Improved Production of Kynurenic Acid by Yarrowia lipolytica in Media Containing Different Honeys
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Conditions of Cultures
2.3. Determination of Dried Yeast Biomass
2.3.1. KYNA Detection via HPLC Method
2.3.2. Analysis of Protein Content and Amino Acids Profile in Yeast Biomass
2.3.3. Analysis of Fatty Acids in Yeast Biomass
2.3.4. Statistical Analysis
3. Results and Discussion
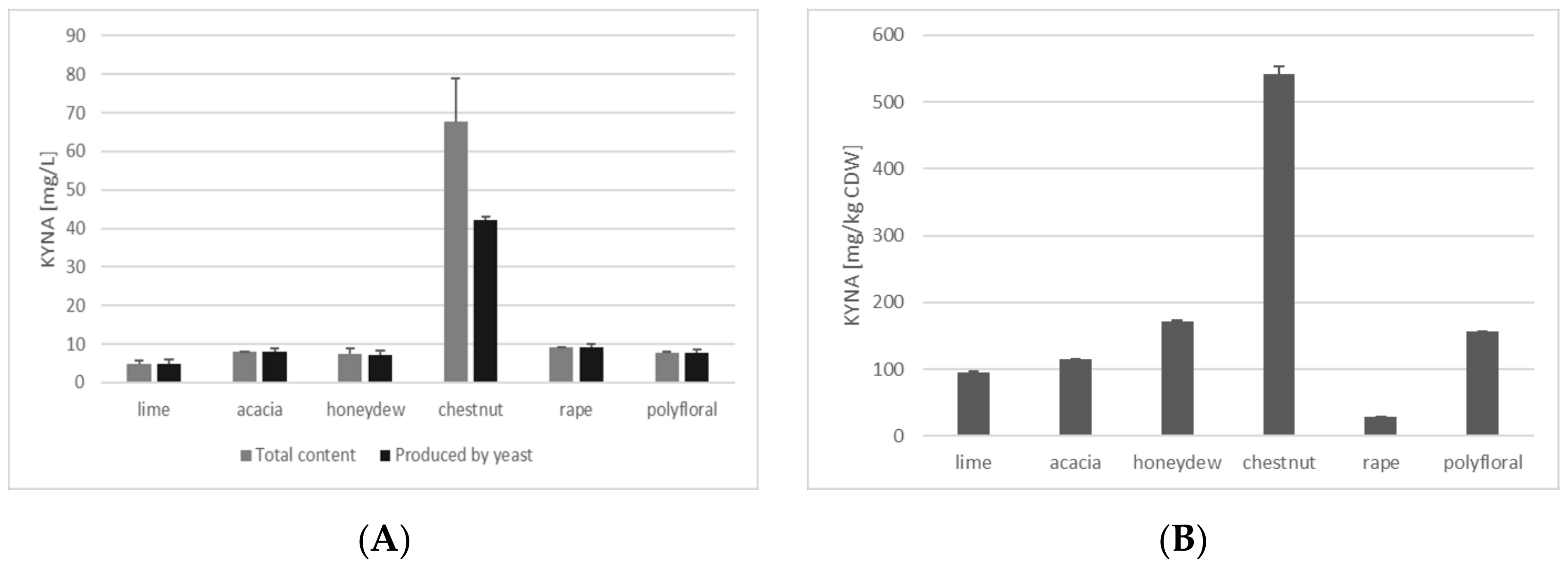
3.1. KYNA Production in Media Containing Different Honeys
3.2. Analysis of Sugar in Medium Containing Chestnut Honey
3.3. Analysis of Lipid Profile of Yeast Producing KYNA
3.4. Protein Content and Amino Acids Profile of Yeast Y. lipolytica S12 Producing KYNA
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gemperlein, K.; Dietrich, D.; Kohlstedt, M.; Zipf, G.; Bernauer, H.S.; Wittmann, C.; Wenzel, S.C.; Müller, R. Polyunsaturated fatty acid production by Yarrowia lipolytica employing designed myxobacterial PUFA synthases. Nat. Commun. 2019, 10, 4055. [Google Scholar] [CrossRef] [PubMed]
- Juszczyk, P.; Rymowicz, W. Characterization of Microbial Biomass Production from Glyc-Erin Waste by Various Yeast Strains; Aggelis, G., Ed.; Microbial Conversions of Raw Glycerol; Nova Science Publishers: New York, NY, USA, 2009; pp. 125–135. [Google Scholar]
- Rakicka, M.; Wolniak, J.; Lazar, Z.; Rymowicz, W. Production of high titer of citric acid from inulin. BMC Biotechnol. 2019, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Rywińska, A.; Juszczyk, P.; Wojtatowicz, M.; Rymowicz, W. Chemostat study of citric acid production from glycerol by Yarrowia lipolytica. J. Biotechnol. 2011, 152, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Mirończuk, A.M.; Biegalska, A.; Dobrowolski, A. Functional overexpression of genes in-volved in erythritol synthesis in the yeast Yarrowia lipolytica. Biotechnol. Biofuels 2017, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Wróbel-Kwiatkowska, M.; Turski, W.; Kocki, T.; Rakicka-Pustułka, M.; Rymowicz, W. An efficient method for production of kynurenic acid by Yarrowia lipolytica. Yeast 2020, 1–7. [Google Scholar] [CrossRef]
- Walczak, K.; Wnorowski, A.; Turski, W.A.; Plech, T. Kynurenic acid and cancer: Facts and controversies. Cell. Mol. Life Sci. 2020, 77, 1531–1550. [Google Scholar] [CrossRef]
- Walczak, K.; Turski, W.A.; Rajtar, G. Kynurenic acid inhibits colon cancer proliferation in vitro: Effects on signaling pathways. Amino Acids 2014, 46, 2393–2401. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Cai, T.; Tagle, D.A.; Li, J. Structure, expression, and function of kynurenine aminotransferases in human and rodent brains. Cell. Mol. Life Sci. 2010, 67, 353–368. [Google Scholar] [CrossRef]
- Dhakar, N.K.; Caldera, F.; Bessone, F.; Cecone, C.; Pedrazzo, A.R.; Cavalli, R.; Dianzani, C.; Trotta, F. Evaluation of solubility enhancement, antioxidant activity, and cytotoxicity studies of kynurenic acid loaded cyclodextrin nanosponge. Carbohydr. Polym. 2019, 224, 115168. [Google Scholar] [CrossRef]
- Nicaud, J.M. Yarrowia lipolytica. Yeast 2012, 29, 409–418. [Google Scholar] [CrossRef]
- Rywińska, A.; Juszczyk, P.; Wojtatowicz, M.; Robak, M.; Lazar, Z.; Tomaszewska, L.; Rymowicz, W. Glycerol as a promising substrate for Yarrowia lipolytica biotechnological applications. Biomass Bioenerg. 2013, 48, 148–166. [Google Scholar] [CrossRef]
- Turski, M.P.; Chwil, S.; Turska, M.; Chwil, M.; Kocki, T.; Rajtar, T.; Parada-Turska, J. An exceptionally high content of kynurenic acid in chestnut honey and flowers of chestnut tree. J. Food Compos. Anal. 2016, 48, 67–72. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 16th ed.; AOAC: Gaithersburg, MD, USA, 1996. [Google Scholar]
- Simpson, R.J.; Neuberger, M.R.; Lin, T.Y. Complete amino acid analysis of proteins from a single hydrolysate. J. Biol. Chem. 1976, 251, 1936–1940. [Google Scholar] [PubMed]
- Spackman, D.H.; Stein, W.H.; Moore, S. Automatic recording apparatus for use in the chromatography amino acid. Anal. Chem. 1958, 30, 1190–1200. [Google Scholar] [CrossRef]
- Browse, J.; McCourt, P.J.; Somerville, C.R. Fatty acid composition of leaf lipids determined after combined digestion and fatty acid methyl ester formation from fresh tissue. Anal. Biochem. 1986, 152, 141–145. [Google Scholar] [CrossRef]
- Yılmaz, C.; Gökmen, V. Determination of tryptophan derivatives in kynurenine pathway in fermented foods using liquid chromatography tandem mass spectrometry. Food Chem. 2018, 243, 420–427. [Google Scholar] [CrossRef]
- Zgrajka, W.; Turska, M.; Rajtar, G.; Majdan, M.; Parada-Turska, J. Kynurenic acid content in anti-rheumatic herbs. Ann. Agric. Environ. Med. 2013, 20, 800–802. [Google Scholar]
- Turski, M.P.; Kamiński, P.; Zgrajka, W.; Turska, M.; Turski, W.A. Potato—An important source of nutritional kynurenic acid. Plant Foods Hum. Nutr. 2012, 67, 17–23. [Google Scholar] [CrossRef]
- Gao, R.; Li, Z.; Zhou, X.; Bao, W.; Cheng, S.; Zheng, L. Enhanced lipid production by Yarrowia lipolytica cultured with synthetic and waste-derived high-content volatile fatty acids under alkaline conditions. Biotechnol. Biofuels 2020, 13, 3. [Google Scholar] [CrossRef]
- Arous, F.; Azabou, S.; Jaouani, A.; Zouari-Mechichi, H.; Nasri, M.; Mechichi, T. Biosynthe-sis of single-cell biomass from olive mill wastewater by newly isolated yeasts. Environ. Sci. Pollut. Res. 2016, 23, 6783–6792. [Google Scholar] [CrossRef]
- Jach, M.; Baj, T.; Juda, M.; Świder, R.; Mickowska, B.; Malm, A. Statistical evaluation of growth parameters in biofuel waste as a culture medium for improved production of single cell protein and amino acids by Yarrowia lipolytica. AMB Express 2020, 35, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Boze, H.; Moulin, G.; Galzy, P. Production of food and fodder yeasts. Crit. Rev. Biotechnol. 1992, 12, 65–86. [Google Scholar] [CrossRef] [PubMed]
- Juszczyk, P.; Tomaszewska, L.; Kita, A.; Rymowicz, W. Biomass production by novel strains of Yarrowia lipolytica using raw glycerol, derived from biodiesel production. Bioresour. Technol. 2013, 137, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Michalik, B.; Biel, W.; Lubowicki, R.; Jacyno, E. Chemical composition and biological value of proteins of the yeast Yarrowia lipolytica growing on industrial glycerol. Can. J. Anim. Sci. 2014, 94, 99–104. [Google Scholar] [CrossRef][Green Version]
- Duarte, S.H.; De Andrade, C.C.P.; Ghiselli, G.; Maugeri, F. Exploration of Brazilian biodi-versity and selection of a new oleaginous yeast strain cultivated in raw glycerol. Bioresour. Technol. 2013, 138, 377–381. [Google Scholar] [CrossRef]
- Juszczyk, P.; Rymowicz, W.; Kita, A.; Rywińska, A. Biomass production by Yarrowia lip-olytica yeast using waste derived from the production of ethyl esters of polyunsaturated fatty acids of flaxseed oil. Ind. Crop. Prod. 2019, 138, 111590. [Google Scholar] [CrossRef]
- Stone, C.W. Yeast Products in the Feed Industry: A Practical Guide for Feed Professionals; Diamond V Mills, Inc.: Cedar Rapids, IA, USA, 1998. [Google Scholar]
- Rodrìguez-Flores, S.; Escuredo, O.; Seijo, M.C. Characterization and antioxidant capacity of sweet chestnut honey produced in North-West Spain. J. Apic. Sci. 2016, 60, 19–29. [Google Scholar] [CrossRef]
- Ronsisvalle, S.; Lissandrello, E.; Fuochi, V.; Petronio, G.; Straquadanio, C.; Crascì, L.; Panico, A.; Milito, M.; Cova, A.M.; Tempera, G.; et al. Antioxidant and antimicrobial properties of Casteanea sativa Miller chestnut honey produced on Mount Etna (Sicily). Nat. Prod. Res. 2019, 33, 843–850. [Google Scholar] [CrossRef]
| Time [h] | Fructose [g/L] | Glucose [g/L] |
|---|---|---|
| 0 | 13.96 | 7.85 |
| 24 | 4.21 | n.d. |
| 48 | n.d. | n.d. |
| 72 | n.d. | n.d. |
| 96 | n.d. | n.d. |
| 120 | n.d. | n.d. |
| 168 | n.d. | n.d. |
| Product | KYNA Content (µg/g) | Reference |
|---|---|---|
| Yeast biomass (Y. lipolytica S12) | 542 | present study |
| Culture broth (Y. lipolytica S12) | 67.67 * | present study |
| Italian chestnut honey | 576 | [10] |
| Basil (leaves) | 14.08 | [19] |
| Thyme (leaves, branches) | 8.87 | [19] |
| Cacao powder | 4.48 | [18] |
| Peppermint (leaves) | 3.82 | [19] |
| Parsley (leaves) | 0.76 | [19] |
| Cumin (seeds) | 0.64 | [19] |
| Potato tubers | 0.24–3.24 | [10] |
| Kefir | 0.17 | [18] |
| Fatty Acid | Lipid Number | S12 | ||
|---|---|---|---|---|
| 40 g/L Fructose A | 80 g/L Fructose B | 50 g/L Chestnut Honey C | ||
| Palmitic acid | 16:0 | 9.98 ± 0.414 BC | 8.17 ± 0.076 AC | 26.93 ± 0.174 AB |
| Palmitoleic acid | 16:1 | 5.47 ± 0.214 C | 5.30 ± 0.131 C | 12.52 ± 0.182 AB |
| Stearic acid | 18:0 | 7.11 ± 0.032 BC | 5.37 ± 0.094 AC | 14.41 ± 0.018 AB |
| Oleic acid | 18:1 | 33.13 ± 0.142 BC | 55.54 ± 0.056 AC | 31.18 ± 0.075 AB |
| Linoleic acid | 18:2 | 13.67 ± 0.165 C | 13.10 ± 0.213 C | 9.27 ± 0.143 AB |
| Linolenic acid | 18:3 | 3.93 ± 0.045 B | 1.14 ± 0.011 A | n.d. |
| Gadoleic acid | 20:1 | 5.01 ± 0.187 C | 6.03 ± 0.076 C | 2.28 ± 0.087 AB |
| Erucic acid | 22:1 | 5.47 ± 0.322 B | 2.17 ± 0.151 A | n.d. |
| Lignoceric acid | 24:0 | 10.83 ± 0.157 BC | 1.57 ± 0.074 AC | 3.36 ± 0.011 BC |
| YL/X | 7.47 | 8.75 | 4.73 | |
| Amino Acid [g/100 g of Protein] | Substrate | Whole Egg 1 | |||
|---|---|---|---|---|---|
| Honeydew Honey | French Chestnut Honey | Italian Chestnut Honey | Fructose 40 g/L+Trp 200 mg/L | ||
| A | B | C | D | ||
| Isoleucine | 1.42 ± 0.05 BCD | 3.36 ± 0.16 A | 3.76 ± 0.17 AD | 3.00 ± 0.10 AC | 5.4 |
| Leucine | 9.20 ± 0.13 BCD | 5.14 ± 0.18 AC | 6.50 ± 0.21 ABD | 5.65 ± 0.19 AC | 8.6 |
| Lysine | 4.65 ± 0.09 BCD | 3.45 ± 0.10 AC | 3.93 ± 0.13 ABD | 3.21 ± 0.09 AC | 7.0 |
| Methionine/Cysteine | 2.68 | 2.43 | 2.96 | 2.10 | 5.7 |
| Phenylalanine/Tyrosine | 4.12 | 4.73 | 5.50 | 3.90 | 9.3 |
| Threonine | 4.50 ± 0.11 BCD | 3.34 ± 0.11 A | 3.40 ± 0.09 A | 3.37 ± 0.13 A | 4.7 |
| Tryptophan | 0.93 ± 0.02 BD | 0.69 ± 0.02 AC | 0.86 ± 0.03 ABD | 0.64 ± 0.01 AC | 1.7 |
| Valine | 2.00 ± 0.04 BCD | 1.34 ± 0.05 A | 1.43 ± 0.06 A | 1.36 ± 0.04 A | 6.6 |
| Protein [%] | 24.6 | 31.7 | 33.3 | 32.3 | |
| Nutritional values: | |||||
| CS isoleucine | 26.3 | - | - | - | - |
| CS valine | - | 20.3 | 21.7 | 20.7 | - |
| EAAI | 52.5 | 46.9 | 54.0 | 44.2 | - |
| ∑EAA | 29.5 | 24.5 | 28.3 | 23.2 | 49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróbel-Kwiatkowska, M.; Turski, W.; Juszczyk, P.; Kita, A.; Rymowicz, W. Improved Production of Kynurenic Acid by Yarrowia lipolytica in Media Containing Different Honeys. Sustainability 2020, 12, 9424. https://doi.org/10.3390/su12229424
Wróbel-Kwiatkowska M, Turski W, Juszczyk P, Kita A, Rymowicz W. Improved Production of Kynurenic Acid by Yarrowia lipolytica in Media Containing Different Honeys. Sustainability. 2020; 12(22):9424. https://doi.org/10.3390/su12229424
Chicago/Turabian StyleWróbel-Kwiatkowska, Magdalena, Waldemar Turski, Piotr Juszczyk, Agnieszka Kita, and Waldemar Rymowicz. 2020. "Improved Production of Kynurenic Acid by Yarrowia lipolytica in Media Containing Different Honeys" Sustainability 12, no. 22: 9424. https://doi.org/10.3390/su12229424
APA StyleWróbel-Kwiatkowska, M., Turski, W., Juszczyk, P., Kita, A., & Rymowicz, W. (2020). Improved Production of Kynurenic Acid by Yarrowia lipolytica in Media Containing Different Honeys. Sustainability, 12(22), 9424. https://doi.org/10.3390/su12229424





