1. Introduction
Coastal development, including aquaculture and mariculture development, has significantly changed wetland ecosystems around the world, consequently reducing habitats and affecting biotic communities [
1,
2,
3,
4]. Migratory water birds, relying on coastal wetlands, are among the most vulnerable groups of organisms in coastal areas and have continuously declined in numbers of species on the East Asian—Australasian Flyway [
5,
6]. To resolve conflicts between food production and biodiversity, the Convention of Biological Diversity declared that Satoyama landscapes are effective for keeping production in harmony with nature [
7]. Many examples of traditional agriculture systems around the world not only provide food products but also maintain diverse landscapes and biodiversity [
8,
9]. Few examples, however, are present in the coastal aquacultural areas [
10]. To facilitate the harmony between aquaculture and water birds, we need to find traditional and/or innovative solutions before formulating conservation policy.
In Southeast Asia, the alternate timing of fish cultivation and non-cultivation periods of traditional shallow-pond milkfish (
Chanos chanos) aquaculture may help conserve migratory overwintering water birds. This 400-year traditional approach in Taiwan is gradually being replaced by either deep-pond aquaculture or common orient clam (
Meretrix lusoria) aquaculture [
11,
12]. Traditionally, milkfish is cultivated from April through October because it is a tropical fish [
13]. From November through the next March, the overwintering period of migratory water birds, ponds are exposed to drying and re-watering cycles in Taiwan [
13]. During the drying phase, water birds can access these ponds and feed. About half the population of the endangered Black-faced Spoonbill (
Platalea minor) [
14] overwinters in Tainan city and its surroundings on the East Asian—Australasian Flyway [
12,
15]. This traditional operation of shallow ponds by milkfish aquaculture may serve as a conservation approach for migratory water birds, such as Black-faced Spoonbills, and can be used to restore a Satoumi aquaculture landscape (a coastal Satoyama) [
16]. Experimental evidence of this operation, however, was lacking. This experiment adopted seasonality and operation of traditional milkfish aquaculture as guidelines to apply on different types of aquaculture.
Water bird usage of aquaculture ponds may be different from their usage in natural wetlands. In wetlands, size, water depth, and isolation are important factors affecting water bird usage of wetlands [
17,
18,
19]. Shallow water depth usually attracts more species [
17,
20], whereas larger sized wetlands can have more types of species and higher densities [
19,
20]. Aquaculture ponds differ from wetlands in feed supply, topography, human activity and vegetation management. Whether the aforementioned factors are applicable to aquaculture ponds is not clear because there are few studies focusing on the ecology of water birds in aquaculture ponds. Abandoned aquaculture ponds, however, may be more similar to natural wetlands than production aquaculture, and may have similar usage of water birds to natural wetlands.
Food abundance also affects the habitat usage of water birds [
21]. When reduced water in dry seasons forces concentrated prey densities as observed in the Everglades swamp (USA), these abundant accessible foods attract feeding of water birds [
22]. This phenomenon, called resource pulse, is important to nutrition and reproduction of water birds [
23,
24]. Drawdown of aquaculture ponds may also create a resource pulse [
25] because it can concentrate prey and make prey more vulnerable to predation, as in the natural wetlands. This resource pulse is an important food source for water birds in the Florida Everglades [
22,
26] and may also be an important food source for overwintering migratory water birds in coastal areas of Taiwan and adjacent regions. However, few studies have tested whether resource pulses created by water drawdown can attract water birds in different types of aquaculture.
With increasing demand for aquaculture production [
27], reduced habitats in coastal areas are envisioned for water birds, especially migrants. Thus, we applied the operational seasonality of traditional shallow-pond milkfish aquaculture as a guideline for this aquaculture experiment. We tested the following hypotheses: (1) whether experimental water drawdown of aquaculture ponds that created a resource pulse of food concentration could increase feeding usage of water birds; (2) whether water birds responded differently to different types of aquaculture; (3) whether the usage patterns of water birds are related to food abundance. We also examined responses of guilds of water birds in abundance and numbers of species and evaluated the relationships between water depths and bird guilds and selected bird species. We predicted that water drawdown would increase water bird densities and the numbers of different species because prey would be concentrated and become more vulnerable to predation, which is a resource pulse that attracts usage by water birds. We also predicted that guilds of water birds would respond differently in control and drawdown because of the different requirements of water birds. In addition, water birds would positively respond to food abundance in general. Results of this study may demonstrate that the operation of aquaculture ponds following this seasonality can serve as a way to conserve migratory water birds in subtropical/tropical regions while maintaining fish production.
2. Materials and Methods
2.1. Study Area
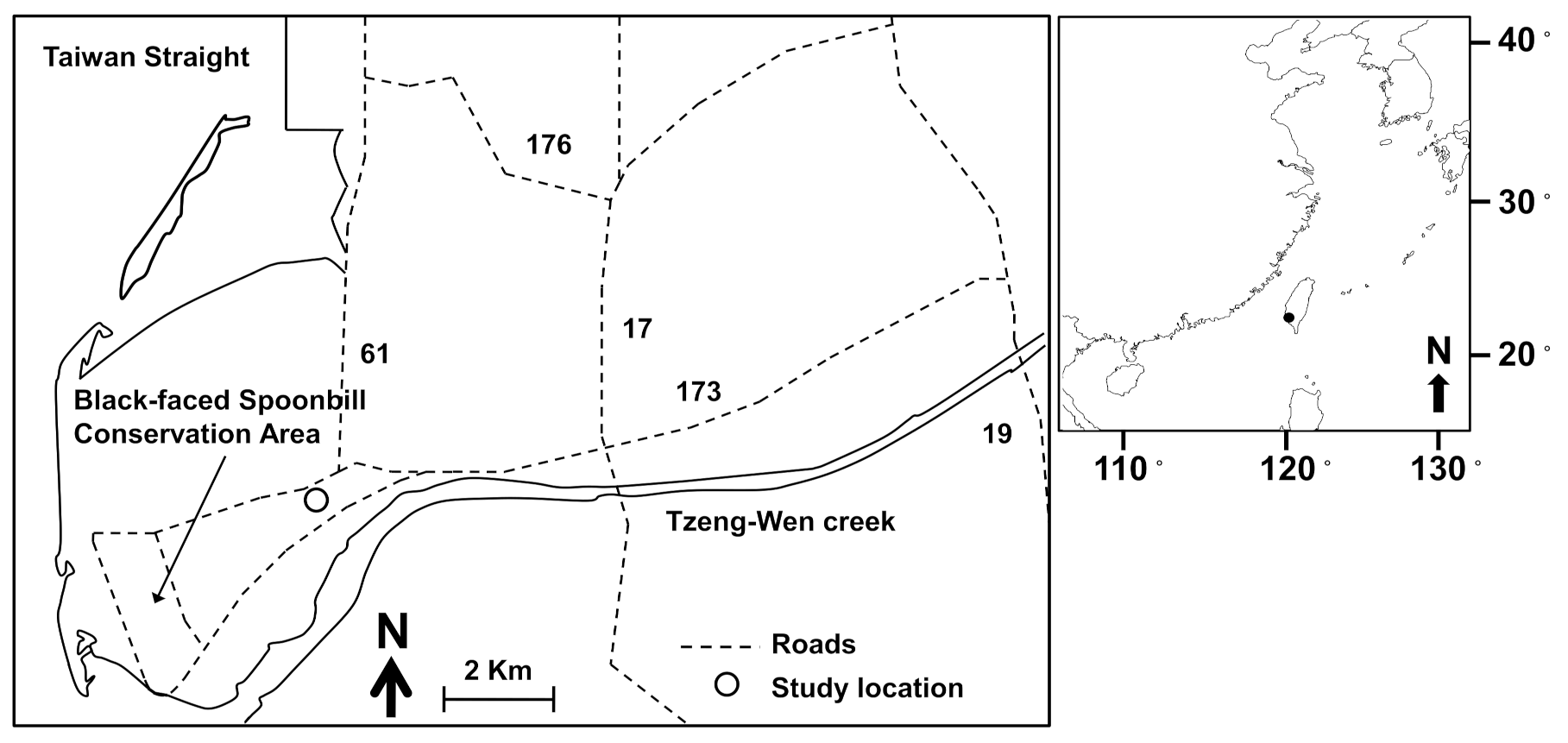
The study area is located in the Cigu region of Tainan City, southern Taiwan. This area harbors the estuary of Tzeng-Wen creek and the Black-faced Spoonbill protection area (
Figure 1). The regional weather is subtropical. The average monthly temperature is highest in July (27.8 ± 0.6 °C; 2011–2016 data of the Cigu weather station of the Central Weather Bureau of Taiwan) and the lowest is in January (17.1 ± 0.9 °C). The wet season usually lasts from April through September in this part of Taiwan, whereas the dry season extends from October to March. The study area is on the East Asia-Australasian migratory flyway [
28]. Migratory birds, including Black-faced Spoonbill, Pied avocet (
Recurvirostra avosetta), Red-necked Stint (
Calidris ruficollis) and Eurasian Wigeon (
Anas penelope), overwinter in this area from September through the next April [
29].
The studied aquaculture ponds are in the western section of the Cigu campus of the National University of Tainan. The western section of the Cigu campus at about 80 ha is mostly wetlands that were restored from aquaculture ponds by removing banks and reconnecting ponds to tidal canals. The studied aquaculture ponds had relatively intact earthen banks and were rehabilitated for aquaculture functions before this experiment. The water sources of these ponds were from the tidal canals and rainfall. The water in tidal canals had to be pumped into aquaculture ponds because these ponds were disconnected to canals by banks.
2.2. Experiments
In this experiment, we controlled for 2 variables: water-level manipulation and aquaculture type. The experiment had four types of aquaculture: abandoned pond with wild fish (type A), no-feed tilapia (type B), milk fish with feed (type C) and tilapia with feed (type D). The two ponds in each group were side-by-side and similar in size. The areas of ponds A1, A2, B1, B2, C1, C2, D1 and D2 were 5.09, 4.95, 5.47, 5.09, 14.35, 18.6, 7.56 and 7.82 ha, respectively. Abandoned ponds, which are scattered in the region, have the potential to cultivate wild fish or tilapia for water bird usage and to serve as a roosting habitat. Invasive tilapia, which is widely cultivated in this region, is prevalent in canals of this region [
30]. Though feeding water birds with tilapia is an aggressive conservation method, this may be necessary when conserved populations are under a major threat [
31]. Shallow pan milk fish ponds often have large areas in this region. Following the seasonality of shallow-pond milkfish cultivation, we cultivated these four types of aquaculture ponds, but only harvested milkfish around the end of October. Fish feed contained only grain protein and had no protein from the ocean bottom trawl. This experiment was conducted from October to January during the years 2012 to 2015. After milk fish harvest, we started the water-level manipulation, which involved drawdown and control.
The number of tilapia and benthic organisms in ponds, which are food sources for water birds, were estimated before drawdown. Tilapia populations were estimated by using mark-recapture. Captured tilapia were weighed and measured in length. Population estimation used the bias-adjusted Lincoln–Peterson estimator [
32]. The number of benthic organisms, including fish, shrimps, snails and crabs, was estimated with a bottom trawl. This trawl had a trawling width of 2.6 m and a mesh size of 0.7 cm. We pulled the trawl net for 10 m to make a sampling area of 26 m
2. We separated organisms into fish, shrimp and crab as well as snail groups, absorbed their water with towels and weighed them. We then estimated the total benthic animal biomass in each pond based on the trawling data and the area ratio (pond area/trawl area).
After the milkfish harvest, we left the remaining organisms in ponds. We started drawdown for only 1 pond in each type. When the average water level of the pond reached 20 cm, we stopped drawdown. The 2nd pond usually had a water depth of above 60 cm. Water in ponds would gradually dry out depending on weather conditions, such as rain, wind and humidity. After the 1st drawdown, we waited at least 20 days before the 2nd drawdown of the 2nd pond to a 20 cm depth because the evaporation rate was around 1 cm per day. Water meters were set-up at the positions of average depths in ponds, usually close to the center of ponds. The shape of each pond was like a shallow dish with a relatively flat bottom and gradually elevating ground around edges. Depths of ponds were calculated from measurements along X-shaped transects for each pond before drawdown. Water depth of each ponds was recorded to the nearest 5 cm mark during every bird survey.
The bird survey for each pond was conducted every other day after drawdown began. The survey started at about 6 a.m. and lasted 2 to 3 h. Ponds were generally not affected by tides because banks were large and were much higher than high tide flows. We recorded numbers and species of water birds inside banks of each pond. The number of surveys was 37 times for 2012, 50 times for 2013 and 2014 and 40 times for 2015. The survey durations were from November to the next January.
2.3. Data Analysis
Foraging guilds of water bird were assigned in accordance with Ma et al. [
18] and our observations [
30] (
Table 1). Common Greenshank (
Tringa nebularia), Common Redshank (
Tringa tetanus) and Marsh Sandpiper (
Tringa stagnatilis), commonly assigned as shorebirds, were classified as shallow waders because they use shallow water rather than mudflats in our observations. We separated long-legged waders from waders as deep waders because they were often observed in deeper waters. Based on the evaluation of the International Union for Conservation of Nature, the Red-necked Stint is near threatened and the Black-faced Spoonbill is endangered.
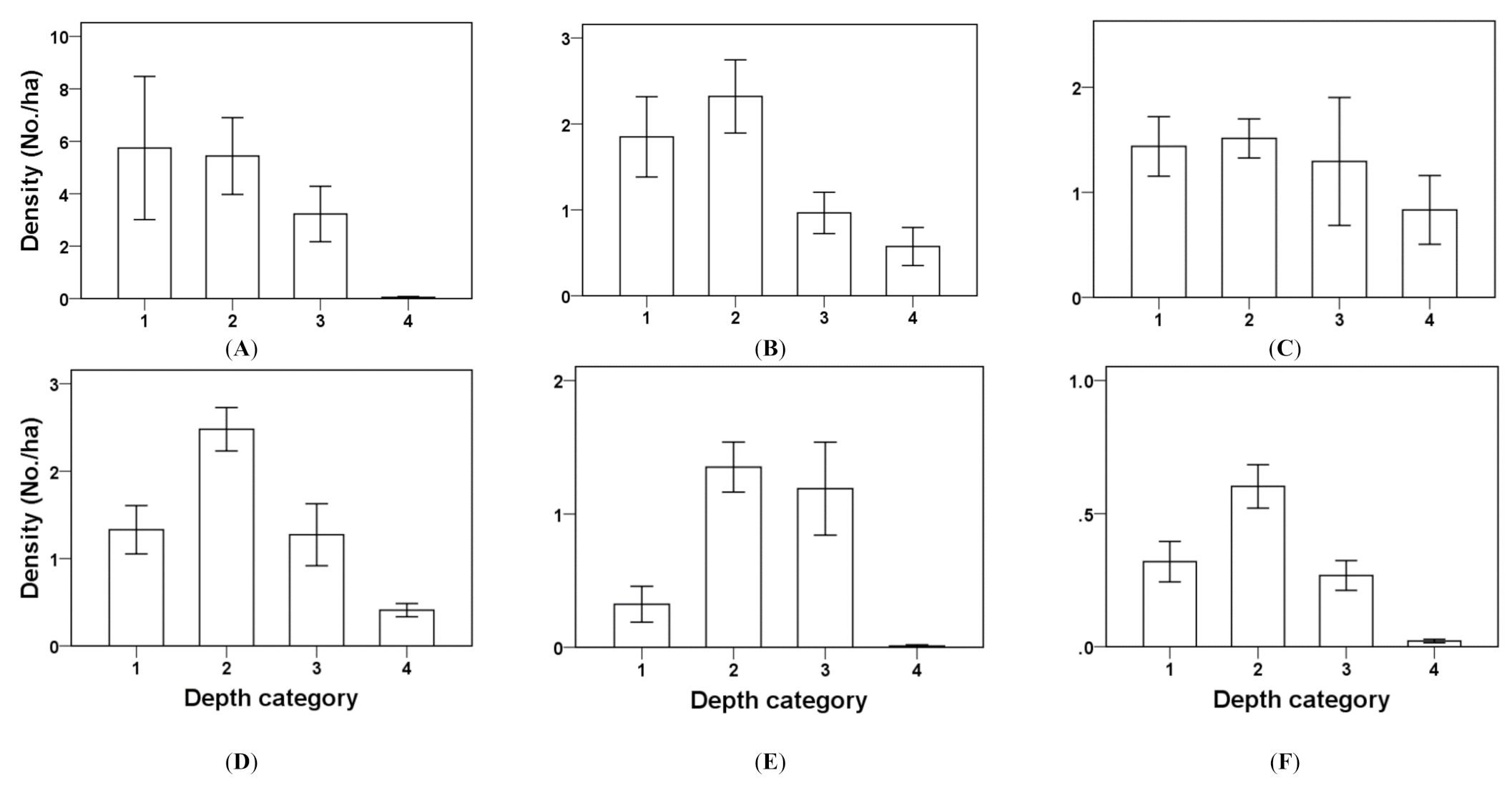
We evaluated foraging guilds by comparing densities and the number of species in each foraging guild in different water depth ranges (≤5, >5 to ≤20, >20 to ≤40 and >40 cm) with one-way analysis of variance (ANOVA) in each aquaculture type. These data were from this experiment and were log-transformed to reach normality. For all cases, the assumption of homogeneity of variance of variables was violated; therefore, Welch’s statistics were adopted [
33]. When ANOVA reached significance using Welch’s statistics, we used the Games-Howell multiple comparison to separate groups of means [
33]. For statistical analyses, we used SPSS 21.0 (IBMTM, Armonk, NY, USA).
To compare the effects of drawdown, aquaculture type as well as the year on density and number of species, we used three-way ANOVA to test these variables and interactions among them with 4-year data. We used Duncan tests for post-hoc analyses. These water bird data were from 10 consecutive surveys after the water level of the drawdown ponds reached 20 cm. We used data from this period because it represented higher usage by water birds. For results with interaction effects, we first analyzed effects of the aquaculture type and water level on densities and the numbers of species for each year with two-way ANOVA. Then, we compared densities and numbers of species of different types in the control and drawdown separately in each year with one-way ANOVA. Additionally, we analyzed effects of water level treatment for each aquaculture type in each year with one-way ANOVA. Densities were log(x + 1) transformed.
To assess responses of guilds to water-level and aquaculture types, we also used 3-way ANOVA to test the effects of guild, aquaculture type and water levels on density and the number of species in the data for each year. We also used Duncan tests for post-hoc analyses. For results with interaction effects, we first analyzed the effects of guilds and water levels on densities and numbers of species for each aquaculture types in each year with two-way ANOVA. Then, we compared densities and numbers of species of different guilds in the control and drawdown separately for each aquaculture type with one-way ANOVA. Additionally, we analyzed the effects of water level treatment in each bird guild for each aquaculture type with one-way ANOVA.
To understand factors related to bird density and number of species, we examined correlations among density, the number of species, bird guild, trawl biomass (including fish, shrimp and crab as well as snail separately) and pond area with Spearman rank analysis. Data of bird density and number of species were also from the same 10 consecutive surveys, because this period of concentrated usage may have a higher potential to respond to associated factors.
In addition, we compared densities of selected species among water depth ranges with one-way ANOVA. The selected species were Black-faced Spoonbills, Great Egrets, Little Egrets, Black-winged Stilts, Dunlins and Kentish Plovers, which represented the deep wader, shallow wader and shorebird guilds and had higher abundance than others in their respective guilds.
5. Conclusions
This study provides results of benefits of the seasonality of shallow-pond milkfish aquaculture for winter migratory water birds on the East Asian—Australasian Flyway. All types of aquaculture, densities and numbers of species of waders and shorebirds were much higher in these shallow drawdown ponds than in the control ponds. Densities and numbers of species of water birds increased with trawled estimated benthic biomass. The numbers for most selected waterbird species, such as Black-faced Spoonbills, also increased with lower water depths. Other areas may deserve further investigations. These include the effects of drawdown speeds on usage of water birds, waterbird usage of drawdown ponds in different seasons and whether maintaining constant low water levels can maximize Black-faced Spoonbill usage of ponds.
The results of our study provide a potential direction for managing a coastal landscape with harmony between aquaculture and water birds [
7]. We suggest the seasonality of shallow-pond milkfish aquaculture as a guideline for achieving this goal. Conservation policies and programs may use economic incentives to sustain water bird-friendly aquaculture. The involvement of stakeholder farmers is essential for creating a Satoumi landscape. Lastly, a public awareness campaign to encourage consumer involvement is also important.