The Effect of Thermal Treatment on the Physicochemical Properties of Minerals Applied to Heterogeneous Catalytic Ozonation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedures
2.3. Analytical Techniques
3. Results and Discussion
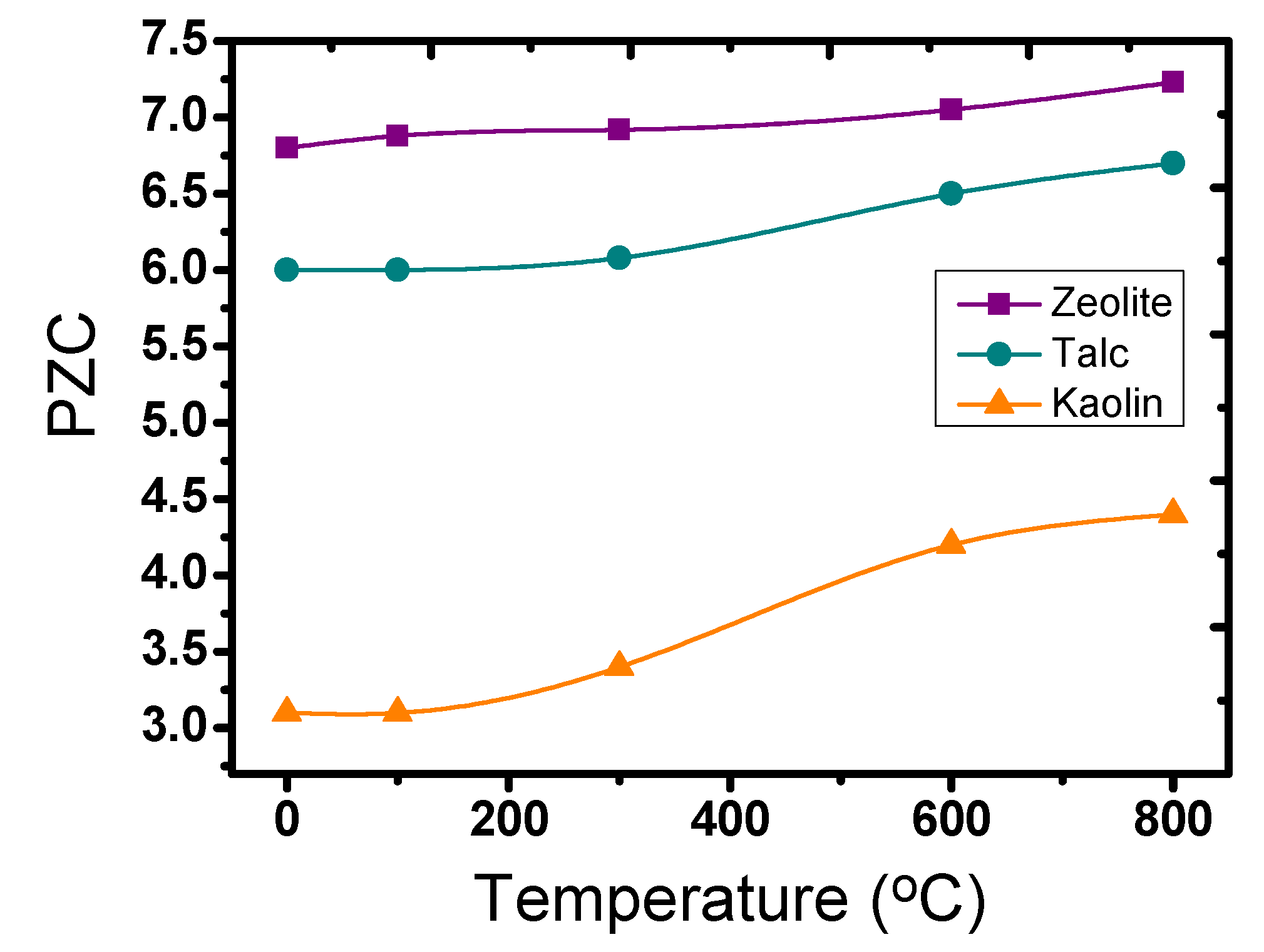
3.1. Catalysts Characterization
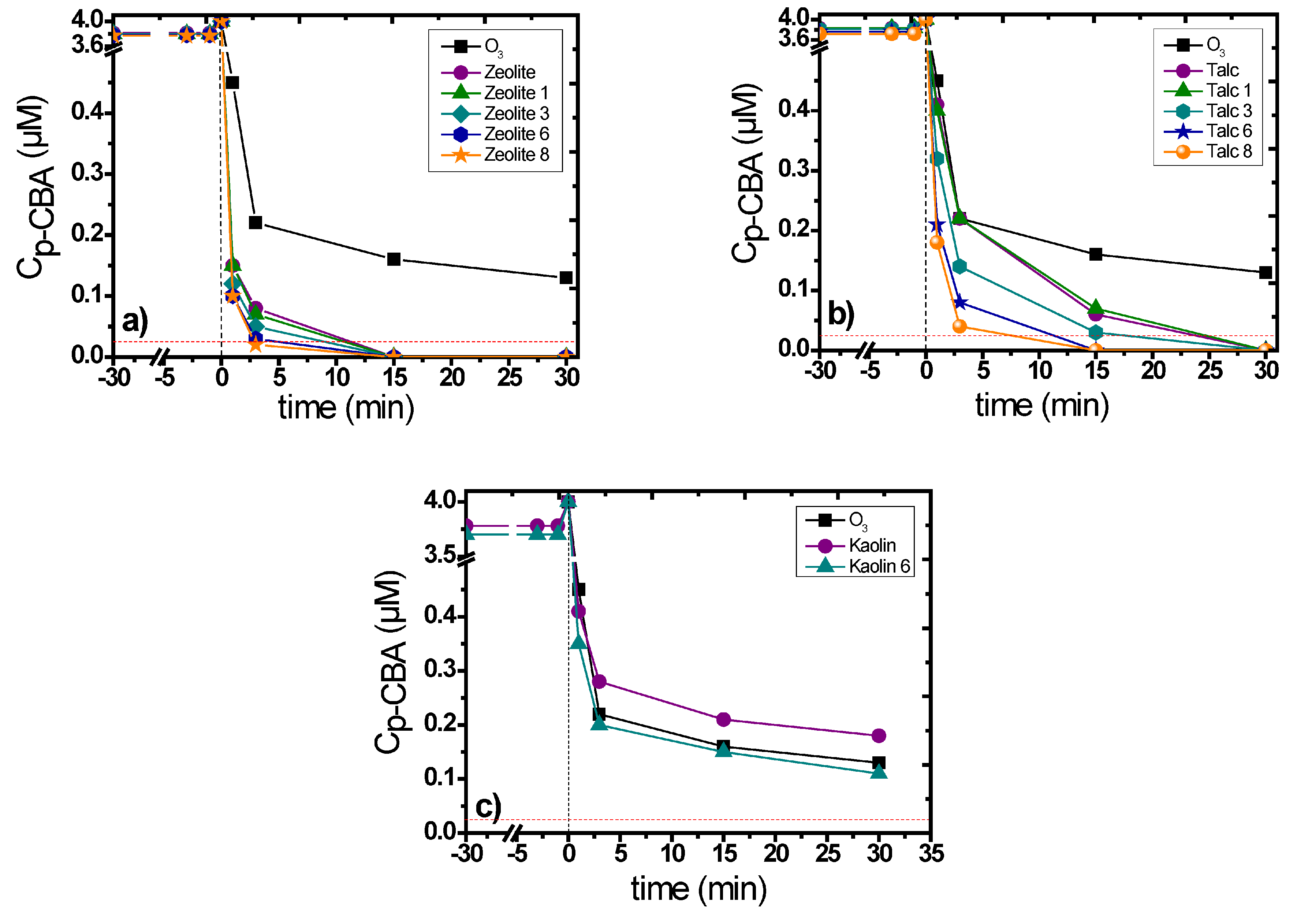
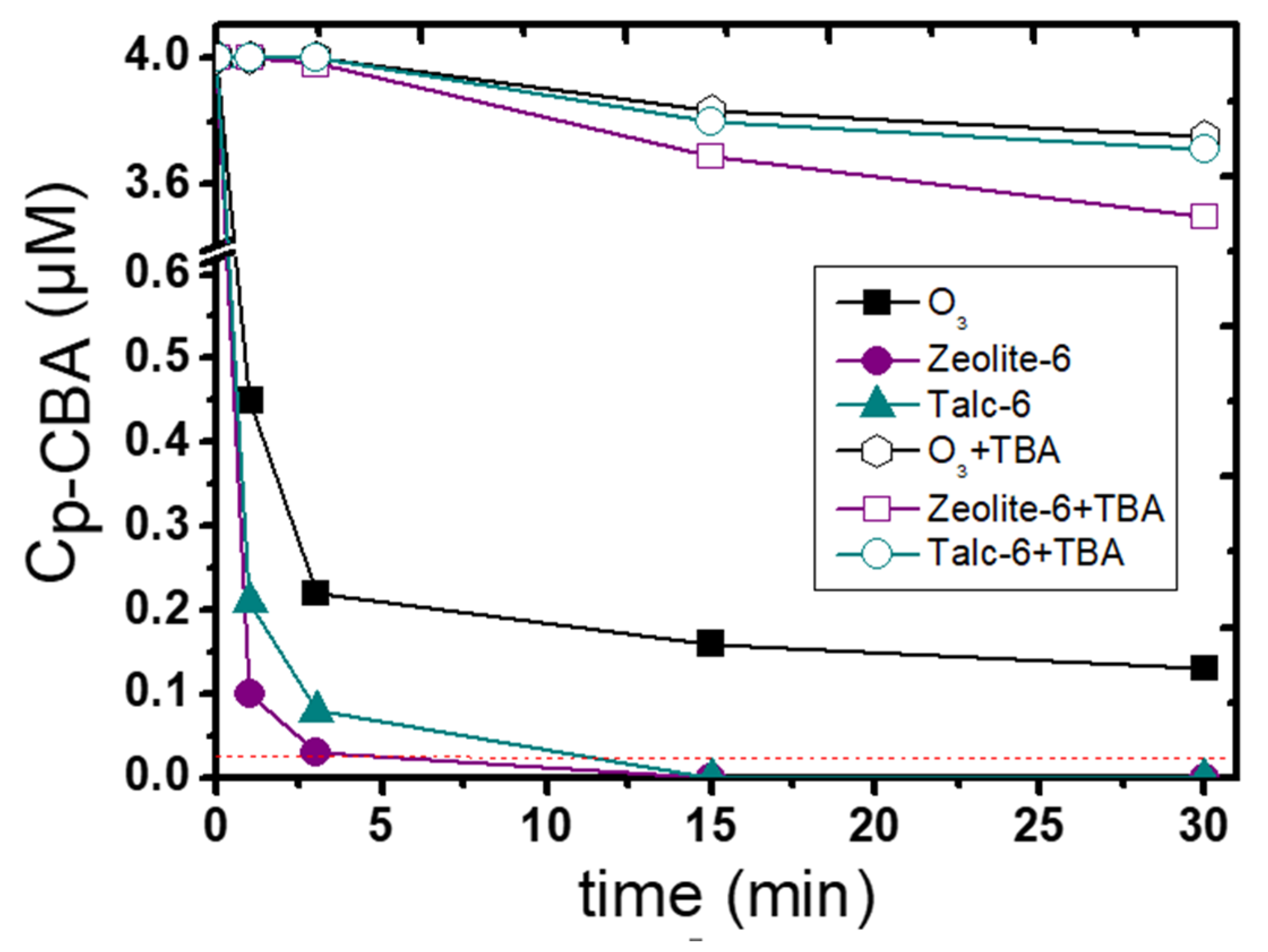
3.2. Catalytic Activity of the Examined Natural Minerals
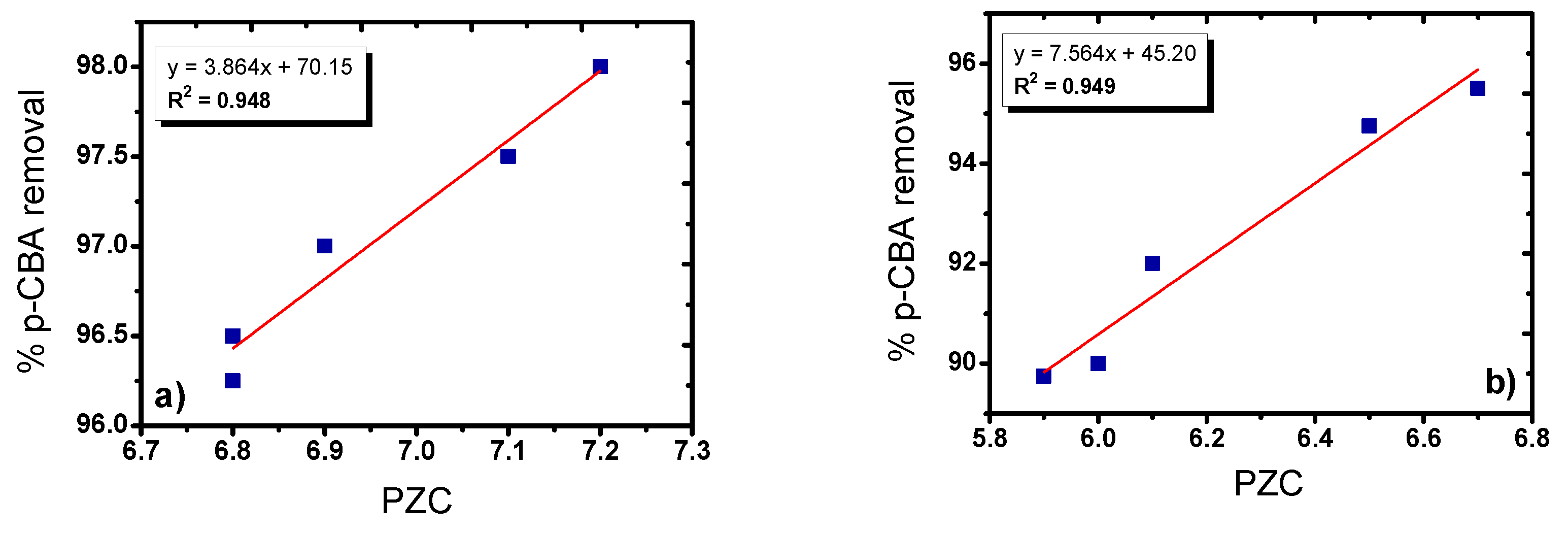
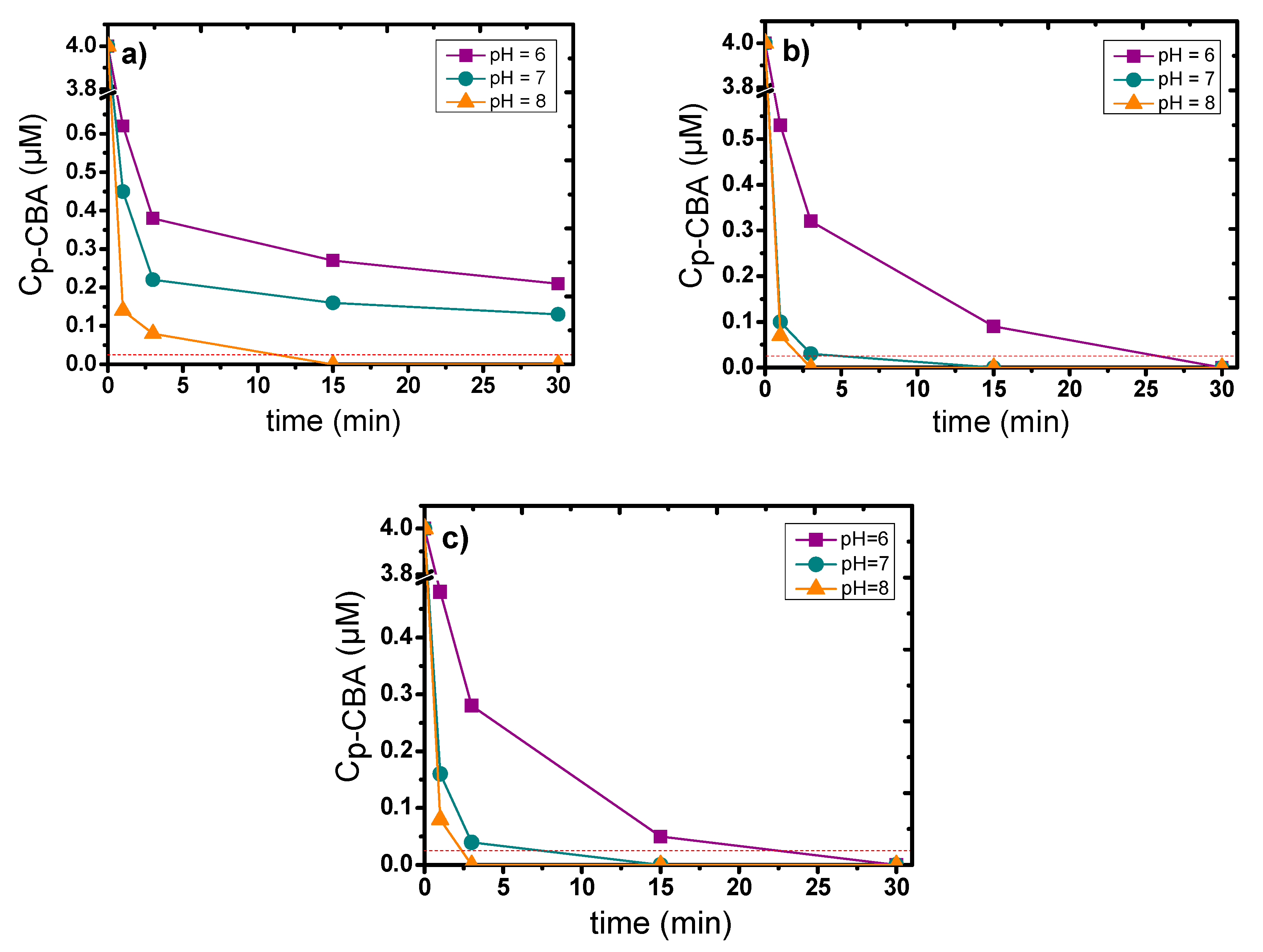
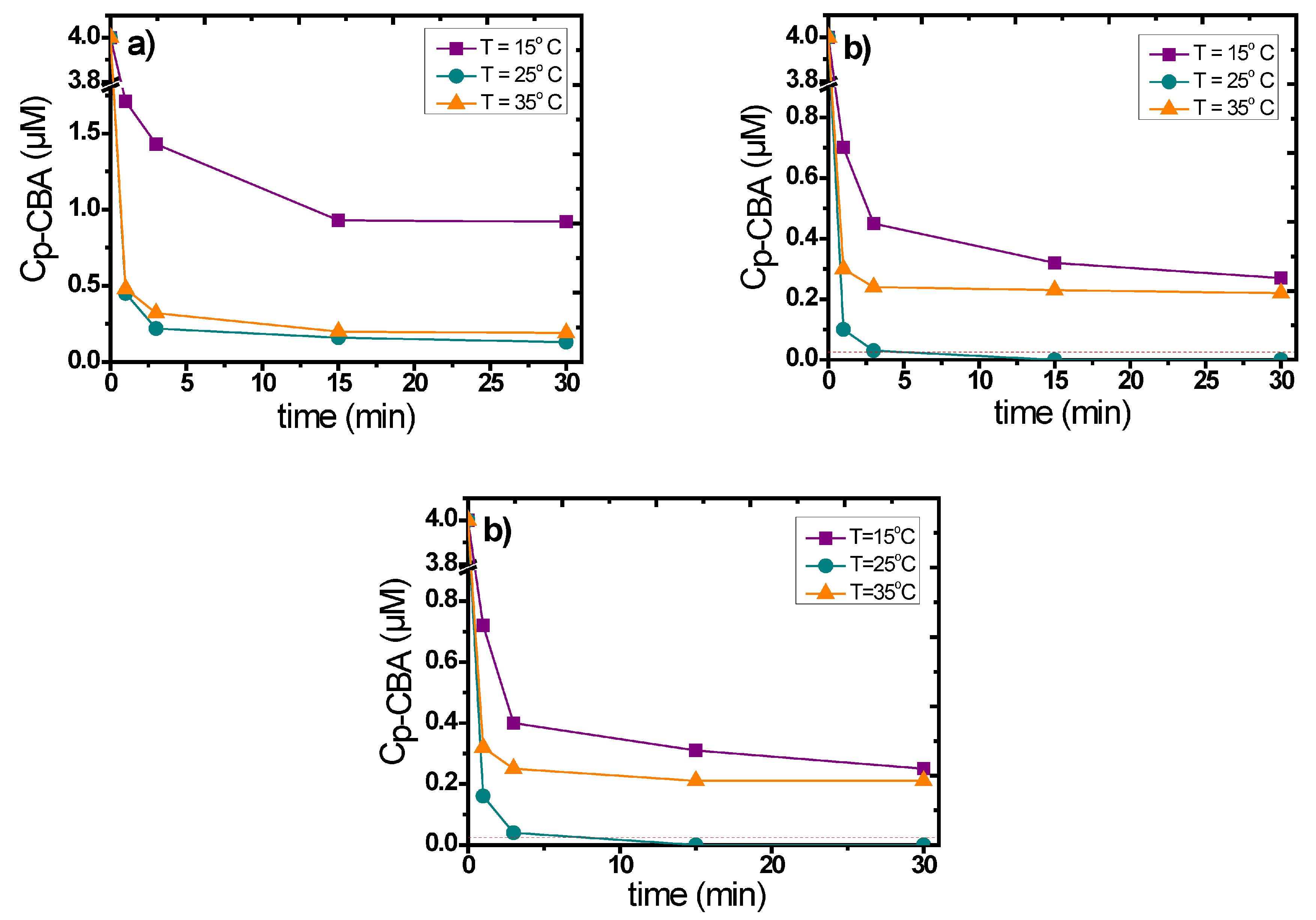
3.3. Influence of the Main Experimental Parameters on the Removal of p-CBA
4. Conclusions
Supplementary Materials
Author Contributions
Funding

Conflicts of Interest
References
- Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernández-González, C. Advanced Oxidation Processes for the Removal of Antibiotics from Water. An Overview. Water 2019, 12, 102. [Google Scholar] [CrossRef]
- Stamm, C.; Räsänen, K.; Burdon, F.J.; Altermatt, F.; Jokela, J.; Joss, A.; Ackermann, M.; Eggen, R.I.L. Unravelling the Impacts of Micropollutants in Aquatic Ecosystems. In Advances in Ecological Research; Elsevier: Amsterdam, The Netherlands, 2016; Volume 55, pp. 183–223. [Google Scholar] [CrossRef]
- Rogowska, J.; Cieszynska-Semenowicz, M.; Ratajczyk, W.; Wolska, L. Micropollutants in Treated Wastewater. Ambio 2020, 49, 487–503. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki, J.; Kasprzyk-Hordern, B. The Efficiency and Mechanisms of Catalytic Ozonation. Appl. Catal. B Environ. 2010, 99, 27–42. [Google Scholar] [CrossRef]
- Gao, G.; Kang, J.; Shen, J.; Chen, Z.; Chu, W. Heterogeneous Catalytic Ozonation of Sulfamethoxazole in Aqueous Solution over Composite Iron–Manganese Silicate Oxide. Ozone Sci. Eng. 2017, 39, 24–32. [Google Scholar] [CrossRef]
- Torres Sánchez, R.M.; Tavani, E.L. Temperature Effects on the Point of Zero Charge and Isoelectric Point of a Red Soil Rich in Kaolinite and Iron Minerals. J. Therm. Anal. 1994, 41, 1129–1139. [Google Scholar] [CrossRef]
- Wang, B.; Xiong, X.; Ren, H.; Huang, Z. Preparation of MgO Nanocrystals and Catalytic Mechanism on Phenol Ozonation. RSC Adv. 2017, 7, 43464–43473. [Google Scholar] [CrossRef]
- Zhu, H.; Ma, W.; Han, H.; Han, Y.; Ma, W. Catalytic Ozonation of Quinoline Using Nano-MgO: Efficacy, Pathways, Mechanisms and Its Application to Real Biologically Pretreated Coal Gasification Wastewater. Chem. Eng. J. 2017, 327, 91–99. [Google Scholar] [CrossRef]
- Yuan, L.; Shen, J.; Chen, Z.; Liu, Y. Pumice-Catalyzed Ozonation Degradation of p-Chloronitrobenzene in Aqueous Solution. Appl. Catal. B Environ. 2012, 117–118, 414–419. [Google Scholar] [CrossRef]
- Gao, G.; Shen, J.; Chu, W.; Chen, Z.; Yuan, L. Mechanism of Enhanced Diclofenac Mineralization by Catalytic Ozonation over Iron Silicate-Loaded Pumice. Sep. Purif. Technol. 2017, 173, 55–62. [Google Scholar] [CrossRef]
- Yuan, L.; Shen, J.; Chen, Z.; Guan, X. Role of Fe/Pumice Composition and Structure in Promoting Ozonation Reactions. Appl. Catal. B Environ. 2016, 180, 707–714. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, Z.; Ma, J.; Liu, H. Enhancement Mechanism of Heterogeneous Catalytic Ozonation by Cordierite-Supported Copper for the Degradation of Nitro benzene in Aqueous Solution. Environ. Sci. Technol. 2009, 43, 2047–2053. [Google Scholar] [CrossRef] [PubMed]
- Sui, M.; Sheng, L.; Lu, K.; Tian, F. FeOOH Catalytic Ozonation of Oxalic Acid and the Effect of Phosphate Binding on Its Catalytic Activity. Appl. Catal. B Environ. 2010, 96, 94–100. [Google Scholar] [CrossRef]
- Kermani, M.; Kakavandi, B.; Farzadkia, M.; Esrafili, A.; Jokandan, S.F.; Shahsavani, A. Catalytic Ozonation of High Concentrations of Catecholover TiO2@Fe3O4 Magnetic Core-Shell Nanocatalyst: Optimization, Toxicity and Degradation Pathway Studies. J. Clean. Prod. 2018, 192, 597–607. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Tu, J.; Wang, Q.; Cui, Y.-H.; Zhang, L.; Wu, X.; Zhang, B.; Ma, J. Catalytic Ozonation of Diethyl Phthalate in Aqueous Solution Using Graphite Supported Zinc Oxide. Sep. Purif. Technol. 2018, 200, 51–58. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, W.; Hu, L.; Zeng, J.; He, C.; Tan, X.; He, Z.; Zhang, Q.; Shu, D. Combined Adsorption and Catalytic Ozonation for Removal of Endocrine Disrupting Compounds over MWCNTs/Fe3O4 Composites. Catal. Today 2017, 297, 143–150. [Google Scholar] [CrossRef]
- Psaltou, S.; Karapatis, A.; Mitrakas, M.; Zouboulis, A. The Role of Metal Ions on P-CBA Degradation by Catalytic Ozonation. J. Environ. Chem. Eng. 2019, 7, 103324. [Google Scholar] [CrossRef]
- Yi, H.; Zhao, Y.; Liu, Y.; Wang, W.; Song, S.; Liu, C.; Li, H.; Zhan, W.; Liu, X. A Novel Method for Surface Wettability Modification of Talc through Thermal Treatment. Appl. Clay Sci. 2019, 176, 21–28. [Google Scholar] [CrossRef]
- Aljibouri, A.K.H.; Wu, J.; Upreti, S.R. Heterogeneous Catalytic Ozonation of Naphthenic Acids in Water. Can. J. Chem. Eng. 2019, 97, 67–73. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Z.; Chai, B.; Cheng, S.; Lu, X.; Bai, X. Heterogeneous Catalytic Ozonation of Natural Organic Matter with Goethite, Cerium Oxide and Magnesium Oxide. RSC Adv. 2016, 6, 14730–14740. [Google Scholar] [CrossRef]
- Clesceri, S.L.; Greenberg, E.A.; Trussell, R.R. Inorganic Nonmetals, in Standard Methods for Examination of Water and Wastewater, 17th ed.; American Public Health Association: Washington, DC, USA, 1989. [Google Scholar]
- Bourikas, K.; Vakros, J.; Kordulis, C.; Lycourghiotis, A. Potentiometric Mass Titrations: Experimental and Theoretical Establishment of a New Technique for Determining the Point of Zero Charge (PZC) of Metal (Hydr) Oxides. J. Phys. Chem. B 2003, 107, 9441–9451. [Google Scholar] [CrossRef]
- Perraki, T.; Kakali, G.; Kontori, E. Characterization and Pozzolanic Activity of Thermally Treated Zeolite. J. Anal. Calorim. 2005, 82, 109–113. [Google Scholar] [CrossRef]
- Christidis, G. Chemical and Thermal Modification of Natural HEU-Type Zeolitic Materials from Armenia, Georgia and Greece. Appl. Clay Sci. 2003, 24, 79–91. [Google Scholar] [CrossRef]
- Palenta, T.; Fuhrmann, S.; Greaves, G.N.; Schwieger, W.; Wondraczek, L. Thermal collapse and hierarchy of polymorphs in a faujasite-type zeolite and its analogous melt-quenched glass. J. Chem. Phys. 2015, 142, 084503. [Google Scholar] [CrossRef] [PubMed]
- Parks, G.A. The Isoelectric Points of Solid Oxides, Solid Hydroxides, and Aqueous Hydroxo Complex Systems. Chem. Rev. 1965, 65, 177–198. [Google Scholar] [CrossRef]
- Ardizzone, S.; Daghetti, A.; Franceschi, L.; Trasatti, S. The Point of Zero Charge of Hydrous RuO2. Colloids Surf. 1989, 35, 85–96. [Google Scholar] [CrossRef]
- Stankovic, J.; Milonjic, S.; Zec, S. The Influence of Chemical and Thermal Treatment on the Point of Zero Charge of Hydrous Zirconium Oxide. J. Serb. Chem. Soc. 2013, 78, 987–995. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Dąbrowska, A.; Świetlik, J.; Nawrocki, J. Ozonation Enhancement with Nonpolar Bonded Alumina Phases. Ozone Sci. Eng. 2004, 26, 367–380. [Google Scholar] [CrossRef]
- Gottschalk, C.; Libra, J.A.; Saupe, A. Ozonation of Water and Waste Water. In A Practical Guide to Understanding Ozone and Its Application; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Wang, D.; Xu, H.; Ma, J.; Lu, X.; Qi, J.; Song, S. Strong Promoted Catalytic Ozonation of Atrazine at Low Temperature Using Tourmaline as Catalyst: Influencing Factors, Reaction Mechanisms and Pathways. Chem. Eng. J. 2018, 354, 113–125. [Google Scholar] [CrossRef]
- Luo, L.; Zou, D.; Lu, D.; Yu, F.; Xin, B.; Ma, J. Study of Catalytic Ozonation for Tetracycline Hydrochloride Degradation in Water by Silicate Ore Supported Co3O4. RSC Adv. 2018, 8, 41109–41116. [Google Scholar] [CrossRef]


| Minerals | Chemical Composition (%) | mg/Kg | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | MgO | CaO | Na2O | K2O | Fe2O3 | LOI | NiO | Cr2O3 | |
| Zeolite | 70.5 | 12.1 | 0.58 | 2.91 | 1.45 | 2.82 | 0.67 | 8.9 a | ND | ND |
| Talc | 65.8 | ND | 28.6 | 0.01 | 0.01 | 0.04 | 0.73 | 4.6 b | 1500 | 20 |
| Kaolin | 50.9 | 33.1 | 0.71 | 0.10 | 0.28 | 1.19 | 0.70 | 12.9 a | 120 | ND |
| Detection Limit | 0.1 | 0.05 | 0.0005 | 0.004 | 0.001 | 0.002 | 0.01 | 0.1 | 100 | 50 |
| Ozone Escape | |||
|---|---|---|---|
| Reaction Time (Min) | 15 °C | 25 °C | 35 °C |
| 0.1 | 3.8% | 4.7% | 5.6% |
| 1 | 5.8% | 7% | 8.3% |
| 3 | 7.7% | 9.4% | 11.1% |
| 15 | 9.6% | 11.7% | 13.9% |
| 30 | 11.5% | 14.1% | 16.7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Psaltou, S.; Kaprara, E.; Kalaitzidou, K.; Mitrakas, M.; Zouboulis, A. The Effect of Thermal Treatment on the Physicochemical Properties of Minerals Applied to Heterogeneous Catalytic Ozonation. Sustainability 2020, 12, 10503. https://doi.org/10.3390/su122410503
Psaltou S, Kaprara E, Kalaitzidou K, Mitrakas M, Zouboulis A. The Effect of Thermal Treatment on the Physicochemical Properties of Minerals Applied to Heterogeneous Catalytic Ozonation. Sustainability. 2020; 12(24):10503. https://doi.org/10.3390/su122410503
Chicago/Turabian StylePsaltou, Savvina, Efthimia Kaprara, Kyriaki Kalaitzidou, Manassis Mitrakas, and Anastasios Zouboulis. 2020. "The Effect of Thermal Treatment on the Physicochemical Properties of Minerals Applied to Heterogeneous Catalytic Ozonation" Sustainability 12, no. 24: 10503. https://doi.org/10.3390/su122410503
APA StylePsaltou, S., Kaprara, E., Kalaitzidou, K., Mitrakas, M., & Zouboulis, A. (2020). The Effect of Thermal Treatment on the Physicochemical Properties of Minerals Applied to Heterogeneous Catalytic Ozonation. Sustainability, 12(24), 10503. https://doi.org/10.3390/su122410503