Waste to Catalyst: Synthesis of Catalysts from Sewage Sludge of the Mining, Steel, and Petroleum Industries
Abstract
1. Introduction
2. Results
2.1. Samples Identification
2.2. Catalyst Preparation
2.3. Characterization of Chemical Properties of Sludge and Catalysts
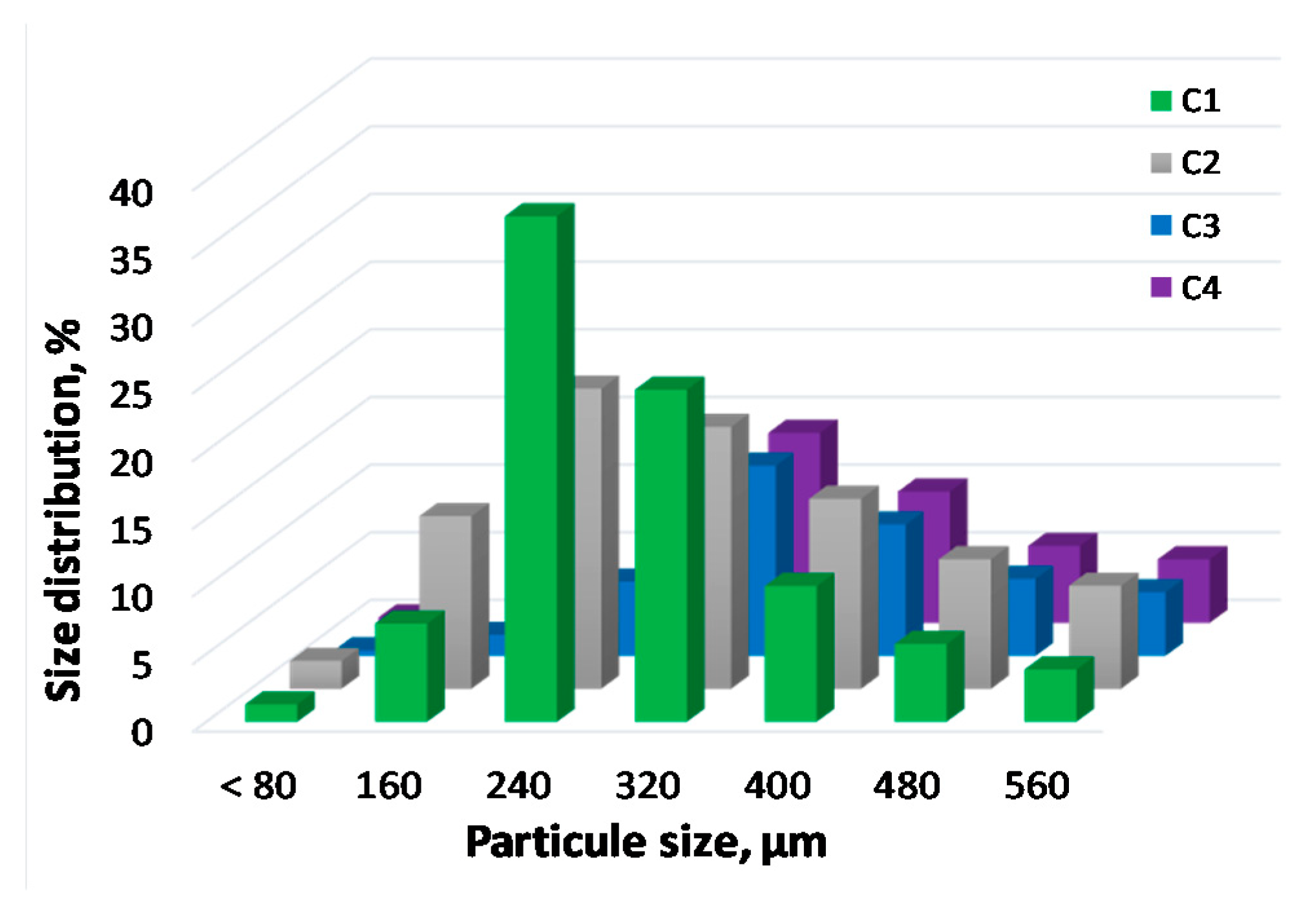
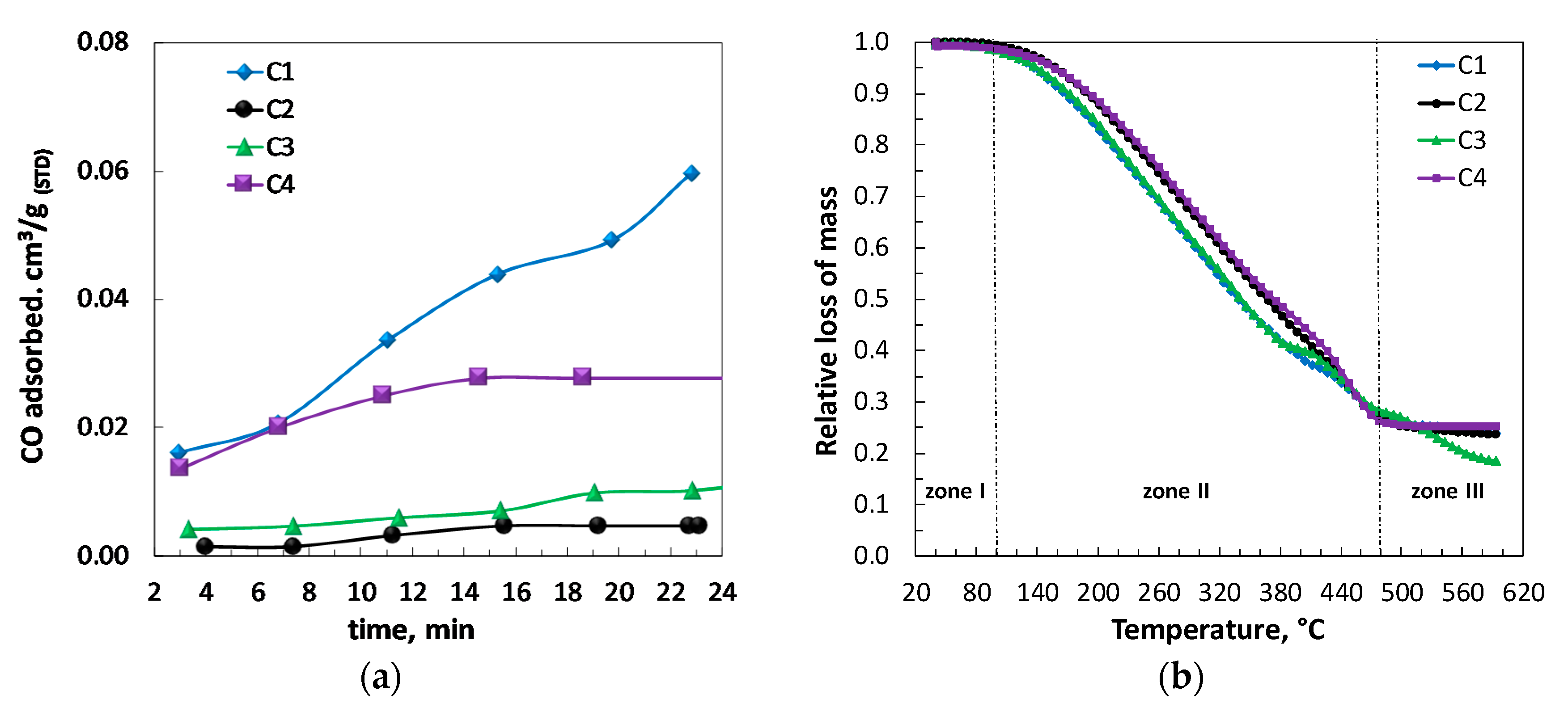
2.4. Physical and Physisorption Properties of Catalysts
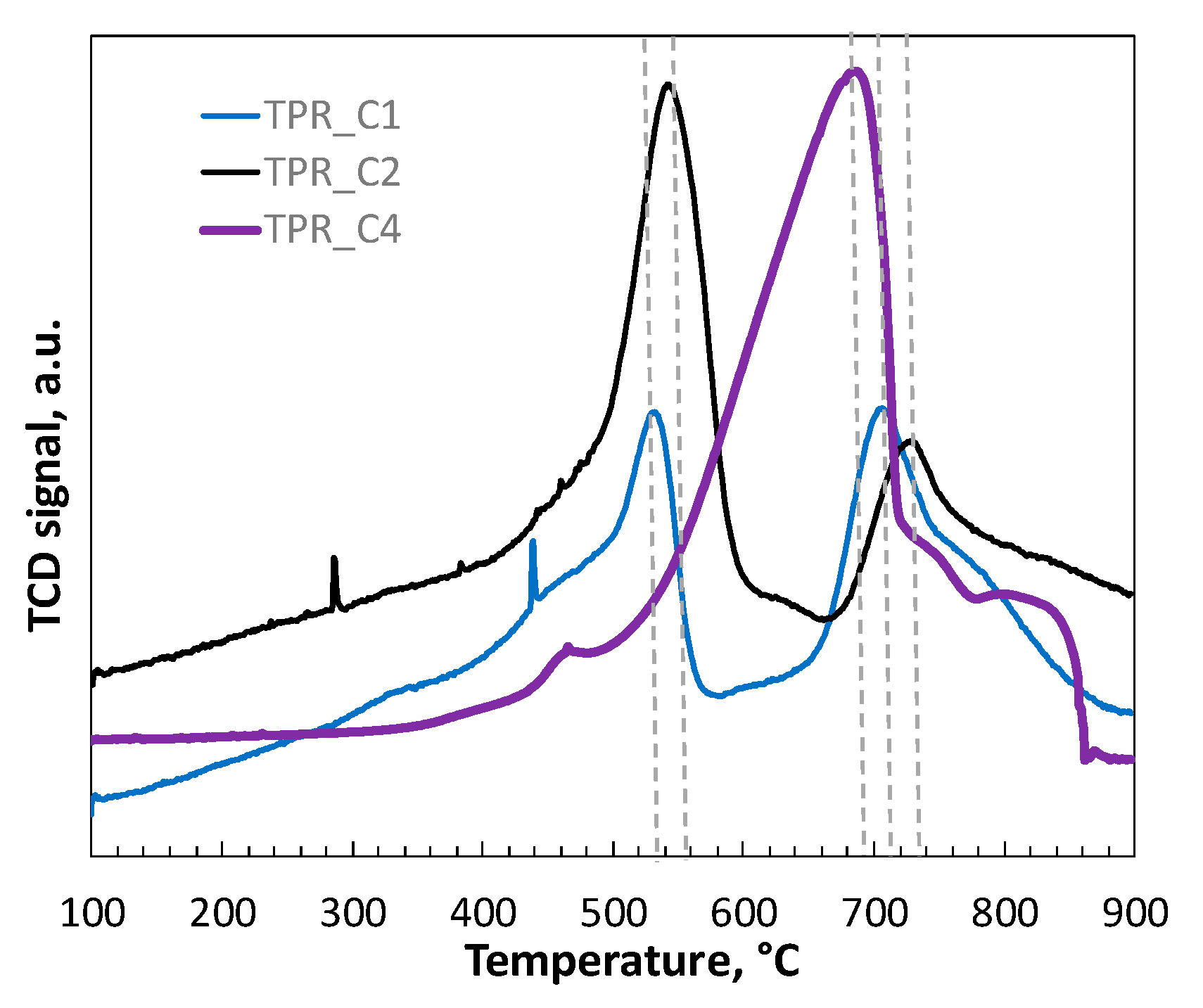
2.5. Temperature-Programmed Reduction (TPR) of Catalysts
2.6. Scanning Electron Microscope with Energy Dispersive X-ray Spectroscopy (SEM–EDS)
2.7. X-ray Diffraction (XRD)
2.8. Characterization Results of Thermoenergetic Properties of Synthesized Materials
- Cp: specific heat, kJ/kg∙K
- Φm sample heat flow rate, W
- Φ0: zero-line heat flow rate, W
- m: mass of sample, g
- β: heating rate, K/s
2.9. Catalytic Activity Evaluation
2.10. Synthesized Material Sustainability
3. Materials and Methods
3.1. Catalyst Characterization
3.2. Catalytic Material Evaluation (Catalytic Tests)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IEA World Energy Outlook—Topics. Available online: https://www.iea.org/topics/world-energy-outlook (accessed on 2 August 2020).
- Ministerio del Ambiente. Ecuador Acuerdo Ministerial 142 Sustancias Peligrosas; Ministerio del Ambiente: Quito, Ecuador, 2012. (In Spanish) [Google Scholar]
- Instituto Nacional de Estadística y Censos-Ecuador (INEC). Módulo Ambiental de la Encuesta Estructural Empresarial (ENESEM), año 2017; Ecuador en cifras: Quito, Ecuador, 2019. (In Spanish) [Google Scholar]
- UNACEM. Resumen del Informe Final de la Consultoria Para la Fase 1 del Libro Blanco de Economía Circular; UNACEM: Guayaquil, Ecuador, 2020. (In Spanish) [Google Scholar]
- United Nations Educational, Scientific and Cultural Organization. Informe Mundial de Naciones Unidas Sobre el Desarrollo de Los Recursos Hídricos 2019: No Dejar a Nadie Atrás; UN: New York, NY, USA, 2019; ISBN 978-92-1-004594-0. (In Spanish) [Google Scholar]
- Suárez-Macías, J.; Terrones-Saeta, J.M.; Iglesias-Godino, F.J.; Corpas-Iglesias, F.A. Retention of Contaminants Elements from Tailings from Lead Mine Washing Plants in Ceramics for Bricks. Minerals 2020, 10, 576. [Google Scholar] [CrossRef]
- Ince, C. Reusing gold-mine tailings in cement mortars: Mechanical properties and socio-economic developments for the Lefke-Xeros area of Cyprus. J. Clean. Prod. 2019, 238, 117871. [Google Scholar] [CrossRef]
- Yildirim Ozen, M.; Moroydor Derun, E. A comparative study: Effects of different nanoparticles on the properties of gold mine tailings containing cement mortars. Constr. Build. Mater. 2019, 202, 396–405. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, Y.; Kim, M.; Park, H. Preparation of high porosity bricks by utilizing red mud and mine tailing. J. Clean. Prod. 2019, 207, 490–497. [Google Scholar] [CrossRef]
- Wołowiec, M.; Komorowska-Kaufman, M.; Pruss, A.; Rzepa, G.; Bajda, T. Removal of Heavy Metals and Metalloids from Water Using Drinking Water Treatment Residuals as Adsorbents: A Review. Minerals 2019, 9, 487. [Google Scholar] [CrossRef]
- Liu, Y.; Khan, A.; Wang, Z.; Chen, Y.; Zhu, S.; Sun, T.; Liang, D.; Yu, H. Upcycling of Electroplating Sludge to Prepare Erdite-Bearing Nanorods for the Adsorption of Heavy Metals from Electroplating Wastewater Effluent. Water 2020, 12, 1027. [Google Scholar] [CrossRef]
- Ouyang, D.; Zhuo, Y.; Hu, L.; Zeng, Q.; Hu, Y.; He, Z. Research on the Adsorption Behavior of Heavy Metal Ions by Porous Material Prepared with Silicate Tailings. Minerals 2019, 9, 291. [Google Scholar] [CrossRef]
- Mombelli, D.; Barella, S.; Gruttadauria, A.; Mapelli, C. Iron Recovery from Bauxite Tailings Red Mud by Thermal Reduction with Blast Furnace Sludge. Appl. Sci. 2019, 9, 4902. [Google Scholar] [CrossRef]
- Ubaldini, S.; Guglietta, D.; Vegliò, F.; Giuliano, V. Valorization of Mining Waste by Application of Innovative Thiosulphate Leaching for Gold Recovery. Metals 2019, 9, 274. [Google Scholar] [CrossRef]
- Carneiro, J.; Tobaldi, D.M.; Capela, M.N.; Seabra, M.P.; Labrincha, J.A. Waste-Based Pigments for Application in Ceramic Glazes and Stoneware Bodies. Materials 2019, 12, 3396. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, C.; Chalouati, S.; Chai, Y.E.; Fantucci, H.; Santos, R.M. Valorization of Kimberlite Tailings by Carbon Capture and Utilization (CCU) Method. Minerals 2020, 10, 611. [Google Scholar] [CrossRef]
- Klose, F.; Scholz, P.; Kreisel, G.; Ondruschka, B.; Kneise, R.; Knopf, U. Catalysts from waste materials. Appl. Catal. B Environ. 2000, 28, 209–221. [Google Scholar] [CrossRef]
- Herrera, S.L.; Hoyos, D.Á.; Palacio, L.A.; Pizarro, J.L.; Aguado, R. Synthesis of Industrial Waste Based Metal Catalysts for Oxidative Dehydrogenation of Propane. Ind. Eng. Chem. Res. 2013, 52, 7341–7349. [Google Scholar] [CrossRef]
- Sanchis, R.; Dejoz, A.; Vázquez, I.; Vilarrasa-García, E.; Jiménez-Jiménez, J.; Rodríguez-Castellón, E.; López Nieto, J.M.; Solsona, B. Ferric sludge derived from the process of water purification as an efficient catalyst and/or support for the removal of volatile organic compounds. Chemosphere 2019, 219, 286–295. [Google Scholar] [CrossRef]
- Yi, X.; Yu, Y.; Huang, F.; Ding, T.; Zhang, Z.; Feng, J.; Baell, J.B.; Huang, H. Turning Waste into Valuable Catalysts: Application of Surface-Modified Sewage Sludge in N–H Insertion Reaction. Ind. Eng. Chem. Res. 2020, 59, 4854–4863. [Google Scholar] [CrossRef]
- Montero, C.; Castañeda, K.; Oña, M.; Flores, D.R.; De La Rosa, A. Catalyst Based on Sludge Derived from Wastewater Treatment of Textile Industry. Chem. Eng. Trans. 2018, 70, 931–936. [Google Scholar] [CrossRef]
- Villamarin-Barriga, E.; Canacuán, J.; Londoño-Larrea, P.; Solís, H.; De La Rosa, A.; Saldarriaga, J.F.; Montero, C. Catalytic Cracking of Heavy Crude Oil over Iron-Based Catalyst Obtained from Galvanic Industry Wastes. Catalysts 2020, 10, 736. [Google Scholar] [CrossRef]
- Colta Ponce, D.X.; Quishpe Bahamontes, L.V. Síntesis de un Material Catalítico a Partir de Lodos Residuales de la Industria de la Curtiembre. Bachelor’s Thesis, Facultad de Ingeniería Química, Universidad Central del Ecuador, Quito, Ecuador, 2017. (In Spanish). [Google Scholar]
- Moreno Moyano, S.K. Descomposición Catalítica de CH4 Utilizando Catalizadores Derivados de lodos Residuales de las Industrias: Textil, Galvanoplastia y Curtiembre. Bachelor’s Thesis, Facultad de Ingeniería Química, Universidad Central del Ecuador, Quito, Ecuador, 2020. (In Spanish). [Google Scholar]
- Xu, B.; Ding, T.; Zhang, Y.; Wen, Y.; Yang, Z.; Zhang, M. A new efficient visible-light-driven composite photocatalyst comprising ZnFe2O4 nanoparticles and conjugated polymer from the dehydrochlorination of polyvinyl chloride. Mater. Lett. 2017, 187, 123–125. [Google Scholar] [CrossRef]
- Gonzalez-Casamachin, D.A.; Rivera De la Rosa, J.; Lucio–Ortiz, C.J.; Sandoval-Rangel, L.; García, C.D. Partial oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid using O2 and a photocatalyst of a composite of ZnO/PPy under visible-light: Electrochemical characterization and kinetic analysis. Chem. Eng. J. 2020, 393, 124699. [Google Scholar] [CrossRef]
- Halasi, G.; Gazsi, A.; Bánsági, T.; Solymosi, F. Catalytic and photocatalytic reactions of H2+CO2 on supported Au catalysts. Appl. Catal. A Gen. 2015, 506, 85–90. [Google Scholar] [CrossRef]
- Zuo, C.; Tian, Y.; Zheng, Y.; Wang, L.; Fu, Z.; Jiao, T.; Wang, M.; Huang, H.; Li, Y. One step oxidative esterification of methacrolein with methanol over Au-CeO2/γ-Al2O3 catalysts. Catal. Commun. 2019, 124, 51–55. [Google Scholar] [CrossRef]
- Liao, X.; Chu, W.; Dai, X.; Pitchon, V. Bimetallic Au–Cu supported on ceria for PROX reaction: Effects of Cu/Au atomic ratios and thermal pretreatments. Appl. Catal. B Environ. 2013, 142–143, 25–37. [Google Scholar] [CrossRef]
- Torres-Luna, J.A.; Carriazo, J.G.; Sanabria-González, N.R. Calcination Temperature Effect on structural and textural properties of Fe(iii)-TiO2. Rev. Fac. Cienc. Básicas 2014, 10, 186–195. [Google Scholar] [CrossRef]
- Guerrero Fajardo, C.A.; Sánchez Castellanos, F.J.; Roger, A.-C.; Courson, C. Síntesis sol-gel de catalizadores de hierro soportados sobre sílice y titania para la oxidación selectiva de metano hasta formaldehído. Ing. Investig. 2008, 28, 72–80. (In Spanish) [Google Scholar]
- Junior, S.A.F.; de Sousa, J.F.; Benachour, M.; Rojas, L.O.A. Wet Oxidation of Phenols using Fe-CeO2, K-MnO2/CeO2/Paligorskite and Fe/Palygorskite Catalysts. Inf. Technol. 2011, 22, 55–68. [Google Scholar] [CrossRef]
- Guerrero Fajardo, C.A.; Sánchez Castellanos, F.J. Síntesis de catalizadores de Fe-Mo soportados sobre sílice para la oxidación selectiva de metano hasta formaldehído. Ing. Investig. 2009, 29, 53–59. (In Spanish) [Google Scholar]
- Santos, J.L.; Reina, T.R.; Ivanov, I.; Penkova, A.; Ivanova, S.; Tabakova, T.; Centeno, M.A.; Idakiev, V.; Odriozola, J.A. Multicomponent Au/Cu-ZnO-Al2O3 catalysts: Robust materials for clean hydrogen production. Appl. Catal. A Gen. 2018, 558, 91–98. [Google Scholar] [CrossRef]
- Mierczynski, P.; Vasilev, K.; Mierczynska, A.; Maniukiewicz, W.; Szynkowska, M.I.; Maniecki, T.P. Bimetallic Au–Cu, Au–Ni catalysts supported on MWCNTs for oxy-steam reforming of methanol. Appl. Catal. B Environ. 2016, 185, 281–294. [Google Scholar] [CrossRef]
- Sun, Z.-X.; Zheng, T.-T.; Bo, Q.-B.; Du, M.; Forsling, W. Effects of calcination temperature on the pore size and wall crystalline structure of mesoporous alumina. J. Colloid Interface Sci. 2008, 319, 247–251. [Google Scholar] [CrossRef]
- Zubaidy, E.A.H.; Abouelnasr, D.M. Fuel recovery from waste oily sludge using solvent extraction. Process Saf. Environ. Prot. 2010, 88, 318–326. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R. Sulfur Poisoning. In Progress in Catalyst Deactivation; Figueiredo, J.L., Ed.; Springer: Dordrecht, The Netherlands, 1982; pp. 209–227. [Google Scholar]
- Zhu, F.; Jiang, H.; Zhang, Z.; Zhao, L.; Wang, J.; Hu, J.; Zhang, H. Research on Drying Effect of Different Additives on Sewage Sludge. Procedia Environ. Sci. 2012, 16, 357–362. [Google Scholar] [CrossRef][Green Version]
- Xia, Y.; Zhan, W.; Guo, Y.; Guo, Y.; Lu, G. Fe-Beta zeolite for selective catalytic reduction of NOx with NH3: Influence of Fe content. Chin. J. Catal. 2016, 37, 2069–2078. [Google Scholar] [CrossRef]
- Issangya, A.; Hays, R.; Cocco, R.; Knowlton, T.; Karry, R.S. An Acoustic Method for the Measurement of Minimum Fluidization and Bubbling Properties of Group a Solids; AIChE: San Francisco, CA, USA, 2013. [Google Scholar]
- Chun, D.H.; Park, J.C.; Lee, H.-T.; Yang, J.-I.; Hong, S.; Jung, H. Effects of SiO2 Incorporation Sequence on the Catalytic Properties of Iron-Based Fischer–Tropsch Catalysts Containing Residual Sodium. Catal. Lett. 2013, 143, 1035–1042. [Google Scholar] [CrossRef]
- Fakeeha, A.; Khan, W.; Ibrahim, A.; Al-Otaibi, R.; Alfatesh, A.; Soliman, M.; Abasaeed, A. Alumina supported iron catalyst for hydrogen production: Calcination study. Int. J. Adv. Chem. Eng. Biol. Sci. 2015, 2, 139–141. [Google Scholar] [CrossRef]
- National Institute for Materials Science NIMS Materials Database (MatNavi). Available online: https://mits.nims.go.jp/index_en.html (accessed on 12 May 2020).
- Rojas-Aguilar, A.; Ginez-Carbajal, F.; Orozco-Guareńo, E.; Flores-Segura, H. Measurement of enthalpies of vaporization of volatile heterocyclic compounds by DSC. J. Therm. Anal. Calorim. 2005, 79, 95–100. [Google Scholar] [CrossRef]
- Pérez, M.C.R.; Herrera, J.A.P.; Priego, F.J.A. Estudio De La Descomposición Térmica De Los Escombros Lateríticos De Moa. Rev. Cuba. Quím. 2005, 17, 104–110. (In Spanish) [Google Scholar]
- Findikakis, A. Heat Capacity Analysis Report; Yucca Mountain Project: Las Vegas, NV, USA, 2004. [Google Scholar]
- Twigg, M.V. Catalyst Handbook; Routledge: Abingdon, UK, 2018; ISBN 978-1-315-13886-2. [Google Scholar]
- Lemire, R.; Palmer, D.; Taylor, P.; Schlenz, H. Chemical Thermodynamics—Chemical Thermodynamics of Iron Part 2; OECD: Paris, France, 2020; Volume 13b. [Google Scholar]
- Dicks, A.P.; Hent, A. The E Factor and Process Mass Intensity. In Green Chemistry Metrics: A Guide to Determining and Evaluating Process Greenness; Dicks, A.P., Hent, A., Eds.; SpringerBriefs in Molecular Science; Springer International Publishing: Cham, Switzerland, 2015; pp. 45–67. ISBN 978-3-319-10500-0. [Google Scholar]
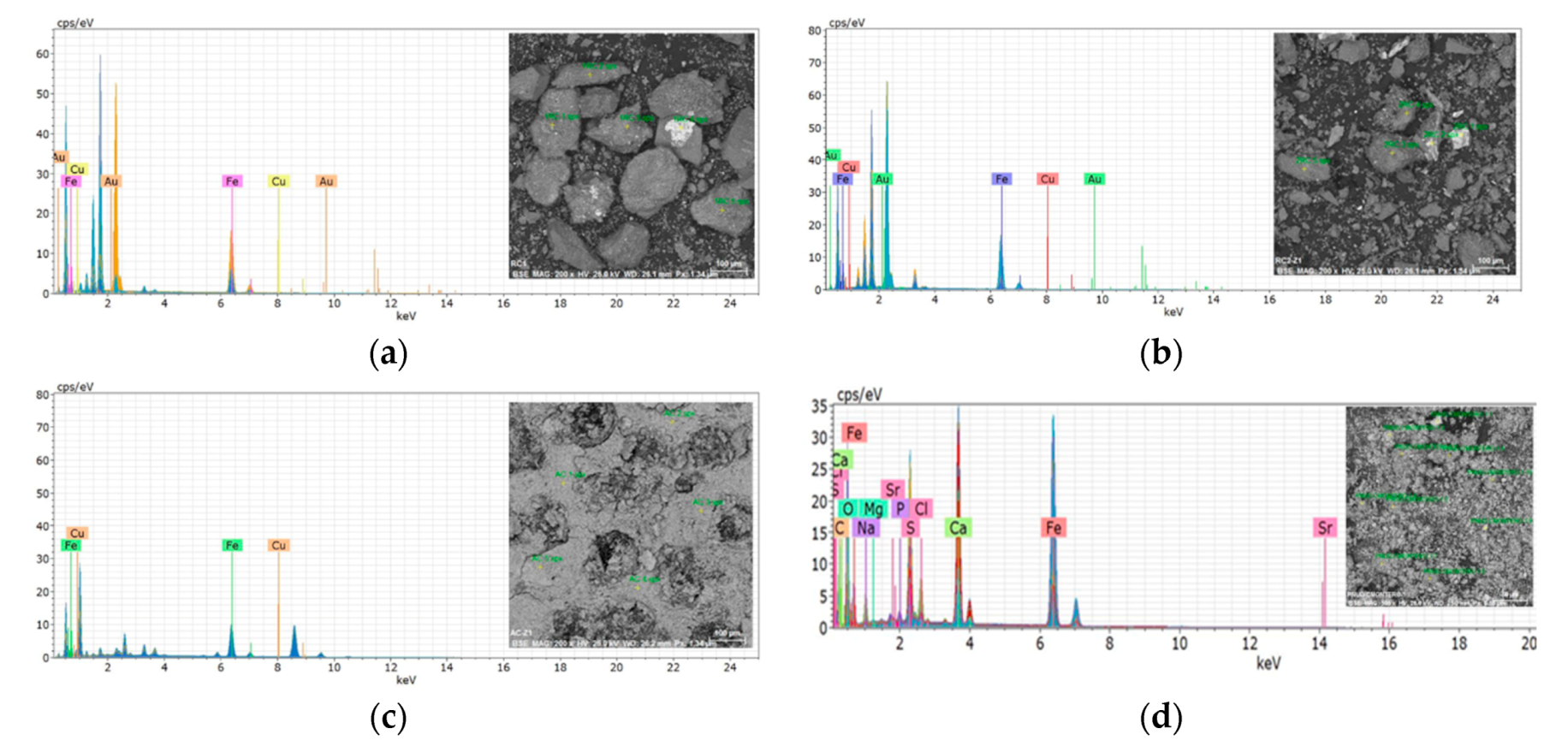
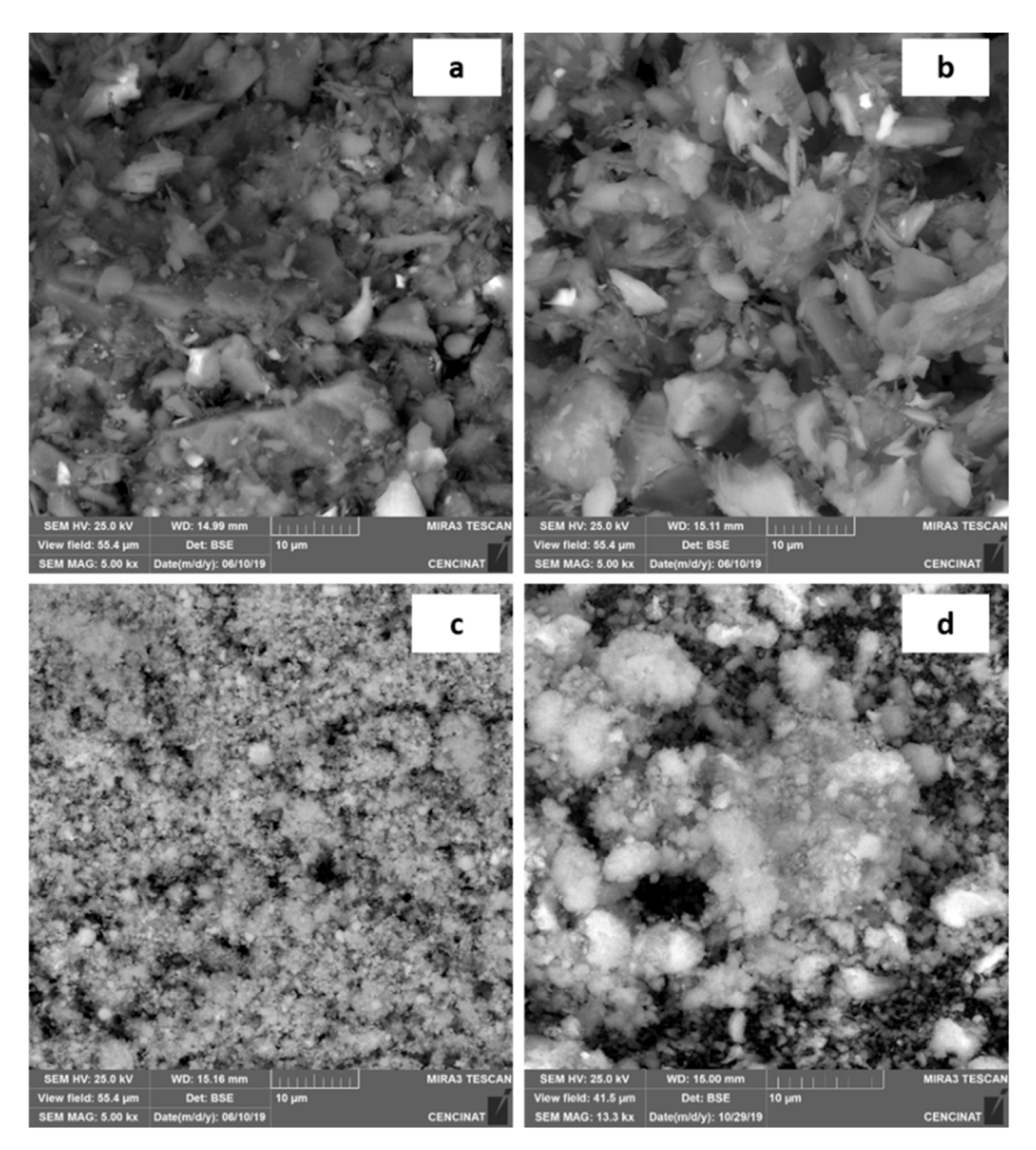
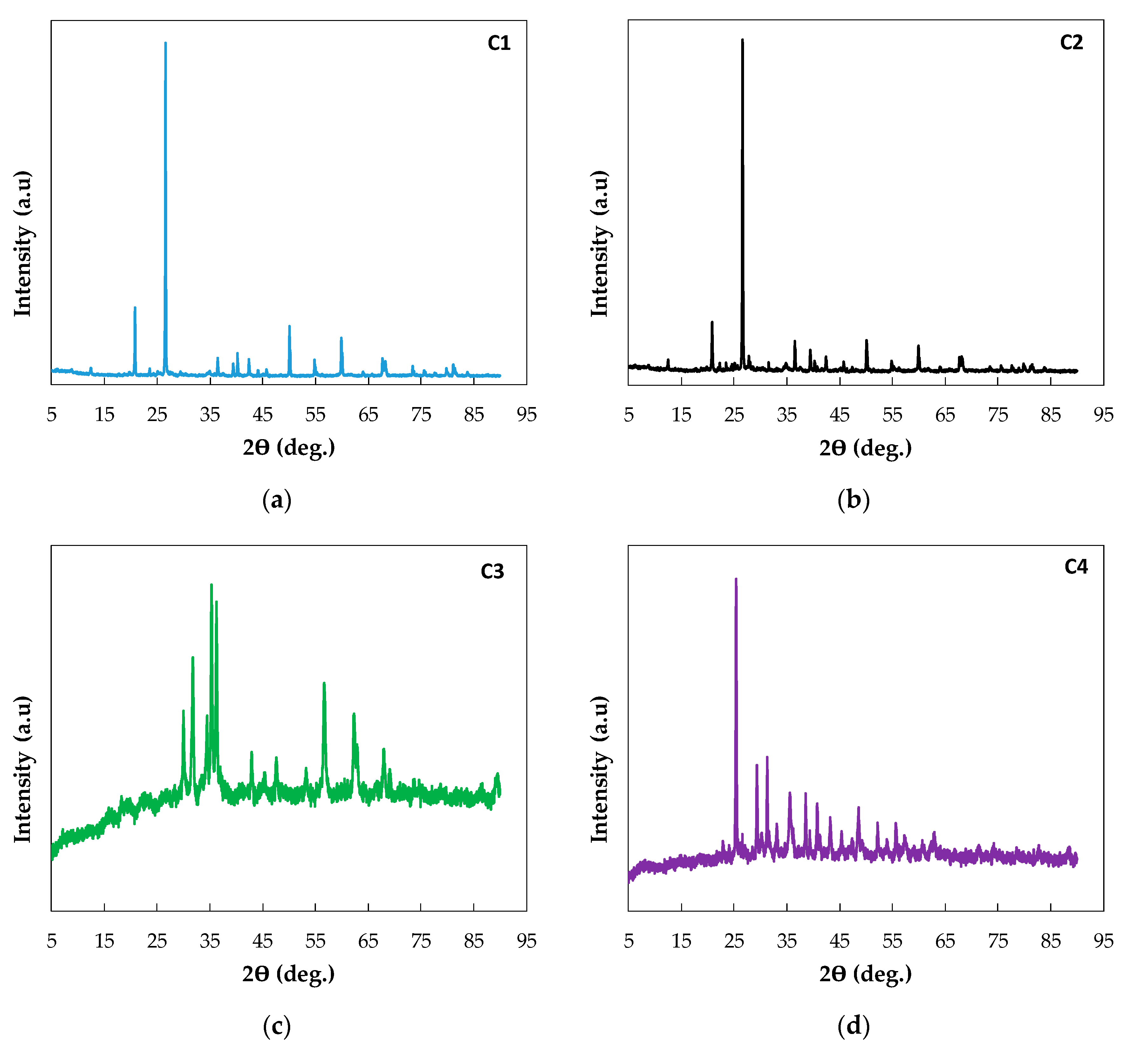
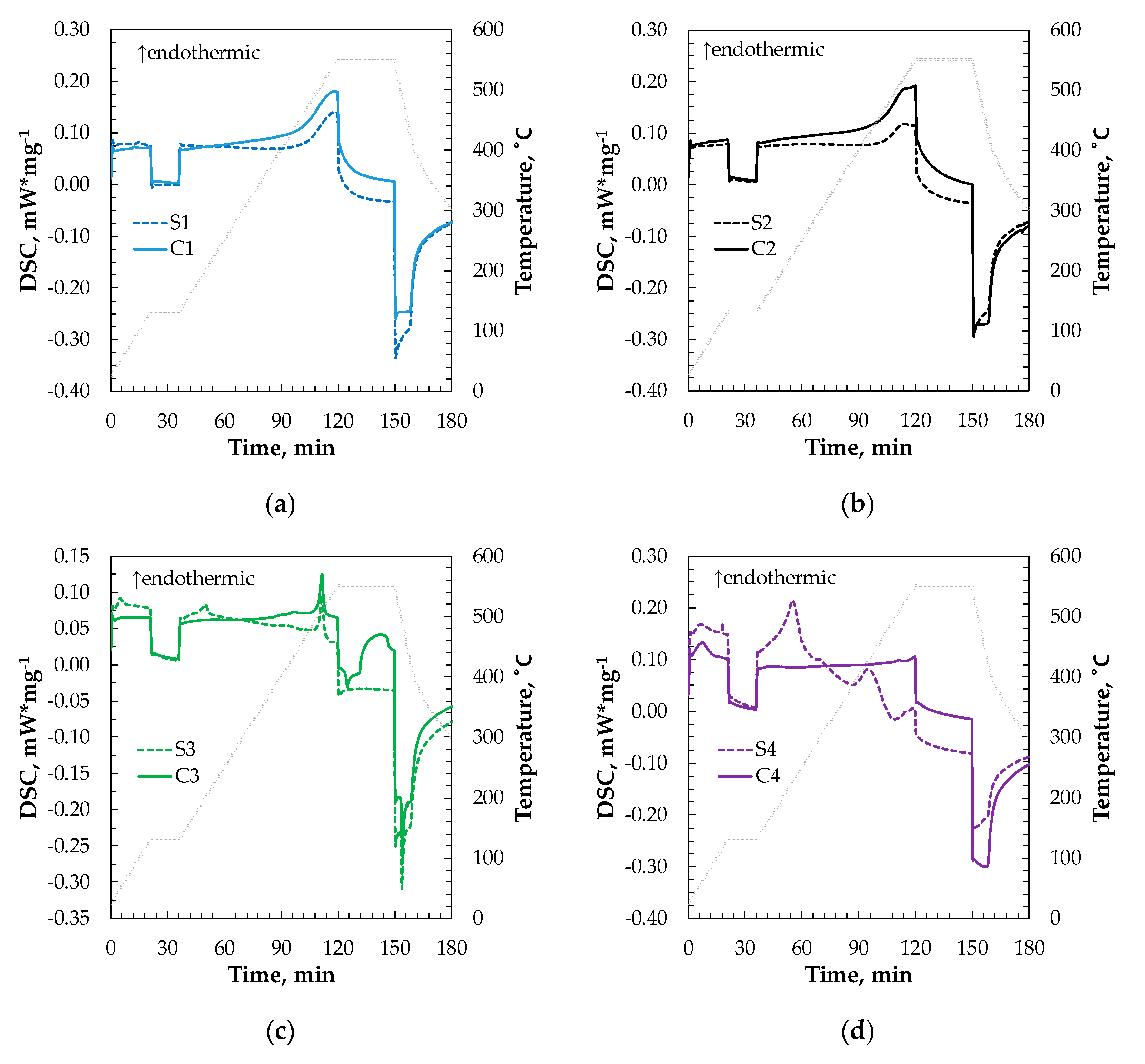
| Company | Type of Sludge | Sludge | Catalytic Material |
|---|---|---|---|
| 1a | Aged mining tailings | S1 | C1 |
| 1b | Fresh mining tailings | S2 | C2 |
| 2 | Wastewater sludge from a steelwork | S3 | C3 |
| 3 | Bottom sludge from petroleum storage tanks | S4 | C4 |
| Company | Catalytic Material | Drying Temperature, °C | Drying Time, h | Calcination Temperature, °C | Calcination Time, h |
|---|---|---|---|---|---|
| 1a | C1 | 110 | 4.5 | 300 | 4 |
| 1b | C2 | 120 | 4.5 | 300 | 4 |
| 2 | C3 | 120 | 4.5 | 500 | 4 |
| 3 | C4 | 130 | 4.5 | 450 | 2 |
| Sample | Nitrogen, wt% | Carbon, wt% | Hydrogen, wt% | Sulfur, wt% |
|---|---|---|---|---|
| S1 | 0.0 | 0.5 | 0.1 | 3.6 |
| C1 | 0.0 | 0.3 | 0.1 | 2.0 |
| S2 | 0.0 | 0.2 | 0.1 | 3.4 |
| C2 | 0.0 | 0.2 | 0.1 | 2.9 |
| S3 | 0.0 | 1.0 | 0.1 | 1.0 |
| C3 | 0.0 | 0.1 | 0.1 | 0.9 |
| S4 | 0.4 | 35.9 | 5.0 | 7.4 |
| C4 | 0.0 | 5.2 | 0.1 | 4.4 |
| Metal | Sample | wt% | Sample | wt% |
|---|---|---|---|---|
| Fe | S1 | 3.7 ± 0.5 | C1 | 3.2 ± 0.5 |
| Fe | S2 | 3.5 ± 0.5 | C2 | 3.8 ± 0.5 |
| Fe | S3 | 7.6 ± 0.5 | C3 | 7.5 ± 0.5 |
| Fe | S4 | 2.6 ± 0.5 | C4 | 2.5 ± 0.5 |
| Catalyst | Specific Surface, m2 g−1 |
|---|---|
| C1 | 4.25 ± 0.04 |
| C2 | 3.03 ± 0.04 |
| C3 | 7.97 ± 0.04 |
| C4 | 16.78 ± 0.04 |
| Sludge | Cp, kJ/kg K | Catalysts | Cp, kJ/kg K |
|---|---|---|---|
| S1 | 0.992 | C1 | 1.155 |
| S2 | 1.018 | C2 | 1.192 |
| S3 | 0.620 | C3 | 0.700 |
| S4 | 1.434 | C4 | 0.951 |
| Samples | Loss of Mass, % | Kinetic Expression | R2 | Rate of Reaction (avg), mgoil. (min.mgFe)−1 |
|---|---|---|---|---|
| Reaction without catalyst | 55.31 | 0.921 | -- | |
| Reaction + C1 | 70.38 | 0.948 | 0.18 | |
| Reaction + C2 | 69.13 | 0.933 | 0.14 | |
| Reaction + C3 | 69.25 | 0.922 | 0.07 | |
| Reaction + C4 | 66.89 | 0.919 | 0.20 | |
| Reaction with commercial catalyst | 62.29 | 0.950 | -- |
| Samples | Initial Sludge Mass, g | Moisture and Volatile Loss, wt% | Organic Loss, wt% | Yield of Catalytic Material, wt% | E-Factor |
|---|---|---|---|---|---|
| 1 | 100.64 | 0.00 | 0.32 | 99.68 | 0.003 |
| 2 | 99.77 | 10.32 | 0.68 | 88.99 | 0.124 |
| 3 | 100.49 | 0.39 | 2.14 | 97.47 | 0.026 |
| 4 | 100.37 | 8.94 | 16.06 | 75.00 | 0.333 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-León, G.; Baquero-Quinteros, E.; Loor, B.G.; Alvear, J.; Montesdeoca Espín, D.E.; De La Rosa, A.; Montero-Calderón, C. Waste to Catalyst: Synthesis of Catalysts from Sewage Sludge of the Mining, Steel, and Petroleum Industries. Sustainability 2020, 12, 9849. https://doi.org/10.3390/su12239849
Castro-León G, Baquero-Quinteros E, Loor BG, Alvear J, Montesdeoca Espín DE, De La Rosa A, Montero-Calderón C. Waste to Catalyst: Synthesis of Catalysts from Sewage Sludge of the Mining, Steel, and Petroleum Industries. Sustainability. 2020; 12(23):9849. https://doi.org/10.3390/su12239849
Chicago/Turabian StyleCastro-León, Gabriela, Erik Baquero-Quinteros, Bryan G. Loor, Jhoselin Alvear, Diego E. Montesdeoca Espín, Andrés De La Rosa, and Carolina Montero-Calderón. 2020. "Waste to Catalyst: Synthesis of Catalysts from Sewage Sludge of the Mining, Steel, and Petroleum Industries" Sustainability 12, no. 23: 9849. https://doi.org/10.3390/su12239849
APA StyleCastro-León, G., Baquero-Quinteros, E., Loor, B. G., Alvear, J., Montesdeoca Espín, D. E., De La Rosa, A., & Montero-Calderón, C. (2020). Waste to Catalyst: Synthesis of Catalysts from Sewage Sludge of the Mining, Steel, and Petroleum Industries. Sustainability, 12(23), 9849. https://doi.org/10.3390/su12239849





