Sustainable Intervention for Health Promotion and Postural Control Improvement: Effects of Home-Based Oculomotor Training
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Recording Procedures
- LFS refers to the path done by the center of pressure during the recording process; it is a non-dimensional parameter.
- APA refers to the accelerations performed by one’s body to keep the orthostatic posture; it is measured in mm/s2.
- LCG represents the total length covered by the center of gravity; it is measured in mm.
- CGA is the surface of the body sway, expressed in mm2, showing the confidence ellipse based on 90% of the sample positions. It indicates the precision of the balance control system. It is measured in mm2.
- BFL indicates the rearfoot load in percentual (%).
2.3. Oculomotor Training
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Ammar, A.; Chtourou, H.; Boukhris, O.; Trabelsi, K.; Masmoudi, L.; Brach, M.; Bouaziz, B.; Bentlage, E.; How, D.; Ahmed, M.; et al. Covid-19 home confinement negatively impacts social participation and life satisfaction: A worldwide multicenter study. Int. J. Environ. Res. Public Health 2020, 17, 6237. [Google Scholar] [CrossRef]
- Tison, G.H.; Avram, R.; Kuhar, P.; Abreau, S.; Marcus, G.M.; Pletcher, M.J.; Olgin, J.E. Worldwide effect of COVID-19 on physical activity: A descriptive study. Ann. Intern. Med. 2020, 173, 767–770. [Google Scholar] [CrossRef]
- Woods, J.; Hutchinson, N.T.; Powers, S.K.; Roberts, W.O.; Gomez-Cabrera, M.C.; Radak, Z.; Berkes, I.; Boros, A.; Boldogh, I.; Leeuwenburgh, C.; et al. The COVID-19 pandemic and physical activity. Sports Med. Health Sci. 2020, 2, 55–64. [Google Scholar] [CrossRef]
- Lear, S.A.; Hu, W.; Rangarajan, S.; Gasevic, D.; Leong, D.; Iqbal, R.; Casanova, A.; Swaminathan, A.R.M.; Kumar, R.; Rosengren, L.; et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: The PURE study. Lancet 2017, 390, 2643–2654. [Google Scholar] [CrossRef]
- Anderson, E.; Durstine, J.L. Physical activity, exercise, and chronic diseases: A brief review. Sports Med. Health Sci. 2019, 1, 3–10. [Google Scholar] [CrossRef]
- Alomari, M.A.; Khabour, O.F.; Alzoubi, K.H. Changes in Physical Activity and Sedentary Behavior Amid Confinement: The BKSQ-COVID-19 Project. Risk Manag. Healthc. Policy 2020, 13, 1757. [Google Scholar] [CrossRef]
- Joy, E.L.; Blair, S.N.; McBride, P.; Sallis, R. Physical activity counselling in sports medicine: A call to action. Br. J. Sports Med. 2013, 47, 49–53. [Google Scholar] [CrossRef]
- Bize, R.; Johnson, J.A.; Plotnikoff, R.C. Physical activity level and health-related quality of life in the general adult population: A systematic review. Prev. Med. 2007, 45, 401–415. [Google Scholar] [CrossRef]
- Maurer, C.; Mergner, T.; Peterka, R.J. Multisensory control of human upright stance. Exp. Brain Res. 2006, 171, 231. [Google Scholar] [CrossRef]
- Meshkati, Z.; Namazizadeh, M.; Salavati, M.; Meshkati, L. The comparison of the role of vision on static postural stability in athletes and nonathletes. Iran. Rehabil. J. 2010, 8, 50–53. [Google Scholar]
- Peterka, R.J. Sensory integration for human balance control. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 159, pp. 27–42. [Google Scholar]
- Jeka, J.; Oie, K.S.; Kiemel, T. Multisensory information for human postural control: Integrating touch and vision. Exp. Brain Res. 2000, 134, 107–125. [Google Scholar] [CrossRef]
- Friedrich, M.; Grein, H.J.; Wicher, C.; Schuetze, J.; Mueller, A.; Lauenroth, A.; Schwesig, R. Influence of pathologic and simulated visual dysfunctions on the postural system. Exp. Brain Res. 2008, 186, 305–314. [Google Scholar] [CrossRef]
- Della Volpe, R.; Popa, T.; Ginanneschi, F.; Spidalieri, R.; Mazzocchio, R.; Rossi, A. Changes in coordination of postural control during dynamic stance in chronic low back pain patients. Gait Posture 2006, 24, 349–355. [Google Scholar] [CrossRef]
- Bonnet, C.T.; Baudry, S.A. functional synergistic model to explain postural control during precise visual tasks. Gait Posture 2016, 50, 120–125. [Google Scholar] [CrossRef]
- Paillard, T. Plasticity of the postural function to sport and/or motor experience. NeuroSci. Biobehav. Rev. 2017, 72, 129–152. [Google Scholar] [CrossRef]
- Paillard, T.; Noe, F. Techniques and methods for testing the postural function in healthy and pathological subjects. Biomed. Res. Int. 2015, 2015, 891390. [Google Scholar] [CrossRef]
- Horak, F.B. Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age Ageing 2006, 35 (Suppl. 2), ii7–ii11. [Google Scholar] [CrossRef]
- Rawstron, J.A.; Burley, C.D.; Elder, M.J. A systematic review of the applicability and efficacy of eye exercises. J. Pediatr. Ophthalmol. Strabismus 2005, 42, 82–88. [Google Scholar] [CrossRef]
- Morimoto, H.; Asai, Y.; Johnson, E.G.; Lohman, E.B.; Khoo, K.; Mizutani, Y.; Mizutani, T. Effect of oculo-motor and gaze stability exercises on postural stability and dynamic visual acuity in healthy young adults. Gait Posture 2011, 33, 600–603. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Vats, M. Effectiveness of gaze stability exercises on balance in healthy elderly population. Int. J. Physiother. Res. 2014, 2, 642–647. [Google Scholar]
- Fischetti, F.; Cataldi, S.; Giunto, A.; Greco, G. Effect of home-based oculomotor exercises on postural stability in healthy female adults. J. Hum. Sports Exerc. 2020, 15, 653–660. [Google Scholar] [CrossRef]
- Minoonejad, H.; Barati, A.H.; Naderifar, H.; Heidari, B.; Kazemi, A.S.; Lashay, A. Effect of four weeks of ocular-motor exercises on dynamic visual acuity and stability limit of female basketball players. Gait Posture 2019, 73, 286–290. [Google Scholar] [CrossRef]
- Stoffregen, T.A.; Pagulayan, R.J.; Bardy, B.G.; Hettinger, L.J. Modulating postural control to facilitate visual performance. Hum. Mov. Sci. 2000, 19, 203–220. [Google Scholar] [CrossRef]
- Carpenter, M.G.; Frank, J.S.; Winter, D.A.; Peysar, G.W. Sampling duration effects on centre of pressure summary measures. Gait Posture 2001, 13, 35–40. [Google Scholar] [CrossRef]
- Bricot, B. La Riprogrammazione Posturale Globale; Sauramps Medicàl: Montpellier, France, 1998. [Google Scholar]
- Clark, N.C.; Röijezon, U.; Treleaven, J. Proprioception in musculoskeletal rehabilitation. Part 2: Clinical assessment and intervention. Man. Ther. 2015, 20, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; L. Lawrence Earlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Glasauer, S.; Schneider, E.; Jahn, K.; Strupp, M.; Brandt, T. How the eyes move the body. Neurology 2005, 65, 1291–1293. [Google Scholar] [CrossRef]
- Manor, B.; Costa, M.D.; Hu, K.; Newton, E.; Starobinets, O.; Kang, H.G.; Peng, C.K.; Novak, V.; Lipsitz, L.A. Physiological complexity and system adaptability: Evidence from postural control dynamics of older adults. J. Appl. Physiol. 2010, 109, 1786–1791. [Google Scholar] [CrossRef]
- Jahn, K.; Strupp, M.; Krafczyk, S.; SchuÈler, O.; Glasauer, S.; Brandt, T. Suppression of eye movements improves balance. Brain 2002, 125, 2005–2011. [Google Scholar] [CrossRef]
- Strupp, M.; Glasauer, S.; Jahn, K.; Schneider, E.; Krafczyk, S.; Brandt, T. Eye movements and balance. Ann. N. Y. Acad. Sci. 2003, 1004, 352–353. [Google Scholar] [CrossRef]
- Pimenta, C.; Correia, A.; Alves, M.; Virella, D. Effects of oculomotor and gaze stability exercises on balance after stroke: Clinical trial protocol. Porto Biomed. J. 2017, 2, 76–80. [Google Scholar] [CrossRef]
- Sell, T.C.; Lephart, S.M. Neuromuscular differences between men and women. In ACL Injuries in the Female Athlete; Springer: Berlin/Heidelberg, Germany, 2012; pp. 133–152. [Google Scholar]
- Hageman, P.A.; Leibowitz, J.M.; Blanke, D. Age and gender effects on postural control measures. Arch. Phys. Med. Rehabil. 1995, 76, 961–965. [Google Scholar] [CrossRef]
- Kahraman, B.O.; Kahraman, T.; Kalemci, O.; Sengul, Y.S. Gender differences in postural control in people with nonspecific chronic low back pain. Gait Posture 2018, 64, 147–151. [Google Scholar] [CrossRef]
- Gauchard, G.C.; Gangloff, P.; Jeandel, C.; Perrin, P.P. Physical activity improves gaze and posture control in the elderly. NeuroSci. Res. 2003, 45, 409–417. [Google Scholar] [CrossRef]
- Barcellona, M.; Giustino, V.; Messina, G.; Battaglia, G.; Fischetti, F.; Palma, A.; Iovane, A. Effects of a specific training protocol on posturographic parameters of a taekwondo elite athlete and implications on injury prevention: A case study. Acta Med. 2018, 34, 1533. [Google Scholar]
- Fischetti, F.; Greco, G. Multilateral methods in Physical Education improve physical capacity and motor skills performance of the youth. J. Physic. Educ. Sport 2017, 17, 2160–2168. [Google Scholar]
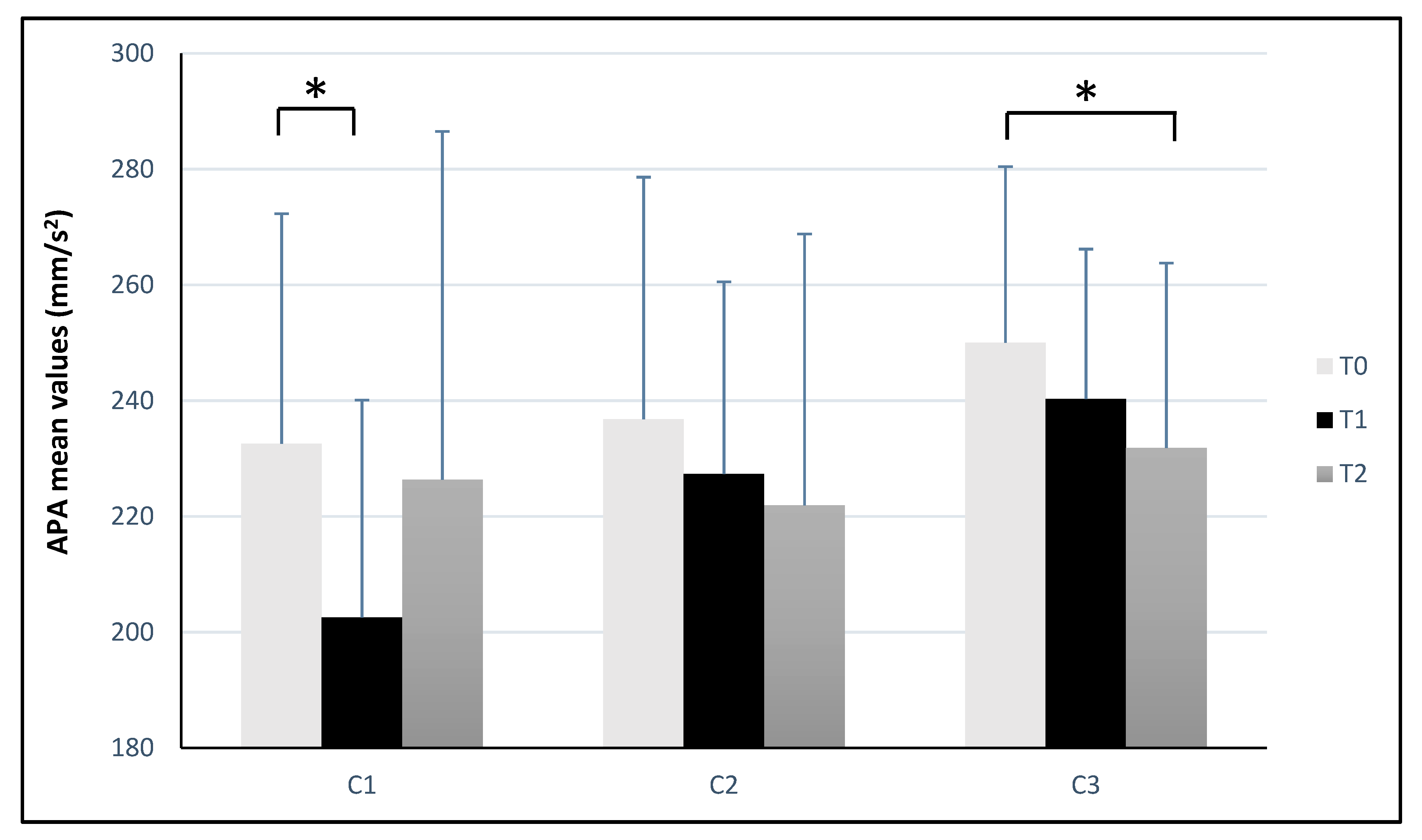
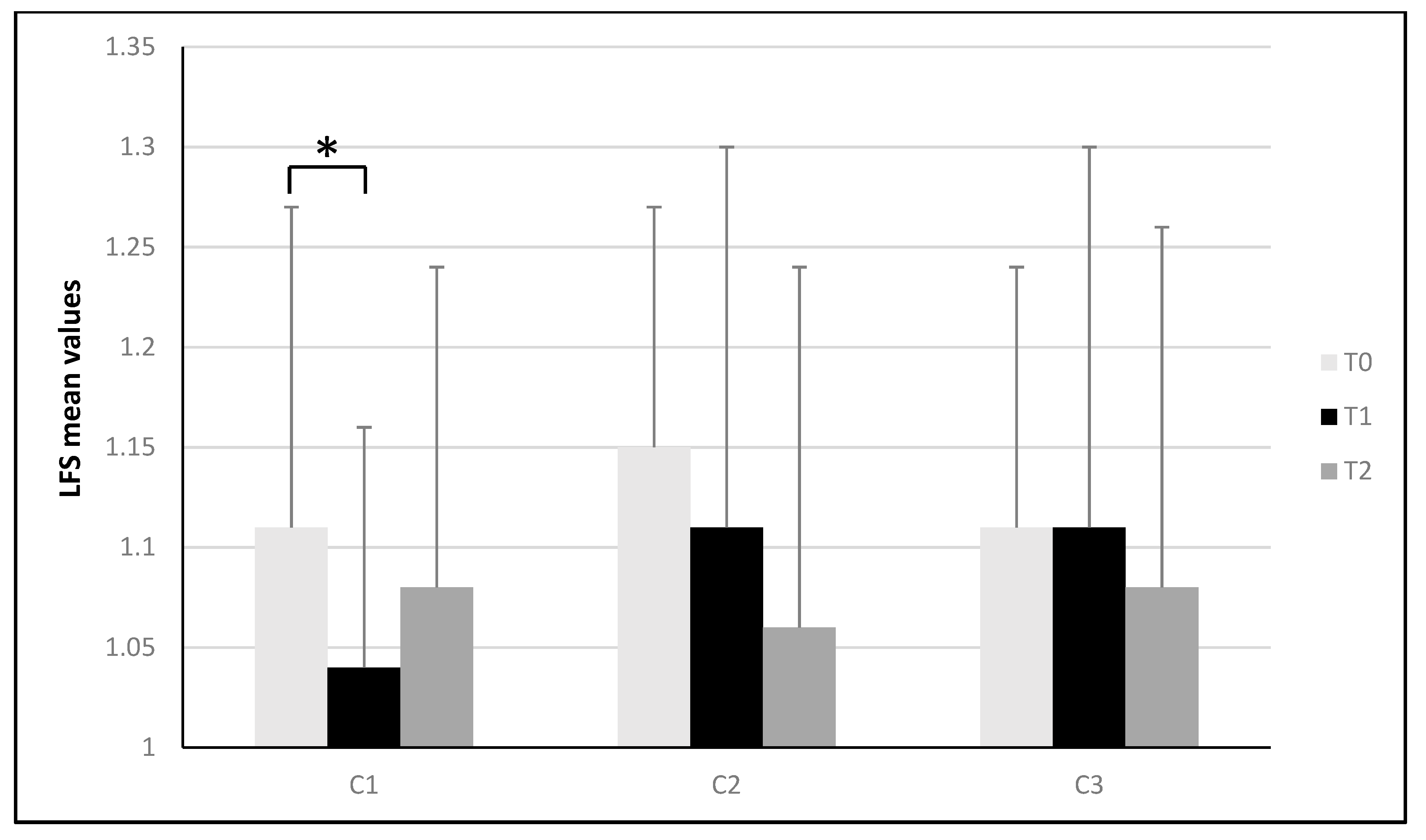

| Panel a | C1 (Counterclockwise) | ||||||||||||
| Parameter | T0 | T1 | ΔT1-T0 | ES | T2 | ΔT2-T0 | ES | ||||||
| LFS | 1.11 | ± | 0.16 | 1.04 * | ± | 0.12 | −0.07 | 0.50 | 1.08 | ± | 0.16 | −0.03 | 0.19 |
| APA (mm/s2) | 232.56 | ± | 39.76 | 202.54 * | ± | 37.57 | −30.02 | 0.78 | 226.39 | ± | 60.11 | −6.17 | 0.12 |
| LCG (mm) | 687.03 | ± | 112.48 | 664.36 | ± | 138.03 | −22.67 | 0.18 | 701.02 | ± | 156.27 | 13.99 | 0.10 |
| CGA (mm2) | 19.77 | ± | 25.90 | 19.33 | ± | 18.22 | −0.44 | 0.02 | 17.80 | ± | 13.37 | −1.97 | 0.10 |
| BFL (%) | 88.74 | ± | 7.85 | 86.94 | ± | 8.88 | −1.80 | 0.22 | 85.02 | ± | 8.93 | −3.72 | 0.44 |
| Panel b | C2 (Clockwise) | ||||||||||||
| Parameter | T0 | T1 | ΔT1-T0 | ES | T2 | ΔT2-T0 | ES | ||||||
| LFS | 1.15 | ± | 0.12 | 1.11 | ± | 0.19 | −0.04 | 0.26 | 1.06 | ± | 0.18 | −0.09 | 0.60 |
| APA (mm/s2) | 236.80 | ± | 41.82 | 227.35 | ± | 33.18 | −9.45 | 0.25 | 221.91 | ± | 46.89 | −14.89 | 0.34 |
| LCG (mm) | 746.79 | ± | 134.26 | 737.83 | ± | 111.91 | −8.96 | 0.07 | 716.89 | ± | 151.21 | −29.90 | 0.21 |
| CGA (mm2) | 24.80 | ± | 36.37 | 25.01 | ± | 40.25 | 0.21 | 0.01 | 15.58 | ± | 11.95 | −9.22 | 0.38 |
| BFL (%) | 90.14 | ± | 9.63 | 87.44 * | ± | 8.81 | −2.70 | 0.29 | 89.19 | ± | 8.77 | −0.95 | 0.10 |
| Panel c | C3 (Mixed) | ||||||||||||
| Parameter | T0 | T1 | ΔT1-T0 | ES | T2 | ΔT2-T0 | ES | ||||||
| LFS | 1.11 | ± | 0.13 | 1.11 | ± | 0.19 | 0 | 0 | 1.08 | ± | 0.18 | −0.03 | 0.19 |
| APA (mm/s2) | 250.03 | ± | 30.39 | 240.28 | ± | 25.91 | −9.75 | 0.35 | 231.82 * | ± | 31.94 | −18.21 | 0.58 |
| LCG (mm) | 737.84 | ± | 162.48 | 715.72 | ± | 157.51 | −22.12 | 0.14 | 721.13 | ± | 155.23 | −16.71 | 0.11 |
| CGA (mm2) | 14.45 | ± | 8.94 | 15.20 | ± | 10.17 | 0.75 | 0.08 | 15.03 | ± | 12.91 | 0.58 | 0.05 |
| BFL (%) | 85.80 | ± | 10.76 | 83.89 | ± | 11.76 | −1.91 | 0.17 | 85.76 | ± | 9.82 | −0.04 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonavolontà, V.; Cataldi, S.; Coluccia, A.; Giunto, A.; Fischetti, F. Sustainable Intervention for Health Promotion and Postural Control Improvement: Effects of Home-Based Oculomotor Training. Sustainability 2020, 12, 10552. https://doi.org/10.3390/su122410552
Bonavolontà V, Cataldi S, Coluccia A, Giunto A, Fischetti F. Sustainable Intervention for Health Promotion and Postural Control Improvement: Effects of Home-Based Oculomotor Training. Sustainability. 2020; 12(24):10552. https://doi.org/10.3390/su122410552
Chicago/Turabian StyleBonavolontà, Valerio, Stefania Cataldi, Adalisa Coluccia, Antonio Giunto, and Francesco Fischetti. 2020. "Sustainable Intervention for Health Promotion and Postural Control Improvement: Effects of Home-Based Oculomotor Training" Sustainability 12, no. 24: 10552. https://doi.org/10.3390/su122410552
APA StyleBonavolontà, V., Cataldi, S., Coluccia, A., Giunto, A., & Fischetti, F. (2020). Sustainable Intervention for Health Promotion and Postural Control Improvement: Effects of Home-Based Oculomotor Training. Sustainability, 12(24), 10552. https://doi.org/10.3390/su122410552








