1. Introduction
Urbanization is increasing worldwide with more and more people moving to expanding cities [
1]. Within these cities the provision of open spaces is limited and unevenly distributed, enhancing the need to provide green infrastructure [
2,
3,
4]. To connect these isolated patches linear structures that function as corridors between them are important [
2,
5,
6]. Such urban greenways benefit residents by providing various regulating and cultural ecosystem services (e.g., experience of nature and improved health and wellness) and support biodiversity [
6,
7,
8,
9]. Most cities are growing rapidly and undergo densification processes, thus isolating remaining open spaces. There is an urgent need for further development of new urban green infrastructure [
10,
11]. In many cities, historic legacies such as military fortifications and former industrial land provide opportunities for establishing new greenways. Examples include the city wall circular greenway in Nanjing [
12], the Ring Boulevard in Vienna [
8,
13] and the Green Belt Berlin established on the grounds of the former Berlin Wall [
6]. Another opportunity for establishing new greenways is disused railway lines including their embankments or changes of the management of these structures. In urban areas, line-side land has a key function in connecting green areas [
5] and a high conservation value has been assigned to a considerable proportion of railway embankments [
14]. For example, over 1000 ha of line-side land in London have been identified as Sites of Importance for Nature Conservation (SINCs; [
15]).
In this study, we focus on the connectivity aspect for supporting biodiversity. The fragmentation of natural habitats by transport infrastructure (highways and other roads with high traffic density, railways) has received a great deal of attention [
16,
17,
18]. Various means to mitigate negative effects of habitat fragmentation have been suggested and the great importance of green bridges and other forms of wildlife passages have been documented repeatedly [
19,
20,
21]. However, roads and railways do not only fragment habitats; their verges and embankments can also provide valuable semi-natural habitats [
22,
23] and serve as dispersal corridors [
5,
17]. In particular, line-side railway land (mainly grassland, but also scrub, woodland and ruderal vegetation) is of considerable importance [
18]. Trackside vegetation prevents erosion, promotes pollinators (wild bees), provides habitat and functions as dispersal corridor for numerous plant and animal species and enhances quality of life to a city’s residents [
23,
24]. However, it should be noted that corridors may also have negative aspects such as dispersal of invasive species, pathogens and diseases [
25].
The gravel bed of a railway line itself may serve as a habitat for specialized invertebrate species. This aspect has so far received little attention. In contrast to line-side railway land and embankments, which are interrupted by bridges, stations or overpasses, the gravel bed continues through these structures. Thus, while the dispersal function of trackside vegetation might be interrupted when railway tracks cross rivers or roads on bridges with no or very little vegetation (e.g., on iron-steel constructions with simple gravel beds), the dispersal function may be less affected for species associated with the gravel bed. To our knowledge it is not known whether existing (extensively used or disused) railway bridges contribute to habitat connectivity by serving as a link between habitats.
Here we present a field study in which animals (vertebrates and invertebrates) crossing a 32 m-long railway bridge were monitored over a period of 9 months using drift fencing with pitfall traps. Not all animals captured on the bridge might have been caught while dispersing. For some invertebrates the gravel bed of a little-used railway track may also serve as habitat and in this way connect similar habitats on either side of the bridge.
Abiotic conditions (pronounced variation in temperature, lack of humidity) in the gravel bed of a railway bridge may act as a filter for dispersing animals. Depending on daily and seasonal activity patterns we expect that some taxonomic groups are less affected by particular conditions. In order to examine these aspects we emptied the traps twice per day separating day- and night-active animals.
Certain morphological or behavioral traits may determine whether the bridge can serve as a connecting habitat or a dispersal corridor for a species. In particular, body size may be connected to dispersal ability through the fit to the scale of interstitial spaces in the gravel bed or because of its relationship with the dispersal pace on the surface. By contrast, the bridge may not serve as a connecting habitat for arboreal species because of a lack of higher vegetation.
In particular, we tested the following hypotheses: (1) a set-aside railway bridge functions as a corridor for small animals providing landscape connectivity across the road. (2) Different taxonomical groups differ in frequency of bridge use. (3) Capture rates on the bridge are higher during the night than during the day, because many species can thus avoid exposure to predation and extreme microclimatic conditions, or are mainly nocturnal. (4) For different taxonomic groups capture rates differ among the seasons. (5) High temperatures and rainfall affect capture rates on the bridge. (6) Taxa with particular combinations of traits are more likely to use the bridge.
2. Materials and Methods
2.1. Set-Aside Railway Bridge
The bridge examined is located on the northeast edge of the railway station Badischer Bahnhof in Basel (Switzerland). The single-track, 32 m long and 6 m wide railway bridge is an iron-steel construction with a simple gravel bed of 30 cm depth (
Figure 1a). The bridge crosses a major road (traffic density of 8000 vehicles per day) at an angle of 45° and at a height of 5.5 m over a distance of 16 m (
Figure 1a). At the southern bridgehead there is a 40 × 100 m grassland adjacent to the wide gravel field of the railway station with 19 tracks. North of the bridge a second track joins at a distance of 60 m and after further 60 m both tracks cross the river Wiese on a 45 m long iron-steel bridge to the 200 m wide and 2 km long gravel covered marshaling yard of the Deutsche Bahn AG, a railway company.
2.2. Trapping Animals
In February 1996 we installed 40 cm-high plastic drift fences from either bridgehead, which led the moving animals from both sides into two separate life traps (40 cm wide, 30 cm long and 50 cm deep) situated in the middle of the bridge (
Figure 1b). This assignment allowed a separation of animals crossing the bridge from north to south from those moving from south to north. The lower edge of the plastic fences was buried in a layer of marl to prevent small animals from slipping under the fence. To separate invertebrates and their potential predators (small mammals, amphibians, reptiles) in the traps, we installed a horizontal net (mesh size 8 mm) 5 cm above the bottom of each trap.
Trapping was conducted between 9 March and 30 November 1996. We checked the traps for captured animals twice daily early in the morning at dawn and in the evening at dusk from 1 March to 31 October. From 1 to 30 November, the traps were emptied every day early in the morning. Vertebrates captured were determined on the spot or photographed, tagged and released immediately on the opposite side of the bridge. Invertebrate animals (arthropods and gastropods) were preserved in 70% alcohol for later species identification by experts (see Acknowledgments and
Supplementary Table S3).
As a result of the set-aside of the bridge, a cover of vegetation developed on the gravel bed in the course of the study.
2.3. Temperature in the Gravel Bed
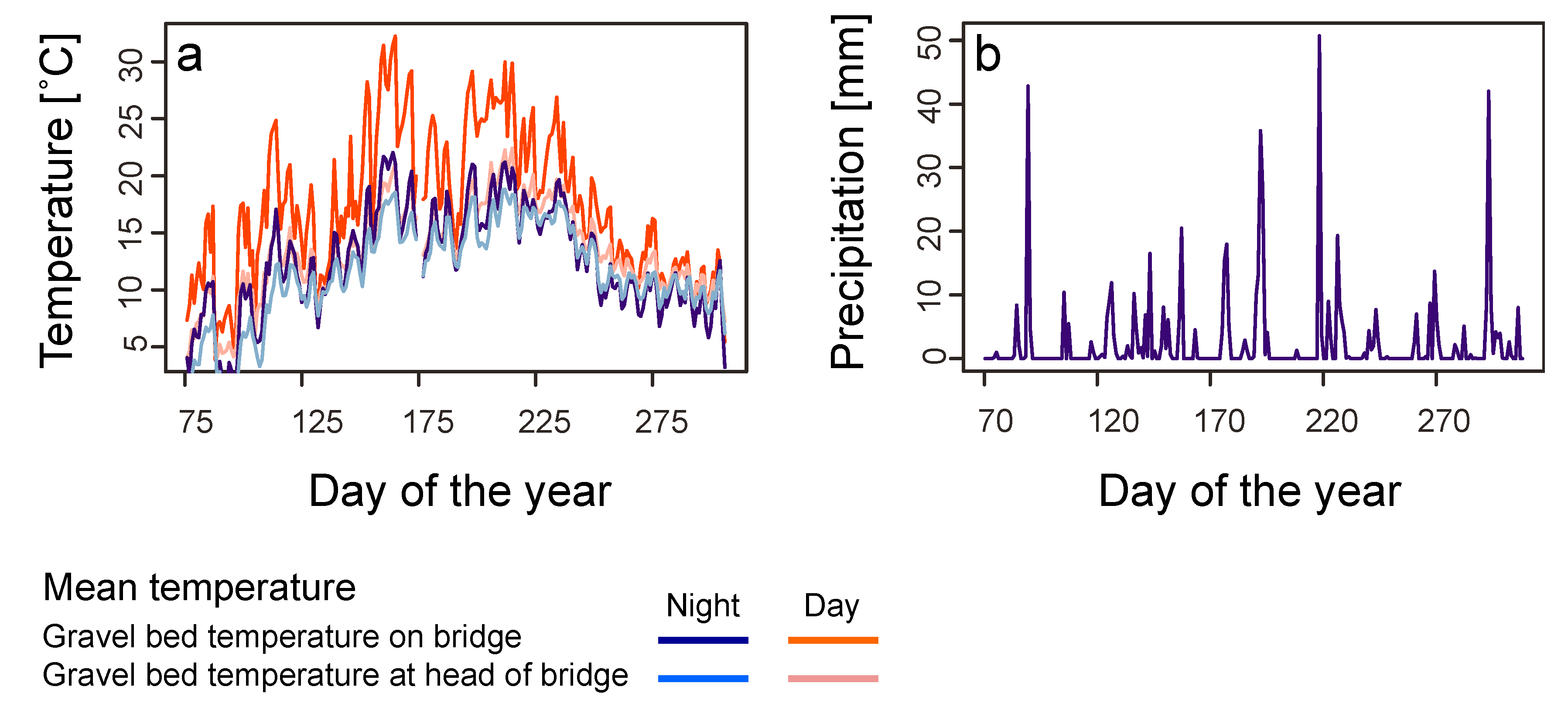
In order to characterize the temperature conditions of the gravel bed, four data loggers (Tiny Talk, Gemini Data Logger, Chichester, West Sussex) were buried in the gravel bed at a depth of 5 cm (two at every bridgehead and two at either end of the bridge between the rails). Temperature was recorded every 30 min between 14 March and 29 November 1996. Temperature readings from the two loggers with the same position from either end of the bridge were averaged for analysis to represent the conditions on the bridge as a whole. A few times loggers malfunctioned and thus only one value was available and was taken in place of the average.
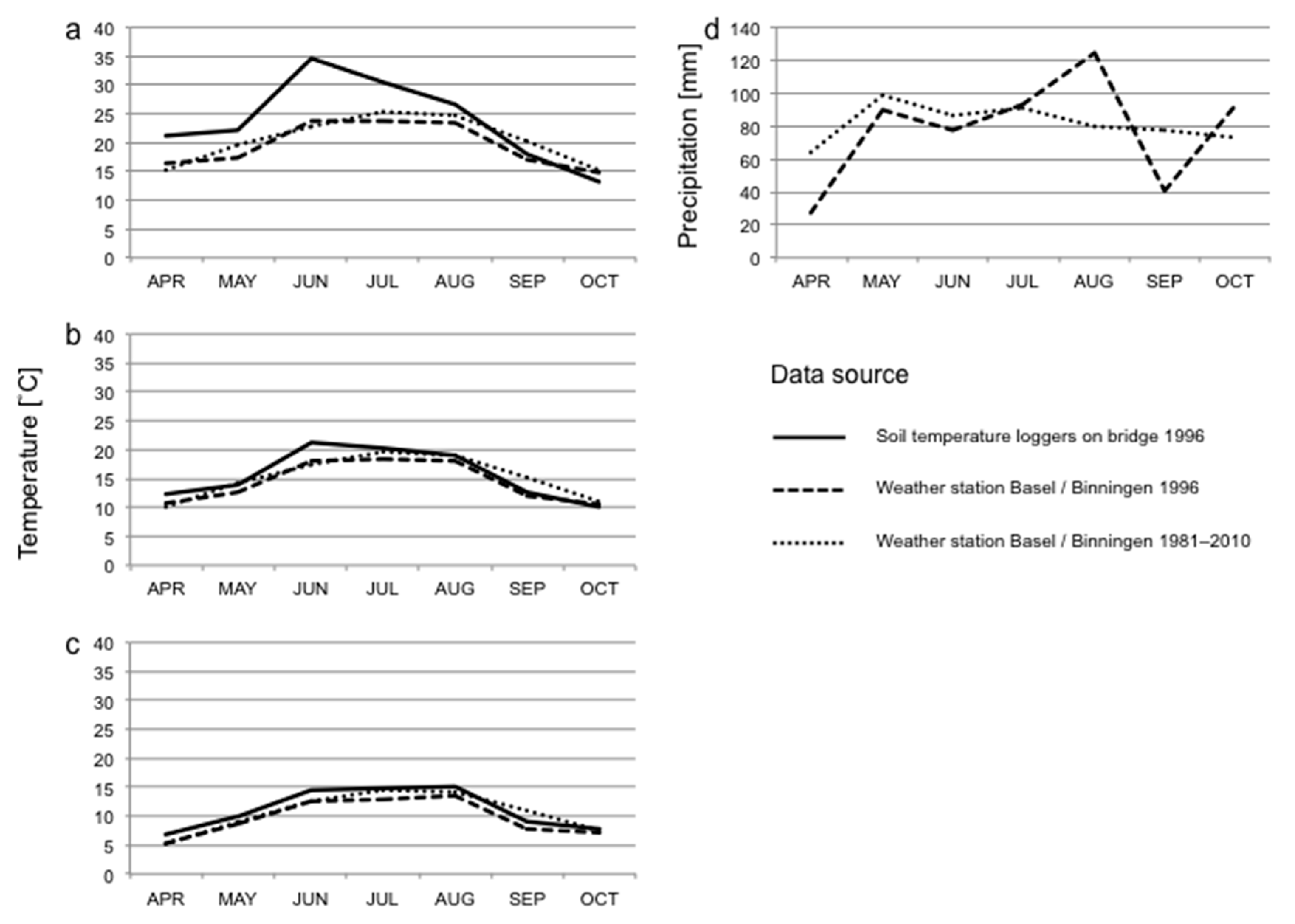
We used data on air temperature (measured 2 m above ground) and precipitation for the sampling period obtained from the weather station at Basel/Binningen (4 km distance from the bridge). Weather data were available as minimum, mean and maximum temperatures and total precipitation per calendar day, as semi-daily totals, or per 24 h period lasting from 5:40 on one day to 5:40 on the next day, as well as 12 h periods between 5:40–17:40 and 17:40–5:40. We used the latter periods rather than data for calendar days for analyses of weather effects on invertebrate abundances in the traps, as a better fit to our day and night collections of invertebrates. Long-term data on temperature and precipitation (climate norms; average of each month from 1981 to 2010) were obtained from the same weather station and compared to monthly values computed for the study period based on the data for calendar days.
2.4. Trait Analysis
The special conditions in the gravel bed on the bridge may function as a filter for dispersing species. Species with particular traits might be more frequently captured. A detailed analysis of species traits can only be done with groups with (1) both a large abundance and a high species diversity, (2) a known species pool for the region and (3) trait information for all species. In our study only two groups fulfilled these criteria: ants and spiders. However, we excluded ants from the trait analysis, because they migrate predominantly by flying.
As spider traits we considered body size (without legs) for both females and males as reported in Nentwig et al. [
26]. Body size is related to the ability of the species to use interstices in the gravel bed and is related to active dispersal on the ground. We further considered the vertical distribution of the species in their habitats (ordinal scale: 0 = beneath stones, in soil, in caves or animal burrows and nests, 1 = on soil surface or in litter layer, 2 = in herb layer, 3 = on bushes, lower twigs of trees, lower part of tree trunk, 4 = on trees, higher boughs, middle part of tree trunk, 5 = in canopy of trees; obtained from Maurer and Hänggi [
27]). Spider species mainly occurring in higher strata (classes 4 and 5) would not find suitable habitat on the bridge itself and thus when found may use the bridge as a dispersal corridor.
The conditions on the bridge (gravel instead of soil, reduced vegetation layer lacking trees) may act as a filter excluding species with certain traits. To examine whether spider species caught on the bridge differ in stratum use and body size from the regional fauna we compared the distributions of these traits to an overall regional species list compiled from several studies that applied pitfall traps in the urban area of Basel (Zoo Basel [
28]; urban forests [
29]; urban gardens: Braschler, Gilgado, Zwahlen, Rusterholz, Buchholz and Baur, unpublished data). The combined list comprised 165 species. However, for 12 species (7.3%) information on stratum use was not available. This included 5 species (12.8% of 39 species), which were caught on the bridge.
2.5. Statistical Analyses
We decided to aggregate data for 7-day periods because in many taxonomic groups no individuals were captured on many days. Accordingly, temperature and precipitation data were compiled to the same 7-day-periods. We used Pearson correlation to examine the correlation between temperatures in the gravel bed and air temperatures from the weather station. One 7-day period had to be omitted from analyses because of missing temperature data for 2 days.
We used generalized linear models (GLM; see
Supplementary Table S1 for details of the models) to examine the effects of weather conditions on the abundance in traps of different taxonomic groups. Different taxonomical groups may react to temperature in different ways. Some groups may respond to mean temperatures, while other groups may be more sensitive to temperature extremes. We used four different variables reflecting different temperature aspects. This enabled us to differentiate between these responses. The analyses had to be run separately for each of these temperature variables, as they were highly intercorrelated. We used the following variables matching the trap collection schedule: grand mean temperature (defined as the mean of daily mean temperatures for a timespan of 7 days, whereby the days ran from the morning emptying of the traps to the emptying of the traps 24 h later on the following morning), maximum mean temperature (the highest daily mean temperature from a 7-day period), mean maximum temperature (the mean of the highest measured temperatures for the 7-day period) and absolute maximum temperature (the highest temperature measured during the 7-day period), all of which were based on the half-hourly measured temperatures from the gravel bed, and precipitation (summed precipitation for the 7-day period starting at 5:40 on the first day, with data obtained from the weather station). Models were also run using corresponding variables for the morning (representing nocturnal activity) and evening (representing diurnal activity) emptying of traps only. The exception was precipitation for which no half-day data were available. For comparison with long-term climatic conditions, weather data based on calendar days was used and aggregated for 7-day periods or monthly. For monthly summaries of the data only the months April to October were used, as temperatures on the bridge were not measured from the start of March. The data from the year of the study was then compared to norm values for the months April through October weather station based data from 1981–2010.
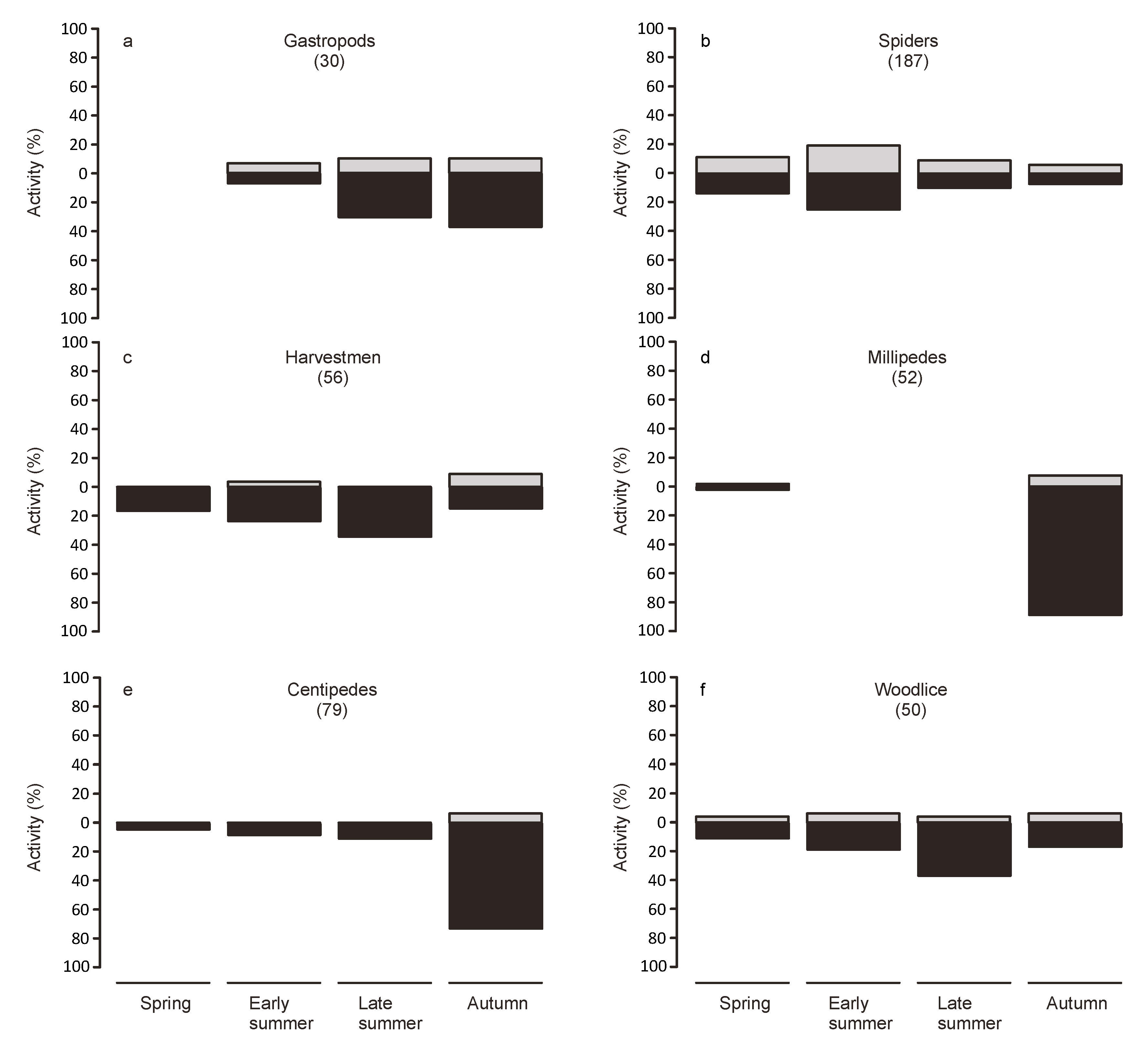
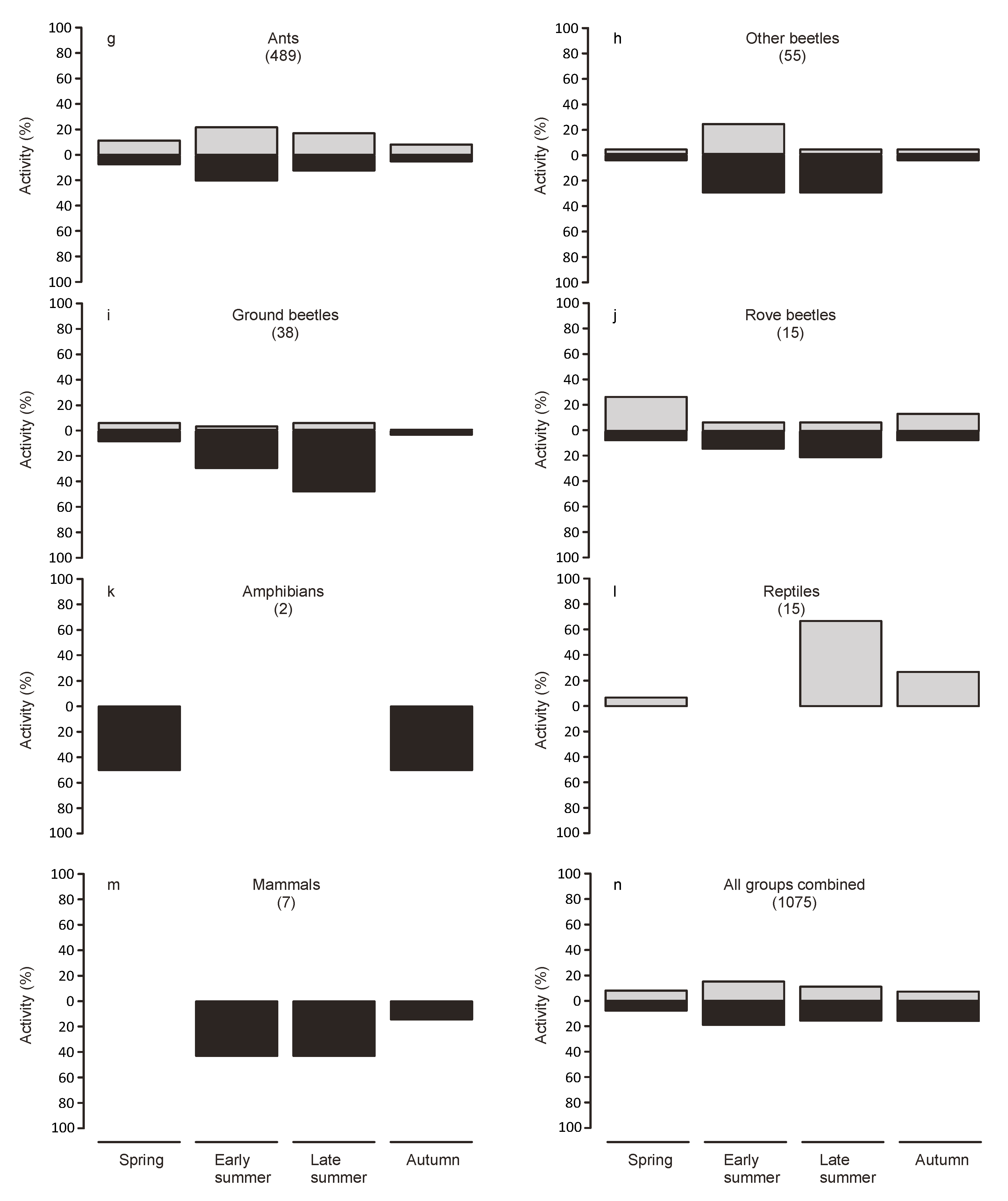
Animals from the collections at dawn were considered to have been active during the night, while animals found in the traps at nightfall were considered to have been active during the day. To examine seasonal variations in activity, we split the study period into four periods of equal length (spring: 7 March to 5 May, early summer: 6 May to 3 July, late summer: 4 July to 1 September, autumn: 2 September to 31 October).
To examine whether spiders caught on the bridge differed in body size from the known spider species pool for Basel, we used t-tests on log-transformed body size data for females and males separately. We compared the potential use of different strata by the spiders caught on the bridge with that of the spiders known for Basel using Fisher’s exact test. For potential strata use we considered all strata listed for a particular species, and combined them for all species to a common frequency distribution. Similarly, we also ran Fisher’s exact tests for the lowest stratum scores and highest stratum scores for the species.
All statistical analyses were performed using R Statistical Software (R ver. 3.3.3) (
www.r-project.org).
4. Discussion
4.1. Greenways and Functional Connectivity
The value of line-side vegetation along railway corridors for citizens and biodiversity is well established [
2,
5,
14]. Though not normally accessible to citizens due to safety concerns, line-side vegetation enhances the attractiveness of the neighborhoods. Local residents feel passionately about the vegetation next to their gardens and the biodiversity harbored in those habitats [
2]. Line-side vegetation also provides services such as a barrier to dust and noise by the passing trains [
2]. The additional value of this habitat as a major natural asset that is important for nature conservation has been recognized in legislation and official documents [
2,
14,
30]. Because of their linear shape, these greenways enhance connectivity for biodiversity within the city [
2,
5,
14]. However, the potential contribution of the gravel bed of the railways to these functions has received far less attention than that of the line-side vegetation. As the gravel bed continues, even where tunnels or overpasses interrupt embankments, railway bridges may serve as important elements in greenways.
Our case study showed that even a seemingly artificial habitat, a relatively thin and uniform gravel layer on a steel construction, is a valuable asset as an element of urban greenways. Besides providing multiple ecosystem services to residents, greenways have an important function as corridors for biodiversity in urban areas. However, their functional connectivity is frequently interrupted when crossing roads and rivers, an aspect that has so far not been investigated for animals. Our case study indicates that the bridge investigated fulfilled the connectivity function for a part of the urban fauna. This included some small vertebrate species as well as species not associated with open spaces and gravel-rich habitats such as arboreal spiders, which may have arrived on the bridge by chance. Indeed, all the small mammal and reptile species recorded on the bridge were previously considered as focal species for ecological network planning [
31,
32]. In addition, the gravel bed on the bridge provided habitat for many resident invertebrate species, highlighting its value as part of a greenway for invertebrates. These findings are somewhat surprising with respect to the rather extreme temperatures measured in the gravel bed with maximum temperatures exceeding the air temperatures by 10 and more °C. For animals adapted to open gravel areas this may not be a problem. However, for other animals it may reduce their likelihood of dispersal.
Tikka et al. [
23] demonstrated the importance of road and railway verges as dispersal corridors for plants. Penone et al. [
5] found that railway edges function as corridors for common grassland plants even in urban environments. Considering embankment gaps, functional connectivity was mainly maintained at railway stations, but not at overpasses. Plant groups differed in response depending on traits related to dispersal [
5]. Similar information is scarce for animals, especially invertebrates. Morón et al. [
24] showed that railway embankments can serve as a new habitat for pollinating insects (bees, butterflies, hoverflies). Similarly, Gonseth [
33] reported a species-rich butterfly community on railway embankments. However, both studies did not consider the gaps in the embankments at bridges.
4.2. Special Conditions in the Gravel Bed
In contrast to railway embankments, the gravel bed is a more artificial habitat exposed to extreme environmental conditions. Gravel beds in general have no vegetation, which would dampen extreme temperature fluctuation. Indeed, temperature conditions in the gravel bed differed considerably from standard measurements from the nearby weather station. Mean and maximum temperatures in the gravel bed were higher than corresponding temperatures measured in the weather station.
To our knowledge, species composition of invertebrates has not been investigated in gravel beds. However, large gravel areas such as in marshaling yards serve as habitats for many xerothermophilic and pioneer species [
34,
35]. Furthermore, some of the railway habitats show a high overlap with species composition of ruderal sites in urban areas [
14,
36]. Interestingly, 65% of the carabid and staphilinid beetle species captured on the bridge were also recorded in natural gravel beds along the river Rhine in the Upper Rhine Valley [
37]. Similar data for other groups are lacking. Railway gravel beds may function as connecting habitat corridors for species occurring in natural or semi-natural gravel-rich habitats and ruderal sites in the urban area.
4.3. Daily and Seasonal Activity Patterns
Twice daily collection allowed us to discern diurnal and nocturnal activity patterns. Our results indicate that the majority of the surface activity happened during the night. However, this differed strongly depending on the taxonomic group. Extreme temperatures during the day may act as a filter for the use as habitat by some groups, which, however, may still disperse on the bridge during more benign conditions. However, many species may be mostly nocturnal independent of the weather conditions. Indeed most of the groups represented in the traps were predominately caught during the night.
These findings are relevant for future management of little-used railway lines in urban areas, particularly for industrial railway lines used for occasional cargo transport. If these cargo transports were restricted to the daytime this would increase the functional connectivity of these railway lines during the night.
4.4. Traits
To understand the function of the railway bridge as part of the greenway, it is necessary to understand whether species using the bridge are representative for the wider species pool of the region or represent a subset of this pool characterized by certain traits or habitat preferences. In our case study, spider species captured on the bridge did not differ in body size and stratum use from the overall species pool of the urban area of Basel. A body size distribution in line with that of the species pool indicates that the bridge is suitable for dispersal for spiders of all sizes. Furthermore, the scale of interstices in the gravel bed may not limit use for spiders because of a reduced amount of fine-grained substrate. In contrast to our expectation, we also found arboreal species on the bridge. Like other invertebrate species, which are typically found in habitats very dissimilar to the open and exposed gravel bed on the bridge, these species were likely dispersing. However, as numbers for these species were low they may also have arrived on the bridge by chance. This highlights the function of the bridge as an important element of a corridor network of linear structures connecting green spaces throughout the urban area.
4.5. Conclusions
Beside a proven dispersal function for vertebrates, we provided evidence for a species-rich invertebrate community in the gravel bed on this set-aside iron-steel railway bridge. It should be noted that in the decade prior to our investigation period the railway bridge was primarily used for irregular goods transports rather than regular passenger traffic. In this respect the railway line investigated may resemble many industrial railway lines. By contrast, information on invertebrate communities in the gravel bed of heavily used tracks is not known. Planned traffic blockages for rail maintenance may allow studying some aspects of gravel-inhabiting invertebrate communities. Newly abandoned railway lines may also provide research opportunities.
Why is the case of our bridge relevant beyond Basel? First, we show the reuse value of set-aside transportation infrastructure for biodiversity. This is of particular importance because of limited budgets for urban greenways. This approach may appeal to transportation managers and politicians alike because of its potential reversibility, allowing flexibility in face of changing demands. Currently, the railway bridge investigated in Basel is still set aside, functioning as a dispersal corridor. Second, the fact that most animals were captured during the night opens an opportunity for biodiversity management of little-used cargo tracks. If industrial railway tracks are exclusively used during the day, individuals of many taxonomic groups would be able to disperse during the night. Such changes in usage may be more easily and faster achievable than the augmentation of existing infrastructure or the construction of new green bridges, and may improve connectivity of urban habitats. Thus, even minor management changes could enhance the connectivity function of greenways.