distilled artisanal alcohol is an element that aggravates the lead exposure situation [
13]. The misusage of these procedures is still widely encountered despite existent regulatory indications on the type and composition of the distillation apparatus or on the composition of the final product. Another element to be analyzed is the causes that determine this social behavior. Beyond the motivation of an ancient custom, there are a number of other factors that amplify this social and medical phenomenon. The low level of medical information and the lack of awareness of the risks of heavy metal use in abnormal conditions with the possibility of toxic effects is the main social cause [
14]. Moreover, following the interview, the investigators stated that one of the reasons for this type of distillation was the low economic level and the fact that the household alcohol production is cheap and achieves a very favorable price ratio in the present investigation [
12]. Additionally, the mostly used raw material came from its own production, thus the cost of the distillate was low. In connection with the use of lead in distillers, the choice of this metal has not been random, because it is easily obtained by recycling some lead devices, such as machine heaters. Often, these recyclable materials were found in the household of the surveyed people. From a technical point of view, the choice of lead to obtain connections or piping of the distiller was determined by the fact that this metal is extremely malleable, being easily molded and not requiring complex industrial processes. An extremely serious element is that most respondents did not know that lead is a toxic metal, which might have important health effects, some of which are irreversible. The users of such distillers have never asked themselves whether there may be any interference between the distilled solution and the metallic elements of the distiller. They have also never considered the possibility of carrying out chemical analyses attesting the quality and safety of the final distillate. Moreover, most of the surveyed people were extremely surprised when they were told that the basis of this poisoning was the distillation and consumption of alcohol in those improvised devices. The patients believed that this habit was a healthy one, while considering that they used natural products from their own household and that they monitored distillation throughout the process. Taking the evolution of recent years into account, with a special emphasis on preserving traditional food habits, a balance must be struck between preserving these habits and food security. At a time when globalization is an element with potential for degradation of national identity, it is necessary to preserve traditions and food habits that actually define and individualize a particular population. However, in order to achieve this, it is necessary to find a formula in which traditional food habits are preserved, but in compliance with the rules of production that will provide consumers with safety. Such measures can be concretized through a legislative framework that precisely defines the way in which these foods are produced, as well as measures whereby food quality and safety can be quantified in an objective manner [
15].
Economic growth does not necessarily mean an improved health status. When discussing health, besides the economic level, other factors are involved, such as: increasing the educational level, the daily standard of living, increasing the performance of the health system, medical innovations, and the easier access of the population to medical services. From a population point of view, improving knowledge and increasing dissemination of information is an important factor that has been reflected in the state of health. Mediating scientific medical information by medical professionals has led the population to understand how medical phenomenon is produced, its causes, but also the fact that it can be curable. Continuous information that will generate awareness of the whole phenomenon by the general population can be utilized to modulate the medical behavior of the population. In the case of lead exposure, an underestimation of the phenomenon can be generated by the inability to determine the level of lead in the territory. All public health policies, medical infrastructure, and high qualified medical staff require significant financial support, without which these goals cannot be applied [
16].
There is an inter-conditionality between health and economic growth as a common factor for the productivity of individuals. In the case of lead exposure, the patient has to be temporarily out of work or have a lower productivity due to neurological symptoms, which are often invalid [
17]. In the case of patients exposed to lead, the number of days of sick leave affects the productivity, but also their individual economic level. On the other hand, this pathology is considered to be a chronic disease requiring periodic investigations and successive treatments, all of which lead to a decrease in self-confidence, with some of these patients requiring psychological or even psychiatric support. For a more objective and in-depth assessment, a number of tools are needed to make correlations between lead exposure, distilled alcohol consumption, socioeconomic and educational level, health status, and impact on work capacity. However, many of these variables are individual and conditioned by the socio-geographic, even zonal environment, but also by customs and traditions. Thus, in 1990, Human Development Index (HDI) was developed as a tool for measuring health [
18,
19]. The Human Development Index (HDI) takes three parameters into account: income, life expectancy, and education. Health modifies individual capacity in two respects: the first is related to the quality of life, and the second to the increase in life expectancy. For example, if we analyze the usefulness of consumption in relation to life expectancy, we could say that if a person knew that he was exposed to a medical risk when consuming distilled artisanal alcohol, he is very likely to resort to this consumption. This is supported by the fact that the interviewed patients were not aware of the risk that they are exposed to when consuming household distilled artisanal alcohol. There is a strong correlation between health and individual income, with the latter requiring the use of distillery artisanal machines to obtain lower production costs. Worldwide, the standard of living is correlated to life expectancy, which is mediated by health. Disability due to lead exposure impacts individual work capacity, with a secondary decline in living standards, which must always be correlated with the increase in costs for the treatment of lead poisoning. While an understanding of the risk that they pose when distilling artisanal alcohol will cause a decrease in the incidence of this type of exposure. The effect will translate into fewer people being affected by lead. Healthy people will have more work, will be more competitive, and will invest more in personal education and development. Additionally, the population will invest more in measures meant to increase health by raising educational attainment and individual income [
20].
At the world level, concerns about reducing lead exposure are extremely intense. In 2008, the U.S. Environmental Protection Agency (EPA) reduced the lead level to 0.15 micrograms per cubic meter of air to protect the general population [
21]. In 2012, the American National Institute of Environmental Health Sciences (NIEHS) concluded that blood lead levels below 10 μg/dL could interfere with a number of physiological processes producing negative effects on the health of the population through a multi-organic affection: the central nervous system and peripheral nervous system, the immune system, the cardiovascular, renal, and reproductive system [
22]. Consequently, in 2013, NIEHS, together with the California Department of Public Health, considered that a level of 5–10 μg/dL could expose the population to medical risk. In 2015, the National Institute for Occupational Safety and Health (NIOSH) concluded that the blood lead level should be below 5 μg/dL [
23].
Lead exposure is a chronic pathology that generates high social costs and, in many cases, exposed and diagnosed lead patients refuse hospitalization and treatment, fearing that they might be made redundant, given the duration of treatment, but especially long periods of incapacity for work due to medical leave. These costs are individually distributed, affecting the financial level of the family, causing malfunctions for the employer. The diagnosis and treatment of these patients increase healthcare costs for healthcare systems.
Lead exposure leads to direct medical costs being represented by inter-hospital transfer when the patient cannot be transported by his/her own means. The costs of hospitalization are determined by diagnosis and treatment. Additional costs are those that are not covered by insurance or cannot be covered by medical insurance, such as: additional diagnostic tests, private consultations, and medicines that are not settled by the Health Insurance House.
Indirect costs mean the loss of work. This has negative effects for an employee who loses his job at a time when medical costs are rising, but also for an employer who loses an active individual in the workforce, thus making a fall in productivity. Patients that are exposed to lead may experience decreases in earnings and shifts from full-time to part-time work. Indirect costs affect the productivity of the individual, but also the loss of leisure, dedicated to recreational activities, with family and friends. The patient exposed to lead needs to change his daily schedule, but also certain behaviors that are caused by physical or mental disability.
Lead produces a series of neurological manifestations, including: sensory effects of muscle pain, visual disturbances, and mental disorders, such as depression, panic attacks, or even dementia [
24]. Among these manifestations, it seems that neuromuscular pain is a prediction factor for the use of long-term medical services, because patients with such manifestations require repeated hospitalizations and complex medical treatments in both primary healthcare systems and specialized centers. However, the costs for the treatment of these manifestations are not high [
25]. Interestingly, patients who declare these neuromuscular and treatment-emergent behaviors generate lower costs when compared to those who are not treated.
Visual disturbances do not cause high medical costs, but the use of medical services is intense for these patients. The risk of depression is increased when patients that are exposed to lead report visual disturbances [
26]. For patients that are exposed to lead and depressive conditions, direct medical costs are not substantial, but the use of medical services is intense. A serious problem is that only 29% of patients with depressive symptoms use a psychiatrist [
27].
Renal impairment can cause acute renal failure when exposure is rapid and with high levels of lead or chronic kidney disease for patients exposed to chronic low lead levels [
28]. The lack of information and knowledge of the disease causes the diagnosis of renal impairment to be delayed, knowing that the obvious symptom appears late in the course of kidney disease. At the time the patient becomes symptomatic, renal impairment is irreversible, thus resulting in final renal disease.
For the most common cardiovascular manifestations, as represented by high blood pressure and acute coronary heart disease, the costs are 1.8 to 3.2 times higher for the treatment of a hypertensive patient that also associates exposure to lead [
29].
By estimating the costs that are generated by each organic event in part, it can be concluded that depression generates the highest total cost, and lead-induced anemia with the highest rates of use of the health system, but both cause an increase in morbidity and mortality [
30].
The indirect costs may be lower, equal, or higher when compared to direct costs. A number of clinical manifestations of lead exposure may have a disproportionate impact on individual leisure time. Musculoskeletal pain, depression, panic attacks, neurological disorders significantly decrease productivity and use of leisure time with family and friends [
31].
For musculoskeletal disorders, the indirect costs outweigh the direct costs [
32]. The decrease in productivity is superior to absenteeism at work [
33]. Additionally, the risk of personal injury at the workplace of exposed persons is higher when compared to a healthy person [
34]. Pain is an element of fear that will limit physical activity at work. These patients may lose up to eight working days a year due to musculoskeletal pain [
35]. These indirect costs can also add costs that are related to personal leisure time or productivity reductions in daily non-professional activities.
Additionally, for depression, indirect costs outweigh the direct costs [
36]. There has been a decline in 11.5-day individual productivity in the workplace over a three-month period, with a 46-day fall in annual productivity and an annual absenteeism of 19.2 days [
37]. In the same way, we can say that these indirect costs of depression add to the impairment of personal spare time or domestic productivity. Anxiety disorders, such as panic attacks, involve indirect costs that far outweigh direct costs. Panic attacks cause absenteeism and reduce productivity by the fact that personal decisions are affected by these psychiatric disorders [
38].
Lead affects every organ of the human body, but the most dangerous is for the central nervous system. High levels of lead can cause irreversible neurological damage. Severe exposures with high levels of lead are rare and usually accompanied by severe manifestations that ease the diagnosis. “Low level exposure” is a medical concept of recent years, which refers to quasi-normal exposures with levels that are close to the normal toxicity level. This type of exposure produces low-intensity, non-systemic signs, and symptoms that delay diagnosis and treatment. It should be noted that these low exposure levels cause toxic effects as dangerous as severe exposures [
39].
The signs and symptoms of lead poisoning depend on a number of parameters, including: exposure/intoxication level, duration, associated pathologies, hormonal status (menopause), and age. Signs and symptoms may occur in any system or organ. These manifestations are the expression of biochemical changes that are induced by lead toxicity in the body. Lack of signs and symptoms does not eliminate the possibility of lead exposure. In recent years, it has been observed that lower levels of lead exposure have effects on the body [
40].
Severe lead poisoning occurs at blood levels exceeding 80 μg/dL [
3]. The symptomatic polymorphism of acute poisoning is manifested by: long-term, unsystematic, diffuse abdominal pain, intestinal transit disorders, mostly constipation, osteoarticular pain, headache, anorexia, concentration and memory disorders, irritability, sleep disorders, fatigue, and neurological disorders, which may range from confusion to seizures or encephalopathy, peripheral neuropathy, anemia, and nephropathy (Fanconi syndrome) [
41]. Peripheral neuropathy is caused by diffuse axonal degeneration, which affects the motor component [
42].
Chronic exposure occurs at much lower levels of lead, even at values that are close to the accepted limit. The signs and symptoms that are associated with this type of chronic exposure retain the same characteristics, but the intensity of the manifestations is much lower and proportionate to the exposure level. However, it should be noted that the diagnosis of chronic exposure is more difficult, given that the signs and symptoms are much more non-specific, with much greater variability and undulant development, which is a diagnostic difficulty for the clinician. For example, there is a correlation between hypertension and chronic lead exposure [
43]. Therefore, the etiologic diagnosis of hypertension should include the toxic cause.
By taking into account all of the issues mentioned above, this research is meant to evaluate the social, economic, and medical effects of lead exposed or poisoned patients to identify causal factors and intervention strategies addressing both primary and secondary or tertiary prevention. The prevention will reduce not only daily exposure to lead, but also the resulting social and economic implications.
The specific objectives consist of:
- (a)
Highlighting the socio-demographic particularities of patients exposed to lead.
- (b)
Identifying special sources of lead in the geographical area of Romania.
- (c)
Determining the correlation between illicit alcohol consumption and lead exposure.
2. Materials and Methods
2.1. Participants
The research was conducted while using a descriptive survey that was based on qualitative and quantitative methods, which included the participants that were registered by the Toxicology Clinic of the Clinical Emergency Hospital Bucharest with the diagnosis of lead exposure/poisoning. The recruitment was done in the period December 2012–December 2015. We decided to include all patients that presented for signs, symptoms, and history suggestive of lead exposure in the study, and division into work groups was performed according to the detected lead levels. In
Figure 1, we highlighted the diagram of the patients’ enrollment and the final sample to be analyzed. All of the procedures performed for patients exposed to/poisoned with lead were also performed for the control group.
The oarticipants were enrolled in the study according to the following criteria:
determinations of serum values for blood lead levels higher than 10 μg/dL for the lead exposed /poisoned patients;
patients with serum values for blood lead levels lower than 10 μg/dL, selected as control group;
absence of prior chelating treatment; and,
signing informed consent to participate in the study.
Participants with the following characteristics were excluded from the research:
patients who have experienced multiple poisoning with other metals (mercury, aluminum, and cadmium);
the impossibility to monitor the patient by not adhering to the study protocol;
previous chelating therapy; and,
lack of informed consent at enrollment.
Informed consent was obtained for each of the participants who participated in the study before enrolling them. The study protocol was approved prior to exposure to the Ethics Commission of the Clinical Emergency Hospital Bucharest. Personal data were completely hidden to avoid potential identification.
2.2. Study Protocol
The study followed an observational retrospective protocol that was based on the analysis of participants under the ATI-Toxicology Clinic for signs and symptoms suggestive of lead exposure/poisoning. Initially, the complex socio-demographic survey of the participants was conducted through the face-to-face interview method. Subsequently, the clinical characteristics of patients were noted and subjected to complex serological investigations by determining the biochemical and hematological parameters. The dosage of blood lead level and lead poisoning level has allowed for the subject to be classified according to the exposure level.
2.3. Socio-Demographic Survey
The socio-demographic context has been assessed by following some indicators: rural/urban environment, educational level (without studies/studies/higher education), economic level of the family (low/medium/high), and age at enrollment. The smoking characteristics involved YES/NO responses for each of the questions about: smoker status at present and the existence of passive smoking. The number of years of smoking and the number of cigarettes on average per day used by the patient were noted. The production of alcohol in their own household represented a particular interest in the present research, patients being asked to declare the existence of this habit at home. They scored with YES/NO responses if they were informed about the risk of using such equipment at home. The patients also reported the type of distillation equipment, the motivation for which they chose lead components.
With respect to educational level, we had three groups: the group without studies included participants who have never attended school or who attended maximum four grades; the second group included those with eight grades or high school, the group of higher education participants included university/master/doctoral students (higher education).
The economic level of the family was assessed on the basis of the respondent’s questionnaire statement, according to the average monthly income per family, based on the gross national minimum wage in payment, as follows: less than two national minimum wages in payment/month, e.g., <1400 RON (low level), between two national minimum wages in payment/month and two national average wages/month, 1400–4044 RON (average level), over two gross salaries/month, >4044 RON (high level).
There were analyzed the additional diagnosis costs of the patients, but also for treatment not paid by the health insurance system, as well as the medical leave period per year, the number of hospitalizations, and the treatment abroad. Finally, we noted the overall impact on the quality of life.
2.4. Clinical Evaluation
Clinical evaluation was performed when entering the hospital and during hospitalization, and the possible symptoms that were associated with lead exposure/poisoning were noted in the patient record: abdominal pain, dyspepsia, anorexia, constipation, fatigue, headache, myalgia, nervousness, and tremor. For patients with severe poisoning, encephalopathy was evaluated. Patient experience of these conditions was also noted during the last three months prior to hospital presentation.
Blood pressure measurement was performed under standard conditions after a 30-minute physical rest in the left upper limb, in a sitting position, with the help of the electronic strain gauge attached to the Nihon intensive care monitors. Two measurements were made for both systolic blood pressure and diastolic blood pressure at a minimum of two minutes between measurements. Systolic blood pressure over 140 mmHg and diastolic blood pressure values that were greater than 90 mmHg were classified as hypertension [
44].
Blood and urine samples were collected for the participants included in the study. After adequately preparing the probes, they were analyzed while using Graphite furnace atomic absorption spectroscopy (GF-AAS). Concentration was expressed in micrograms/ deciliter (μg/dL).
While considering the obtained results, we divided patients in four groups according to the level of lead in order to be able to make correlations and statistical determinations. Group 1 was represented by patients with lead levels up to 10 μg/dL, Group 2 was made up of patients with values between 10 and 40 μg/dL, being considered patients exposed to lead with increased determinations, Group 3 was made up of patients with lead poisoning that ranged between 40 and 80 μg/dL, and in Group 4 we included patients with severe poisoning with lead values that were higher than 80 μg/dL.
We used venous blood sampled on anticoagulant in order to evaluate the effects of lead poisoning exposure on hematological status. The probe was subsequently analyzed by an automated flow cytometry analyzer.
Independently trained personnel collected venous blood for specific serological determinations. From the 6 ml probe the level of liver enzymes, such as: serum aspartate aminotransferase (ASAT) and serum alanine aminotransferase (ALAT) was analyzed.
2.5. Statistical Analysis and Ethical Considerations
The data were statistically processed in Microsoft Excel 2013 and SPSS Statistics 22. Qualitative variables were expressed in absolute and relative frequencies (expressed as a percentage). The Kolmogorov–Smirnov test was applied for the test of normality, which was confirmed, and thus justified the use of the more powerful parametric techniques. We used the t-Student test to compare numerical data on the randomly selected sample, having the unknown population dispersion; Chi square test or Fischer test, depending on the size of the analyzed groups. At the end of the analysis, the value for p less than 0.05 was considered to be statistically significant. We used the one-way ANOVA test to compare between different variables, followed by post-hoc Bonferroni analysis for the selected groups to compare the control group with the other evaluated groups. The numerical results are presented as average ± standard deviation.
3. Results
3.1. General Characteristics of the Studied Group
The final study involved 115 patients. In Group 1 (control group), we included 19 patients, as compared with 96 participants in exposed/lead poisoned Groups. The overall gender distribution indicated the presence of 20 females and 95 males.
We divided the analyzed group by taking the alcohol production in the household into account, following the statements of the patients; thus, in the non-alcoholic group there were seven female and 39 male participants; in the group of alcohol-producing participants, there were 13 women and 56 males.
A total of 87 participants came from the rural area and the remaining 28 belonged to the urban environment.
A number of 69 rural patients responded positively to the question of their own alcohol production in the household, while no urban patient said anything about such a home procedure (
p < 0.005).
Table 1 is a contingency table obtained after performing the Chi-square test to analyze the difference between the distribution frequencies of patients in urban and rural areas who are/are not alcohol producers.
The big difference (reported by the very small value of p) appears because, in the rural area, there are 69 alcohol producers, while in the urban area there is none.
We categorized the population according to the educational level of the participants and the classification indicated that 18 participants had a low level of training, most of them were in the middle-level group (81) and only 13 of them attended the courses of a faculty. A total of 16 patients were enrolled as having a low socio-economic level, and only three patients belonged to social environments with high economic level, the remaining 96 patients having average social conditions.
Of the patients who declared their own household alcohol production, 48.7% belong to social environments with average income, 2.61% were classified as having a high social and economic level, and 8.7% were considered to have a low standard of living.
We analyzed the general characteristics of the control group compared to the lead-exposed/poisoned group in order to obtain a comparison between the two groups of patients (independent samples t-test).
Table 2 shows the resulting statistical data.
The average age of the total sample of participants enrolled in the study was 48.97 ± 1.12 years, with the extremes being represented by individuals with the ages of 24 and 78.
A percentage of 62% of the participants declared trades in the field of agriculture, public services, and consumer goods production activities; 12% of participants did not declare activities or a stable job. Of these, 44% reported income placing them in the middle class regarding social inclusion, according to the income statement, and 56% declared low incomes, being included in the economically low-income area. The employment rate was 88%; of those employed, 65% reported more than 40 working hours per week.
A percentage of 12.17% of respondents were informed about the risk.
Most of the participants considered the low cost of production as the main reason for the chosen method.
54.78% of respondents recognized the use of their own distillation equipment, while 60% admit that they used lead to make the above-mentioned device (for various reasons, the most common being malleability, affordability, and previous recommendations).
3.2. Anthropometric Evaluation
In the analyzed group, the distribution according to the body mass index indicated that 8.7% of participants were obese and 48.7% were overweight.
In models with multiple determinations, we analyzed the changes in average values of systolic and diastolic blood pressure (SBP and DBP) after the exclusion of patients while using antihypertensive medication (
Table 3). The values were statistically significant after the correlation between the lead poisoning value and the blood pressure values. The patient group with lead poisoning value between 0 and 10 μg/dL had average SBP values of 119.95 ± 1.41 mmHg and for DBP of 71.89 ± 1.49 mmHg; for group 2, with a lead poisoning value between 10 and 40 μg/dL, the values were 128.74 ± 1.57 and 74.87 ± 0.98, respectively.
We obtained statistically significant results in the sense that the blood pressure values of the patients in Group 1 were significantly lower than those of Groups 2, 3, and 4, with increases in systolic and diastolic blood pressure, depending on the lead poisoning value.
3.3. Toxicological Determinations
The group distribution that was based on blood lead level found at the admission of patients indicated that most of them, 44.35% (51 participants) had lead levels between 40 and 80 μg/dL. We also had a group of participants with severe lead poisoning, consisting of 14 patients (12.17%), for whom lead levels above 80 μg/dL were found.
Table 4 shows the mean values for the serological determination of lead. Patients in Group 4 had mean values of 114.94 ± 9.18 μg/dL.
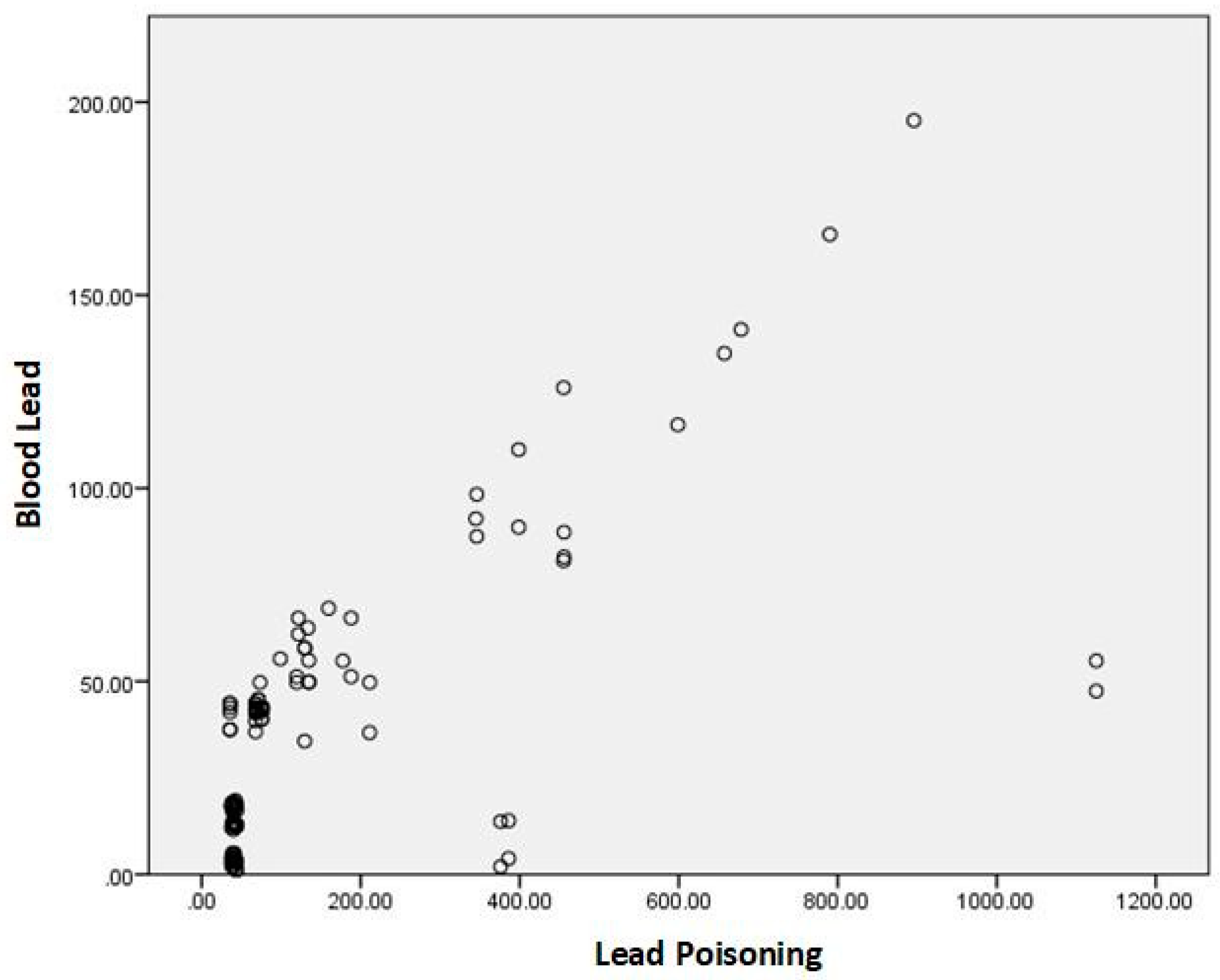
For each exposed/poisoned group, we analyzed the blood lead values based on lead poisoning and identified statistically significant correlations.
Figure 2 is the graphic representation of the correlation between lead values and lead poisoning values.
We analyzed the mean blood lead values according to the patient’s environment and found statistically significant results for the patients who came from the rural area compared to the urban environment; the serum level for the former was 43.25 ± 37.02 μg/dL, and for urban participants it was 32.23 ± 28.21 μg/dL, p < 0.001.
We compared the mean blood lead levels depending on the socio-economic level of the studied population using the ANOVA test and we noticed that there are statistically significant differences between the groups involved,
p < 0.05. Subsequently, through post-hoc analysis, we identified that participants with lower economic levels had higher blood lead values compared to patients with a high economic level (47.41± 5.99 μg/dL vs. 3.23 ± 0.55 μg/dL), and the difference is statistically significant.
Table 5 describes the statistical analysis.
The patients who reported illicit production of their own distilled household alcohol had statistically significant higher levels of lead than those who had other sources of lead exposure (p = 0.0001). For blood lead values, relative to the same indicators, the obtained correlations were statistically less significant, but they maintained the same correlation.
3.4. Hematologic Evaluation
We analyzed the variation of hematological values for the studied group, according to the mean blood lead values, for each Group. Regarding the mean hemoglobin, this was 14.77 ± 0.18 g/dL for the participants in the control group, while, for the participants with blood lead values between 10 and 40 μg/dL, the hemoglobin was 11.38 ± 0.52 g/dL, but the difference was not statistically significant. The post-hoc analysis identified that the control group patients had statistically significantly higher values for the total number of red blood cells when compared to the participants included in Groups 3 and 4 (
p = 0.0001). Additionally, hematocrit values in the control group were 50 ± 0.70%, as compared to 42.81 ± 1.16, 38.01 ± 0.60, and 33.50 ± 0.57, respectively, for Groups 2, 3, and 4 (
p = 0.0001).
Table 6 shows the mean values and the resulting statistical data.
The prevalence of anemia in lead exposed/poisoned groups was different. In the group of patients with normal blood lead levels, we did not identify patients with anemia, while its prevalence was statistically significantly higher in groups 3 and 4 (p = 0.0001). Differences in hemoglobin are not explained by the lead level; the results are not statistically significant (p > 0.05).
3.5. Biochemical Evaluation
We analyzed the average values for the biochemical constants that were included in the study. The mean aspartate amino transferase (ASAT) values according to the respective poisoned group were 23.14 ±.66 U/L, 32.99 ± 1.21 U/L, 41.01 ± 1.30 U/L, and 81.87 ± 2.45 U/L, respectively. The differences were statistically significant for each of the values (
p = 0.0001). In order to compare the identified constants, we used the ANOVA test and the post-hoc analysis through which we obtained statistically significantly lower values for serum iron level, ASAT, alanine amino transferase (ALAT), alkaline phosphatase (ALP), total direct, and indirect bilirubin, in the case of patients who had lower blood lead levels.
Table 7 describes the statistical analysis.
Participants having higher level of lead also have statistically significant higher values for ASAT and ALAT when compared to those included in Group 1, lead level <10 μg/dL (p < 0.05).
Additionally, participants producing household alcohol have statistically significant higher values for ASAT and ALAT as compared to those who do not have this habit (
p < 0.05).
Table 8 shows the results.
4. Discussion
A number of elements gives the relevance of our results: access to a group of consumers, possibility of medical testing, patients’ consent to respond, access to specialized literature, and big interest in our society for reducing work absenteeism. However, we should also take into account some limitations, such as the lack of a database for continuing this work and the low budgets allocated to information and education. Additionally, except for the medical results, the other information was based on participants’ declarations and veracity could not be verified. Therefore, a relation between illicit alcohol consumption and lead exposure seems to exist, but we could not determine the correlation between the two variables, as it was stated in the third objective of our research.
The analysis of the socio-economic determinants of the lead exposed/poisoned status indicated that it does not vary according to gender, environment, educational level, and declared economic status; instead, the number of smoking years and the number of cigarettes consumed per day were statistically significantly lower for the control group when compared to the exposed/intoxicated group; the number of years of smoking was higher in the exposed/intoxicated group than in the control group (25.73 ± 1.12 vs. 8.89 ± 0.98, p = 0.0001).
It is known that implementing preventive measures regarding sources of lead exposure is much less costly than therapy itself, especially if such interventions occur at an early stage of exposure. Therefore, it is necessary to implement measures to control production methods for artisanal alcohol identified as a source of lead in our study. Integrated cost-efficiency regulations and monitoring the exposure and active implementation of these measures is part of the strategy that has expanded regionally in selected target groups of interest.
Once nationally identified, vulnerable groups can be monitored through non-invasive determination of urinary delta-aminolevulinic acid.
Social prevention and health problems can only be achieved by influencing the habits, the behaviors, despite resistance factors through continuous education [
45].
A series of improvements are necessary: involving the political competence, creating licenses for programs that shift the domestic behavior on certain habits that have proven to be harmful to health, and for which bearing the treatment costs can be a higher burden than prevention itself.
Implementing national programs and legislative measures would be crucial in order to inform regarding the risks that are involved in drinking illegally distilled alcohol from artisan distillation boilers. Additionally, studies among consumers of marijuana should be initiated, in order to identify the level of lead exposure in this population, because of the high risk that is involved.
There are some ideas to be taken into account. First of all, the approach must be personalized, given that the use of the health system in terms of prevention, diagnosis, and treatment is variable (the principle of inequality of opportunity). Second, the level of risk is different, depending on the economic environment, on origin, and on the level of education. There is a genetically determined individual variability, which everybody responds to environmental aggressions, particularly selected. These genetic particularities are amplified by the economic environment (social vulnerability). It requires an innovative approach, both scientific and legislative, in order to understand, evaluate, and solve national cumulative effects, exposure to lead, according to socioeconomic status. Methodological challenges in this regard require a real assessment and characterization of health effects on the vulnerable population through informational instruments at the national level, data storage. Selected exposure−risk relationship analysis should consider the following aspects: the strength of clinical data, availability of quantitative evaluation information of serum-level association clinical effect, and whether these effects are noticeable in terms of diagnosis and curative.
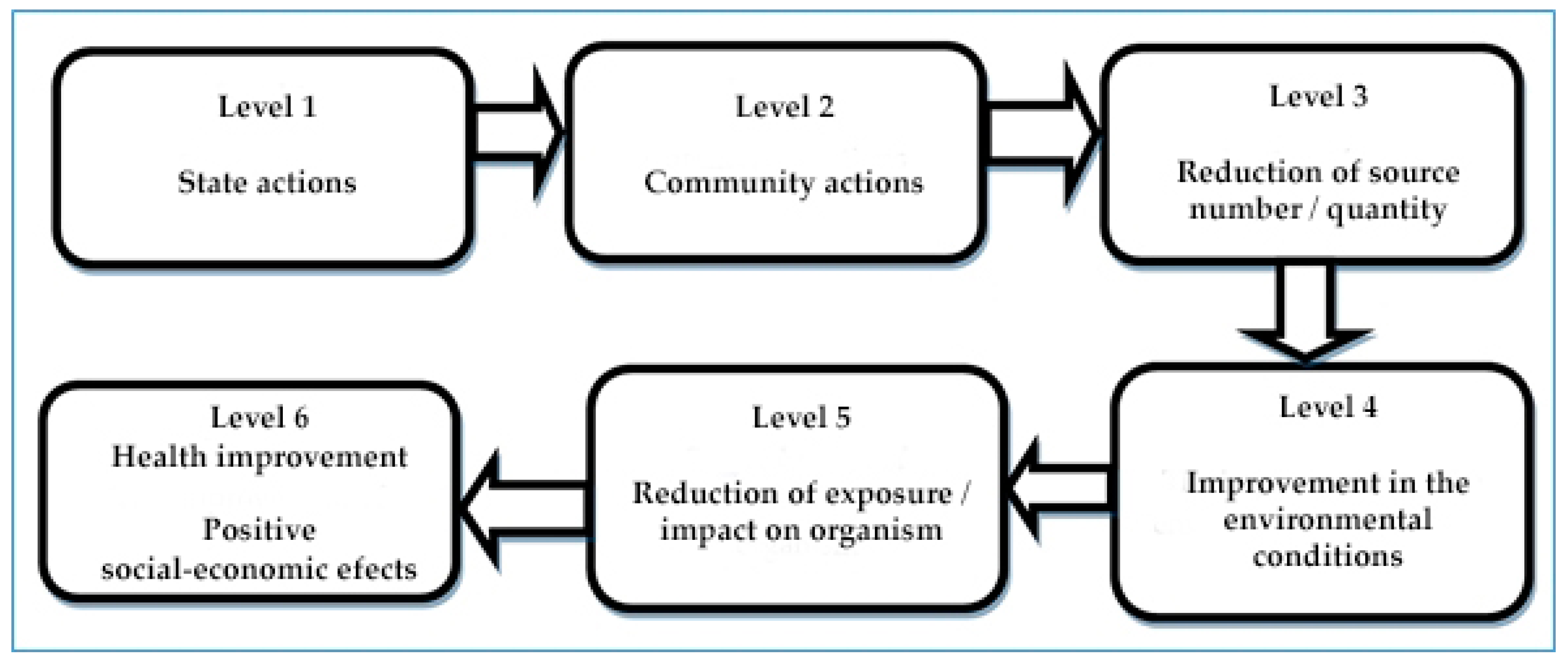
Figure 3 highlights the steps on different levels, aimed at reducing the exposure to lead, and culminating with an improvement in the quality of life. The involvement of academic medical research activities in the legislation is the only measure by which innovations are sustained.
Preventive policy must be done, especially at the primary level, which influences the event before it takes place. So far, the main effort is aimed at identifying and warning of obvious sources of lead, usually domestic related to child development. The direction of interest must acquire a national dimension, followed by the cost-effectiveness evaluation. This can be achieved by working in different sectors and mechanisms of health and social infrastructure devices, including imposing public-private partnerships. Public agencies must be connected with the health sector for identifying vulnerable communities and population groups in terms of lead exposure. Health providers may impose a screening system of rapid diagnostic evaluation of individuals in society.
The question arises as to whether the economic level can determine the health status in a certain way, given that the majority of patients exposed to lead as a result of artisanal alcohol distillation have a low economic level. Interestingly, patients who distill artisanal alcohol have mostly stated that they have chosen this method of distillation for two main reasons: the first is related to the millenary tradition they inherited from their parents, and the second is correlated with the fact that this type of distillation is cheap and available to anyone. In addition, by means of distillation, multiple sources of raw material can be used and the obtained quantities are high, thus generating a very favorable price/quantity ratio. We cannot say the same about the price/quality ratio, because most of the respondents said that they used fruit scraps or wine lees to get the artisanal distillate. All of the aspects mentioned above suggest that the standard of living determines the state of health, which has been stated since 1975 by Preston, who observed that between 1930–1960 the life expectancy increased by 30% [
46]. This increase was attributed to improving living standards [
47].
The use of artisanal distillation equipment made from recyclable metal scrap is a significant risk factor for the lead exposure of the population. Lead is the “ideal” material for such devices, since it is an easy-to-mold metal, being especially used for pipes and elbows. This type of exposure is an important source of intoxication of the population, especially in rural areas. A solution to this particular type of exposure is to inform the public of the risk that they are exposed to when distilling artisanal alcohol. Secondly, the metal parts in the distillation equipment that come in contact with alcohol vapors that are hot and have high pressure must be removed. This desideratum can be achieved by using distillation machines that are made of glass. But such a device involves high costs and can cost up to 700–1000 euros, depending on capacity.
Even in terms of this cost, local policies can be thought of, so local public authorities can be involved in explaining the risks to the population and proposing the purchase of glass distilleries to be used by the local community against modest taxes to cover equipment maintenance. Laws should be developed to regulate this practice to reduce the risk of exposure to lead, associated with the consumption of distilled artisanal alcohol. Banning it does not solve the problem, but the surveillance of the phenomenon with the active involvement of local communities is the way in which a reduction in exposure can be recorded. The sale of distilled alcoholic beverages should only be allowed when the distillation equipment is authorized and verified by the authorities. In this way, the risk of spreading a lead-contaminated alcohol will decrease, and the phenomenon of exposure will only remain an individual or a family one. Uncontrolled marketing of distilled alcohol can cause widespread exposure at the population level. However, we strongly believe the main instrument to be used remains information, in order to raise awareness, by taking into account the fact that the analyzed phenomenon is not under the control of the regulations regarding food production standards and food quality control (as the alcohol is obtained by artisan for own consumption).
5. Conclusions
Our research highlights the reasons why it is important to be aware of the socio-economic impact of lead exposure in the Romanian context and to properly act to find better ways of preventing unhealthy habits. One of the proposed objectives was to identify the local peculiarities of lead exposure on the territory of Romania, allowing for an individualization of health protection public policies and finding the right solutions to prevent and reduce the risk of lead poisoning. The study highlights some peculiarities that can modulate the phenomenon of lead exposure, including: educational level, economic level, geographical, and cultural area. In addition, the processed data have highlighted the fact that the basis of the lead exposure is the lack of knowledge and medical information that could enable the population to correctly and accurately identify the risks to which they are exposed, as well as the fact that the individual economic level is the real condition for the production and consumption of distilled artisanal alcohol.
The sample used provides the basis for further research aimed at evaluating Romanian population in a more general and comprehensive way. To begin with, the present study identifies the communities that are at risk of poisoning, and then we have made a general population evaluation meant to allow for an accurate analysis by means of which the establishment of a level of population exposure representing a reference level was possible. This level needs to be identified in order to assess the effectiveness of prevention measures later on, as well as the fluctuations in the blood lead level that might occur at certain times, such as: toxic substance spills or increased pollution from already identified sources. It has to be noted that asymptomatic patients with toxic lead levels have been identified in this study, indicating the possibility of a silent lead exposure at the population level, which will cause toxic effects over time. The current research is not only for medical professionals or the Ministry of Health, but it is also useful in related fields of activity, including agriculture, industry, and culture. From a general utility point of view, the work becomes useful for the population when they discuss the prevention strategies that are disseminated through the media or local authorities in areas that are identified as areas of risk. The media, audio-visual institutions, and, at the local level, City Halls and County Councils, Public Health Directorates, or other non-governmental agencies, can be involved in these prevention strategies.
The present research is a pioneering work in the Romanian area. The medical data obtained was a starting point for a much more detailed interdisciplinary research, including the socio-demographic and economic impact of this type of toxic exposure. The identification of medical and socio-economic conclusions allows for the projection of some prevention recommendations to correct the medical data resulting from the statistical analysis. In addition, the present paper does not follow general research lines, leading to general principles of prevention, as it has been customized since the elaboration of the study protocol for the local particularities of Romania.
The structure of the work respects the requirements of scientific research by observing the research norms, by carrying out statistical analyses and interpreting the results in a scientific and objective way. The findings of the research allowed for a series of unique correlations, making a bridge between the medical and other fields of interest: economic, social, and cultural.
In the future, research in this area should be continued, precisely to overcome the boundaries that the current research has encountered. The main limitations of our study are represented by the fact that an exhaustive population evaluation could not be obtained, with a national average exposure to lead. Future perspectives for research are firstly represented by the evaluation of prevention strategies as well as the achievement of a national evaluation aimed at the lead exposure of the population in Romania.