Potential of Octanol and Octanal from Heracleum sosnowskyi Fruits for the Control of Fusarium oxysporum f. sp. lycopersici
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Samples
2.2. Fungal Isolates
2.3. Screening of Antifungal Activity
2.4. Antifungal Bioassay of Authentic Volatile Compounds
2.5. Measuring the Actual Volatile Concentration
2.6. Determination of the EC50 Values
2.7. Biofumigation Assay
2.7.1. Preparation of Conidial Suspension
2.7.2. Experimental Setup for Biofumigation Assay
2.8. Counting the Conidial Density from Different Biofumigated Soil
2.9. Data Analysis
3. Results
3.1. Screening of Antifungal Activity
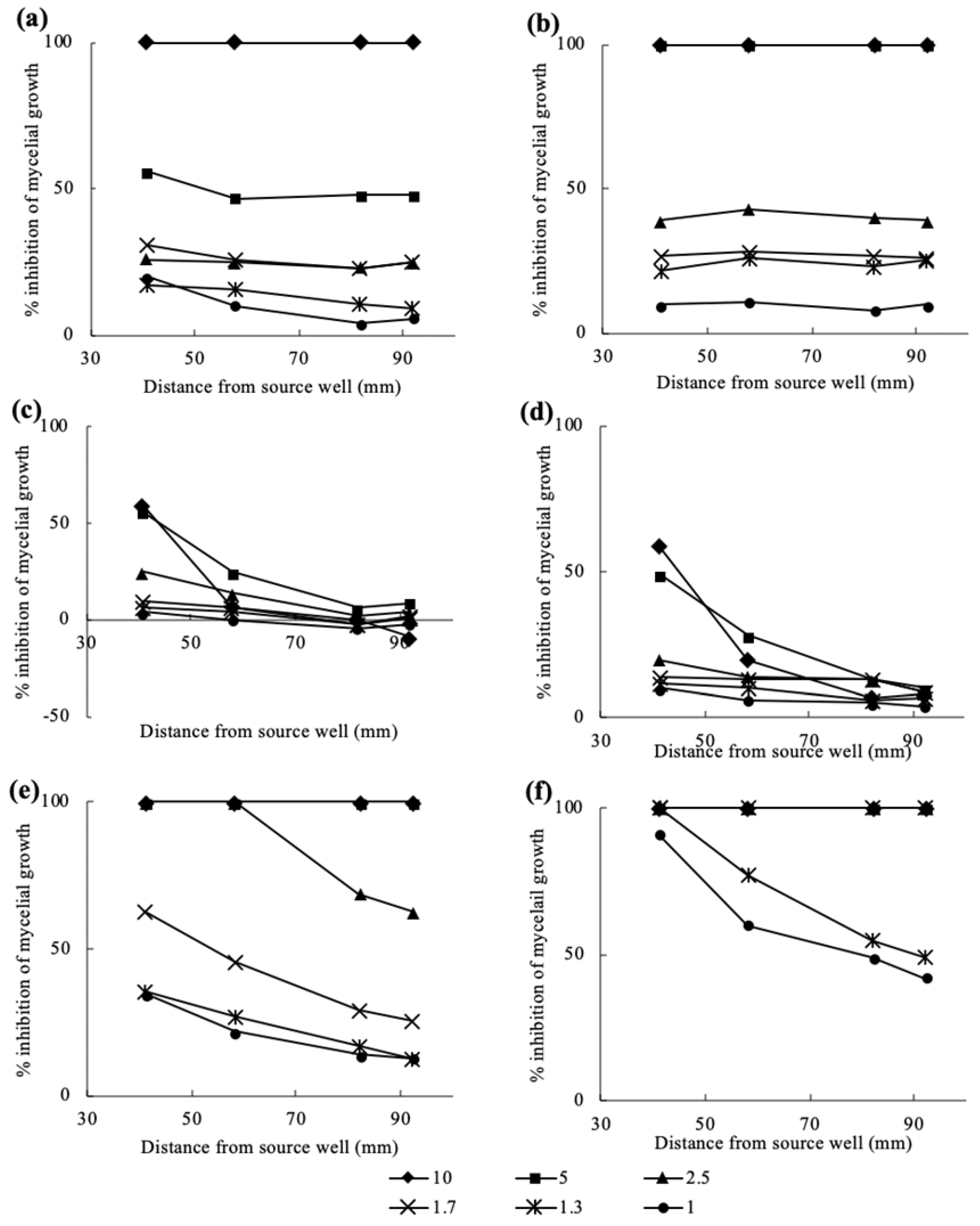
3.2. Antifungal Bioassay of Authentic Volatile Compounds
3.3. Biofumigation Assay
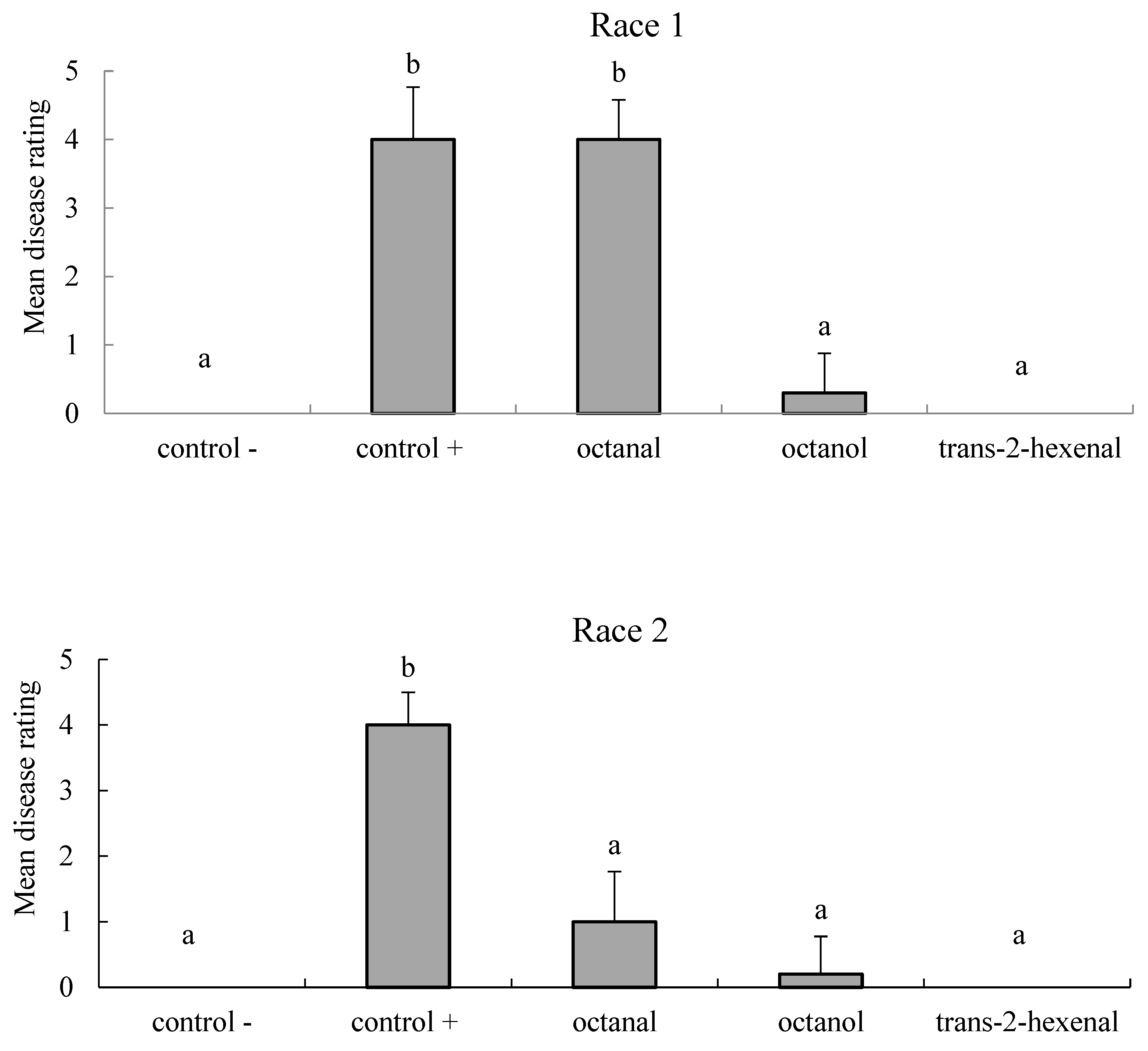
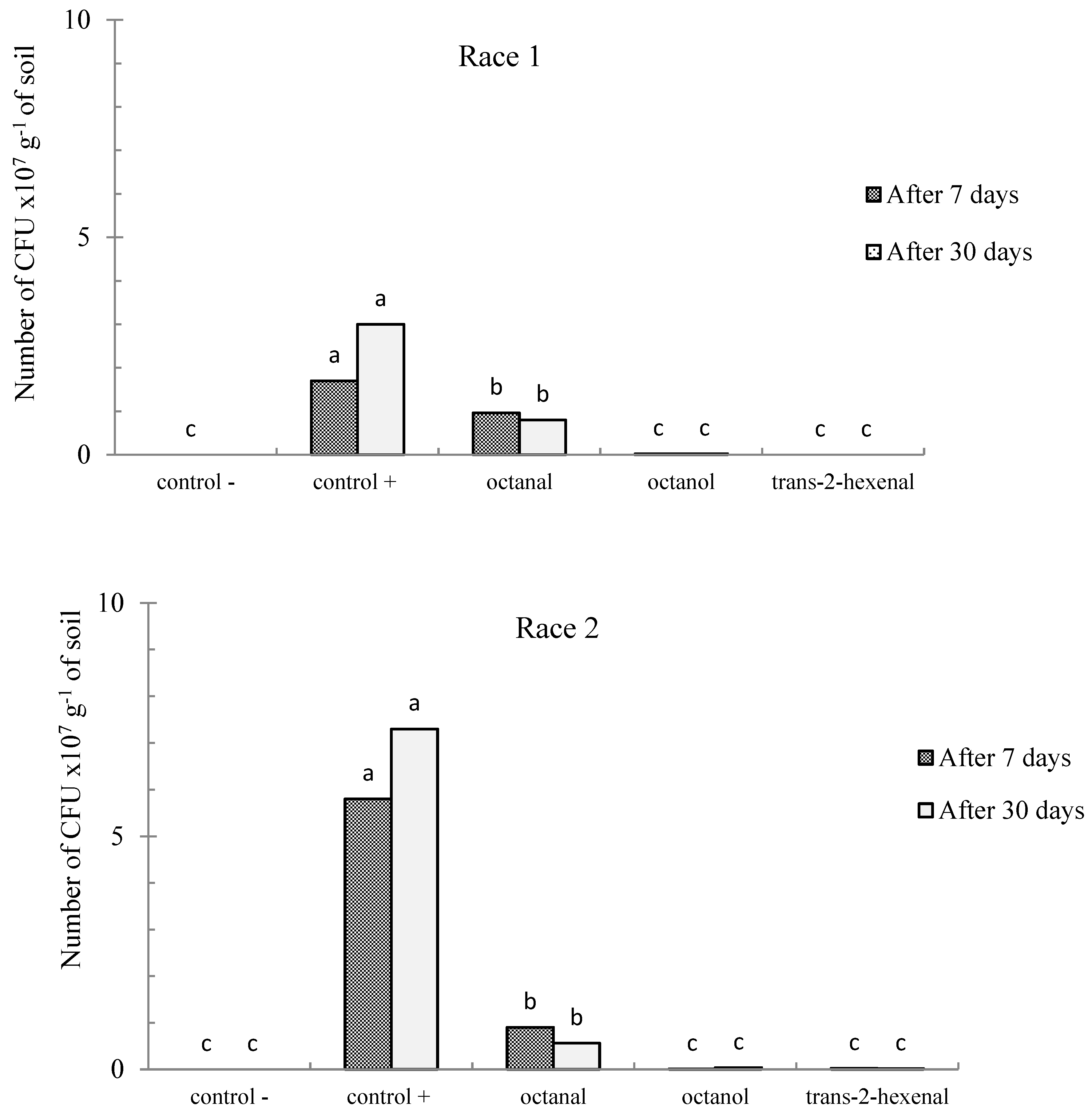
3.3.1. Biofumigation Assay of FOL Race 1
3.3.2. Biofumigation Assay on FOL Race 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vengurlekar, S.; Sharma, R.; Trivedi, P. Efficacy of some natural compounds as antifungal agents. Pharmacogn. Rev. 2012, 6, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Wallace, R.J. Antimicrobial properties of plant secondary metabolites. Proc. Nutr. Soc. 2004, 63, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Rattan, R.S. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot. 2010, 29, 913–920. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Engelmeier, D.; Hadacek, F. Antifungal natural products: Assays and applications. Adv. Phytomed. 2006, 3, 423–467. [Google Scholar]
- Isaac, G.; Abu-Tahon, M. In vitro antifugal activity of medicinal plant extract against Fusarium oxysporum f. sp. lycopersici race 3 the causal agent of tomato wilt. Acta Biol. Hung. 2014, 65, 107–118. [Google Scholar] [CrossRef]
- Della Pepa, T.; Elshafie, H.S.; Capasso, R.; De Feo, V.; Camele, I.; Nazzaro, F.; Scognamiglio, M.R.; Caputo, L. Antimicrobial and phytotoxic activity of Origanum heracleoticum and O. majorana essential oils growing in Cilento (Southern Italy). Molecules 2019, 24, 2576. [Google Scholar] [CrossRef]
- Soylu, E.M.; Soylu, S.; Kurt, S. Antimicrobial activities of the essential oils of various plants against tomato Late Blight disease agent Phytophthora infestans. Mycopathologia 2006, 161, 119–128. [Google Scholar] [CrossRef]
- De Corato, U.; Maccioni, O.; Trupo, M.; Sanzo, G.D. Use of essential oil of Laurus nobilis obtained by means of a supercritical carbon dioxide technique against postharvest spoilage fungi. Crop Prot. 2010, 29, 142–147. [Google Scholar] [CrossRef]
- Sekine, T.; Sugano, M.; Majid, A.; Fujii, Y. Antifungal effects of volatile compounds from black zira (Bunium persicum) and other spices and herbs. J. Chem. Ecol. 2007, 33, 2123–2132. [Google Scholar] [CrossRef]
- Neri, F.; Mari, M.; Brigati, S. Control of Penicillium expansum by plant volatile compounds. Plant Pathol. 2006, 55, 100–105. [Google Scholar] [CrossRef]
- Wood, E.M.; Miles, T.D.; Wharton, P.S. The use of natural plant volatile compounds for the control of the potato postharvest diseases, black dot, silver scurf and soft rot. Biol. Control 2013, 64, 152–159. [Google Scholar] [CrossRef]
- Ignjatov, M.; Milošević, D.; Nikolić, Z.; Gvozdanović-Varga, J.; Jovičić, D.; Zdjelar, G. Fusarium oxysporum as causal agent of tomato wilt and fruit rot. Pestic. Phytomed. (Belgrade) 2012, 27, 25–31. [Google Scholar] [CrossRef]
- de la Isla, A.L.; Macías-Sánchez, K.L. Fusarium oxysporum f. sp. lycopersici: How can we control this fungus? Adv. Biotech. Microbiol. 2017, 4. [Google Scholar] [CrossRef]
- Mes, J.J.; Weststeijn, E.A.; Herlaar, F.; Lambalk, J.J.M.; Wijbrandi, J.; Haring, M.A.; Cornelissen, B.J.C. Biological and molecular characterization of Fusarium oxysporum f. sp. lycopersici divides Race 1 isolates into separate virulence groups. Phytopathology 1999, 89, 156–160. [Google Scholar] [CrossRef]
- Hanan Aref, H. Biology and integrated control of tomato wilt caused by Fusarium oxysporum lycopersici: A comprehensive review under the light of recent advancements. J. Bot. Res. 2020, 3, 84–99. [Google Scholar] [CrossRef]
- Booth, C. The Genus Fusarium; Commonwealth Mycological Institute: Kew, Surrey, 1971. [Google Scholar]
- Stall, R.E. Development of Fusarium wilt on resistant varieties of tomato caused by a strain different from race 1 and 2 of Fusarium oxysporum f. sp. lycopersici. Plant Dis. Rep. 1961, 45, 12–15. [Google Scholar]
- Alexander, L.J.; Tucker, C.M. Physiologic specialization in the tomato wilt fungus Fusarium oxysporum f. sp. lycopersici. 1. J. Agric. Res. 1945, 70, 303–313. [Google Scholar]
- Grattidge, R.; O’Brien, R.G. Occurrence of a third race of Fusarium wilt of tomatoes in Queensland. Plant Dis. 1982, 66, 165–166. [Google Scholar] [CrossRef]
- Baysal, Ö.; Siragusa, M.; İkten, H.; Polat, İ.; Gümrükcü, E.; Yigit, F.; Carimi, F.; Teixeira da Silva, J.A. Fusarium oxysporum f. sp. lycopersici races and their genetic discrimination by molecular markers in West Mediterranean region of Turkey. Physiol. Mol. Plant Pathol. 2009, 74, 68–75. [Google Scholar] [CrossRef]
- Gullino, M.; Minuto, A.; Gilardi, G.; Garibaldi, A.; Ajwa, H.; Duafala, T. Efficacy of preplant soil fumigation with chloropicrin for tomato production in Italy. Crop Prot. 2002, 21, 741–749. [Google Scholar] [CrossRef]
- Tripathi, P.; Dubey, N. Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharvest Biol. Technol. 2004, 32, 235–245. [Google Scholar] [CrossRef]
- Fujii, Y.; Matsuyama, M.; Hiradate, S.; Shimozawa, H. Dish pack method: A new bioassay for volatile allelopathy. Thymus 2005, 2, 493–497. [Google Scholar]
- Skidmore, A.M.; Dickinson, C.H. Colony interactions and hyphal interference between Septoria nodorum and phylloplane fungi. Trans. Br. Mycol. Soc. 1976, 66, 57–64. [Google Scholar] [CrossRef]
- Mishyna, M.; Laman, N.; Prokhorov, V.; Maninang, J.S.; Fujii, Y. Identification of octanal as plant growth inhibitory volatile compound released from Heracleum sosnowskyi fruit. Nat. Prod. Commun. 2015, 10, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Tao, N.; Jia, L.; Zhou, H.; He, X. Effect of octanal on the mycelial growth of Penicillium italicum and P. digitatum. World J. Microbiol. Biotechnol. 2014, 30, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Gillot, G.; Decourcelle, N.; Dauer, G.; Barbier, G.; Coton, E.; Delmail, D.; Mounier, J. 1-Octanol, a self-inhibitor of spore germination in Penicillium camemberti. Food Microbiol. 2016, 57, 1–7. [Google Scholar] [CrossRef]
- Morid, B.; Hajmansoor, S.; Kakvan, N. Screening of resistance genes to fusarium root rot and fusarium wilt diseases in tomato (Lycopersicon esculentum) cultivars using RAPD and CAPs markers. Eur. J. Exp. Biol. 2012, 2, 931–939. [Google Scholar]
- Kim, J.T.; Park, H.I.; Hahm, Y.I.; Yu, S.H. Crown and root rot of greenhouse tomato caused by Fusarium oxysporum f. sp. radicis-lycopersici in Korea. Plant Pathol. J. 2001, 17, 290–294. [Google Scholar]
- Laman, N.; Prokhorov, V.; Maslovskii, O. Giant Hogweed Is a Dangerious Invasive Species to Natural Systems and the Population of Belarus; National Academy of Sciences of Belarus, Institute of Experimental Botany: Minsk, Belarus, 2009. [Google Scholar]
- Dusko, L.B.; Comic, L.; Solujic-Sukdolak, S. Antibacterial activity of some plants from family Apiaceae in relation to selected phytopathogenic bacteria. Kragujev. J. Sci. 2006, 28, 65–72. [Google Scholar]
- Shokri, H.; Sharifzadeh, A.; Ashrafi Tamai, I. Anti-Candida zeylanoides activity of some Iranian plants used in traditional medicine. J. Mycol. Med. 2012, 22, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Davari, M.; Ezazi, R. Chemical composition and antifungal activity of the essential oil of Zhumeria majdae, Heracleum persicum and Eucalyptus sp. against some important phytopathogenic fungi. J. Mycol. Med. 2017, 27, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Tao, N.; Jia, L. Antifungal activity of citral, octanal and α-terpineol against Geotrichum citri-aurantii. Food Control 2014, 37, 277–283. [Google Scholar] [CrossRef]
- Environmental Protection Agency. 1-octanol; Exemption from the requirement of a tolerance. Fed. Regist. 2015, 80, 25950–25953. [Google Scholar]
- Gardini, F.; Lanciotti, R.; Guerzoni, M.E. Effect of trans-2-hexenal on the growth of Aspergillus flavus in relation to its concentration, temperature and water activity. Lett. Appl. Microbiol. 2001, 33, 50–55. [Google Scholar] [CrossRef]
- Lanciotti, R.; Belletti, N.; Patrignani, F.; Gianotti, A.; Gardini, F.; Guerzoni, M.E. Application of hexanal, (E)-2-Hexenal, and hexyl acetate to improve the safety of fresh-sliced apples. J. Agric. Food Chem. 2003, 51, 2958–2963. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, H.; Sun, H.; Wang, X. Antifungal activity of trans-2-Hexenal against Penicillium cyclopium by a membrane damage mechanism. J. Food Biochem. 2017, 41, e12289. [Google Scholar] [CrossRef]
- Daubert, T.E.; Danner, R.P. Physical and Thermodynamic Properties of Pure Chemicals Data Compilation; Taylor and Francis: Washington, DC, USA, 1989. [Google Scholar]
- Gueldner, R.C.; Wilson, D.M.; Heidt, A.R. Volatile compounds inhibiting Aspergillus flavus. J. Agric. Food Chem. 1985, 33, 411–413. [Google Scholar] [CrossRef]
- Moleyar, V.; Narasimham, P. Antifungal activity of some essential oil components. Food Microbiol. 1986, 3, 331–336. [Google Scholar] [CrossRef]
- Helal, G.A.; Sarhan, M.M.; Abu Shahla, A.N.K.; Abou El-Khair, E.K. Effects of Cymbopogon citratus L. essential oil on the growth, lipid content and morphogenesis of Aspergillus niger ML2-strain. J. Basic Microbiol. 2006, 46, 456–469. [Google Scholar] [CrossRef]
- USEPA/Office of Prevention Pesticides and Toxic Substances. Reregistration Eligibility Decision Document for Aliphatic Alcohols; United States Environmental Protection Agency: Washington, DC, USA, 2007; p. 14.
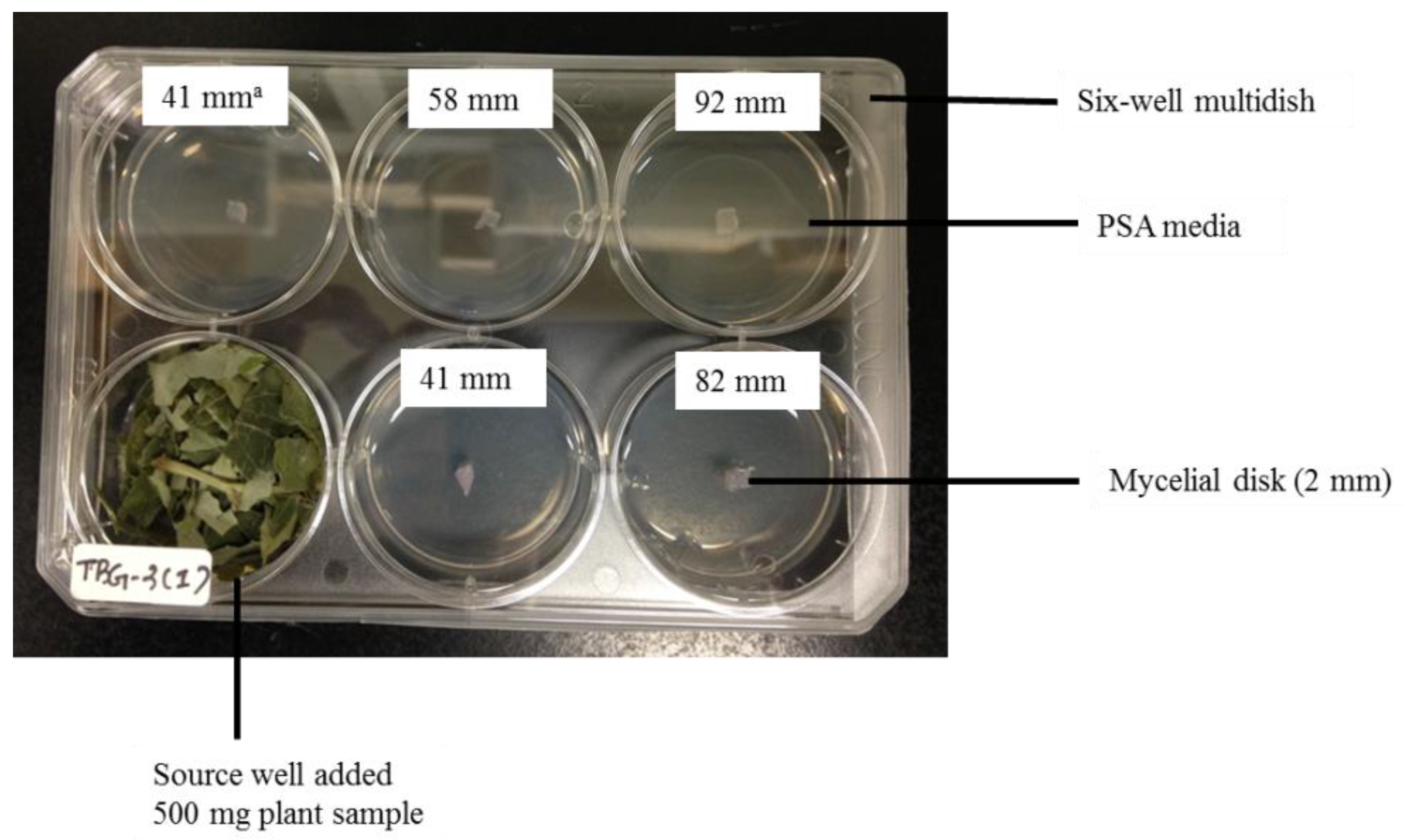
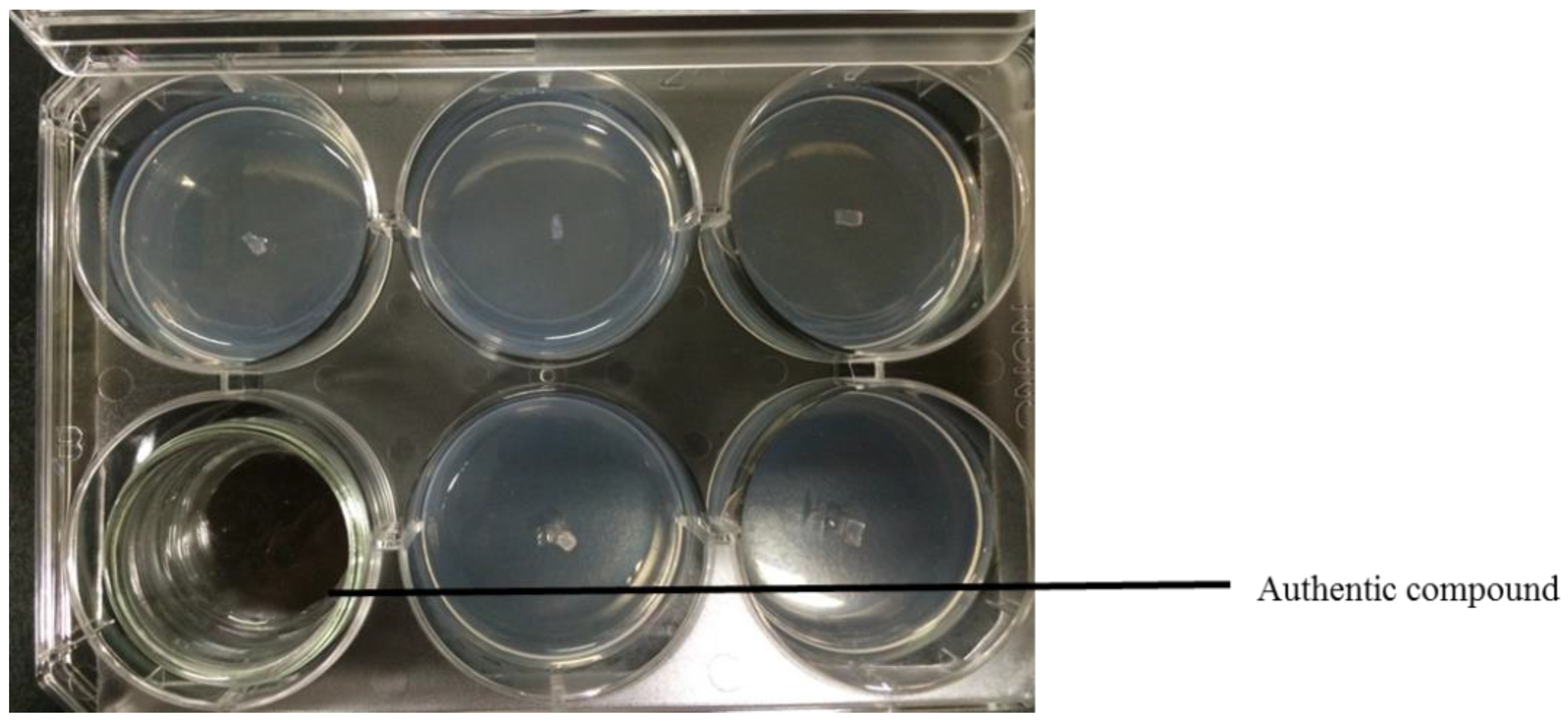
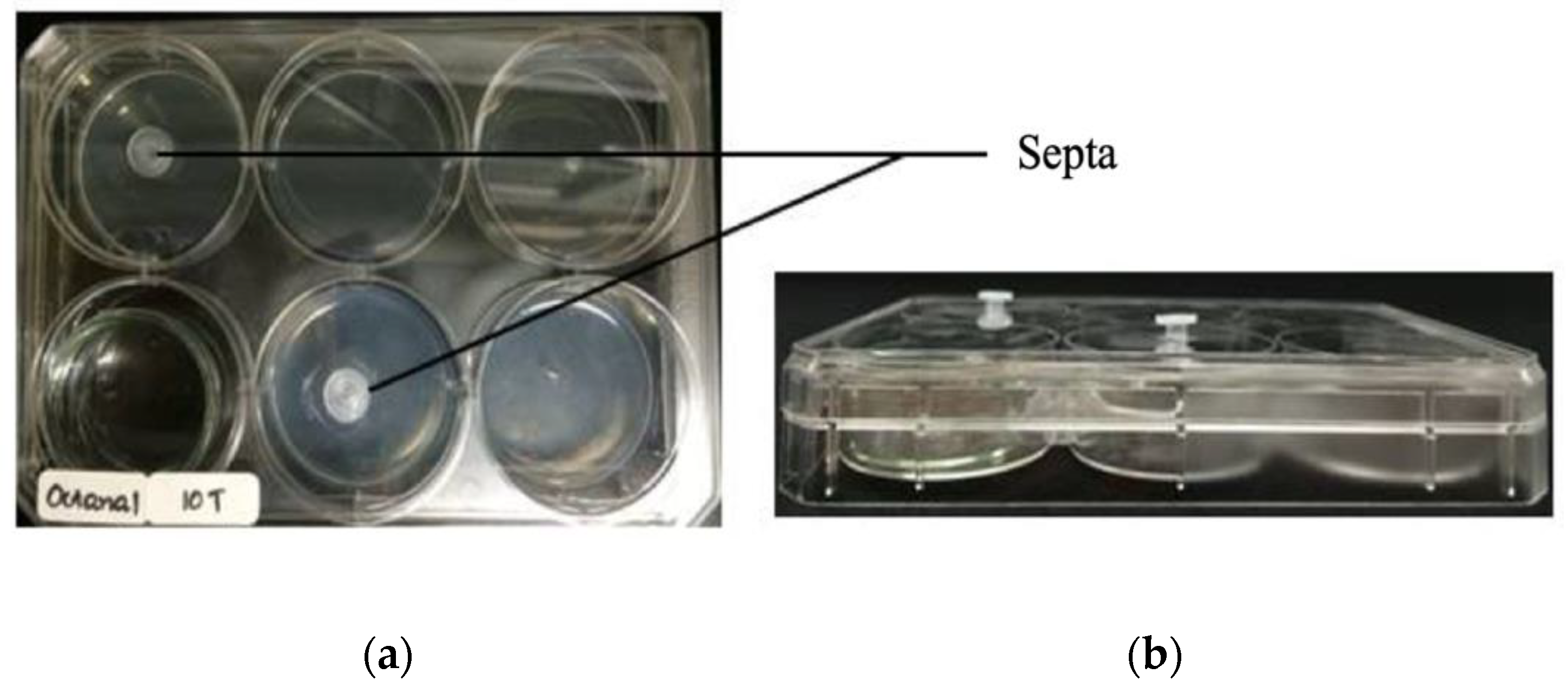
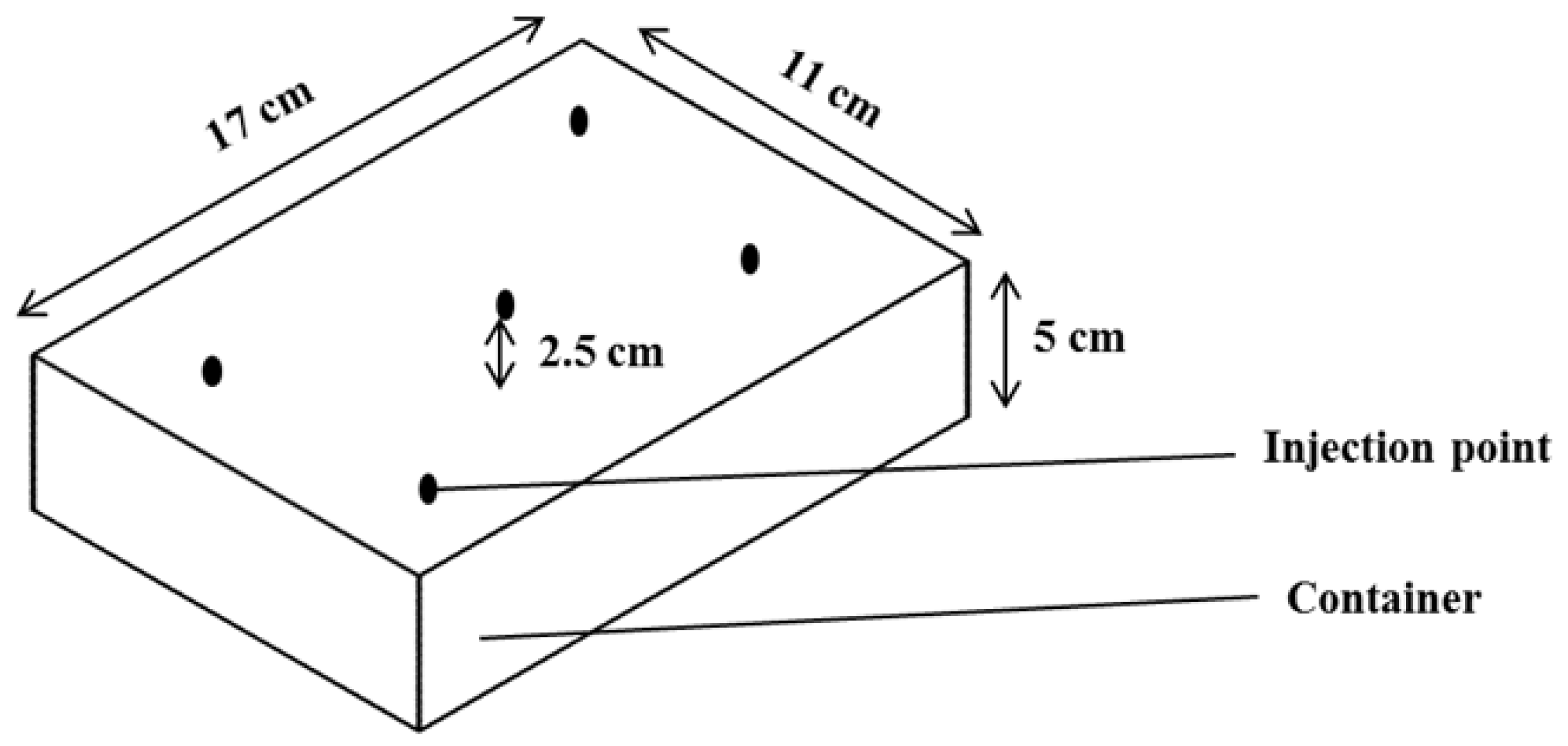

| Plant Species | Part Used | Growth Inhibition (%) | ||
|---|---|---|---|---|
| Common Name | Scientific Name | Family Name | ||
| Sosnowskyi’s hogweed | Heracleum sosnowskyi Manden. | Apiaceae | Fruit | 67 |
| Ostrich fern | Matteuccia struthiopteris (L.) Tod. | Onocleaceae | Leaf | 18 |
| Dokudami | Houttuynia cordata Thunb. | Saururaceae | Rhizome | 15 |
| Woad | Isatis tinctoria L. | Cruciferae | Leaf | 14 |
| Touki | Angelica acutiloba Kitagawa | Apiaceae | Leaf | 12 |
| Wadatsumi-no-ki | Nothapodytes amamianus Nagam. & Mak. Kato | Icacinaceae | Leaf | 12 |
| Black mangrove | Lumnitzera racenosa Willd. | Combretaceae | Leaf | 11 |
| Donan-koban-no-ki | Phyllanthus oligospermus Hayata subsp. donanensis T.Kuros. | Phyllanthaceae | Leaf | 11 |
| Cha-ran (Tea orchid) | Chloranthus spicatus (Thunb.) Makino | Chloranthaceae | Leaf | 9 |
| Peppermint | Pelargonium tomentosum Jacq. | Geraniaceae | Leaf | 9 |
| Macadamia | Macadamia integrifolia Maiden & Betche | Proteaceae | Leaf | 9 |
| Japanese yellow bark | Phellodendron amurense Rupr. | Rutaceae | Leaf | 9 |
| Turmeric | Curcuma longa L. | Zingiberaceae | Leaf | 9 |
| Indian mangrove | Avicennia officinalis L. | Acanthaceae | Leaf | 8 |
| Magic lilly | Lycoris squamigera Maxim. | Amaryllidaceae | Leaf | 8 |
| Mishima-saiko | Bupleurum stenophyllum (Nakai) Kitag. | Apiaceae | Leaf | 8 |
| Sanyo-aoi | Asarum hexalobum F.Maek. | Aristolochiaceae | Leaf | 8 |
| Pei lan | Eupatorium fortunei Turcz. | Asteraceae | Leaf | 8 |
| Yellow starwort | Inula helenium L. | Asteraceae | Leaf | 8 |
| Goat weed | Epimedium grandiflorum C.Morren var. thunbergianum (Miq.) Nakai | Berberidaceae | Leaf | 8 |
| Yellow ginger | Dioscorea zingeberensis C.H.Wright | Dioscoreaceae | Leaf | 8 |
| Rubber bark tree | Eucommia ulmoides Oliv. | Eucommiaceae | Leaf | 8 |
| Quaresmeira | Tibouchina sp. | Melastomataceae | Leaf | 8 |
| Taiyo-fuutou-kadzura | Piper postelsianum Maxim. | Piperaceae | Leaf | 8 |
| Ryukyu-suzukake | Veronicastrum liukiuense (Ohwi) T.Yamaz. | Plantaginaceae | Leaf | 8 |
| Winter rose | Helleborus orientalis Lam. | Ranunculaceae | Leaf | 8 |
| Aromatic ginger | Kaempferia galanga L. | Zingiberaceae | Leaf | 8 |
| Meik-thalin | Zingiber barbatum Wall. | Zingiberaceae | Leaf | 8 |
| Shell ginger | Alpinia zerumbet (Pers.) B.L.Burtt & R.M.Sm. | Zingiberaceae | Leaf | 8 |
| Bitter ginger | Zingiber zerumbet (L.) Roscoe ex Sm. | Zingiberaceae | Leaf | 8 |
| Wild turmeric | Curcuma aromatica Salisb. | Zingiberaceae | Leaf | 8 |
| Blue bush | Maireana sedifolia (F.Muell.) Paul G.Wilson | Amaranthaceae | Leaf | 6 |
| Sosnowskyi’s hogweed | Heracleum sosnowskyi Manden | Apiaceae | Root | 6 |
| Goat horns | Strophanthus divaricatus (Lour.) Hook. & Arn. | Apocynaceae | Leaf | 6 |
| Urashima-sou | Arisaema thunbergii Blume subsp. urashima (H.Hara) H.Ohashi & J.Murata | Araceae | Leaf | 6 |
| Ohba-kan-aoi | Asarum lutchuense T.Itô | Aristolochiaceae | Leaf | 6 |
| Frosted jade | Asarum kumageanum Masam. | Aristolochiaceae | Leaf | 6 |
| Umano-suzukusa | Aristolochia debilis Siebold & Zucc. | Aristolochiaceae | Leaf | 6 |
| Maruyama-shuukaidou | Begonia formosana (Hayata) Masam. | Begoniaceae | Leaf | 6 |
| Cranberry | Vaccinium wrightii A.Gray | Ericaceae | Leaf | 6 |
| Sea derris | Derris trifoliata Lour. | Leguminosae | Leaf | 6 |
| Spear mint | Mentha spicata L. | Lamiaceae | Leaf | 6 |
| Creeping thyme | Thymus quinquecostatus Celak. | Lamiaceae | Leaf | 6 |
| Three-leafed akebia | Akebia trifoliata (Thunb.) Koidz. | Lardizabalaceae | Leaf | 6 |
| Tendai-uyaku | Lindera strychnifolia (Siebold & Zucc.) Fern.-Vill. | Lauraceae | Leaf | 6 |
| Cinnamon | Cinnamomum zeylanicum Blume | Lauraceae | Leaf | 6 |
| Bead tree | Melia azedarach L. | Meliaceae | Leaf | 6 |
| Okinawa-sokei | Jasminum sinense Hemsl. | Oleaceae | Leaf | 6 |
| Lacquered pepper-tree | Piper magnificum Trel. | Piperaceae | Leaf | 6 |
| Veronica | Veronicastrum villosulum (Miq.) T.Yamaz. | Plantaginaceae | Leaf | 6 |
| Yabu-kouji | Ardisia japonica (Thunb.) Blume | Primulaceae | Leaf | 6 |
| Silver dollar fern | Adiantum peruvianum Klotzsch | Pteridaceae | Leaf | 6 |
| Venus’s hair fern | Adiantum anceps Maxon & C.V.Morton | Pteridaceae | 6 | |
| Karatorikabuto | Aconitum carmichaeli Debx. | Ranunculaceae | Leaf | 6 |
| Japanese belladonna | Scopolia japonica Maxim. | Solanaceae | Leaf | 6 |
| Giant Pelican Flower | Aristolochia gigantea Mart. | Aristolochiaceae | Leaf | 5 |
| Asparagus fern | Asparagus densiflorus (Kunth) Jessop | Asparagaceae | Leaf | 5 |
| Japanese silver | Farfugium japonicum (L.) Kitam. | Asteraceae | Leaf | 5 |
| Samoan cup fern | Dennstaedtia samoensis T.Moore | Dennstaedtiaceae | Leaf | 5 |
| Tamarind | Tamarindus indica L. | Leguminosae | Leaf | 5 |
| Lavender Moon | Prostanthera baxteri A.Cunn. ex Benth. | Lamiaceae | Leaf | 5 |
| Lily turf | Liriope muscari (Decne.) L.H.Bailey | Asparagaceae | Leaf | 5 |
| Ooba-ohyama-renge | Magnolia sieboldii K.Koch | Magnoliaceae | Leaf | 5 |
| Bilimbi | Averrhoa bilimbi L. | Oxalidaceae | Leaf | 5 |
| Grape Ivy | Cissus rhombifolia Vahl | Vitaceae | Leaf | 5 |
| Ginger | Zingiber officinale Roscoe | Zingiberaceae | Leaf | 5 |
| Mango-ginger | Curcuma amada Roxb. | Zingiberaceae | Leaf | 5 |
| Cassumunar ginger | Zingiber cassumunar Roxb. | Zingiberaceae | Leaf | 5 |
| Hanamyouga | Alpinia japonica (Thunb.) Miq. | Zingiberaceae | Leaf | 5 |
| River bell | Mackaya bella Harv. | Acanthaceae | Leaf | 3 |
| Sibirian yellow | Achillea sibirica Ledeb. | Asteraceae | Leaf | 3 |
| Silver mound | Artemisia schmidtiana Maxim. | Asteraceae | Leaf | 3 |
| Licorice plant | Helichrysum petiolare Hilliard & B.L.Burtt | Asteraceae | Leaf | 3 |
| May apple | Podophyllum peltatum L. | Berberidaceae | Leaf | 3 |
| Hop | Humulus lupulus L. | Cannabinaceae | Leaf | 3 |
| Celandine-leaved pelargonium | Pelargonium fulgidum L’Hér. | Geraniaceae | Leaf | 3 |
| Chocolate vine | Akebia quinata (Houtt.) Decne. | Lardizabalaceae | Leaf | 3 |
| Giant cigar plant | Cuphea micropetala Kunth | Lythraceae | Leaf | 3 |
| Mouse trap tree | Uncarina grandidieri (Baill.) Stapf | Pedaliaceae | Leaf | 3 |
| Dokudami | Houttuynia cordata Thunb. | Saururaceae | Leaf | 3 |
| Tar bush | Eremophila glabra (R.Br.) Ostenf. | Scrophulariaceae | Leaf | 3 |
| Ginger-lilies | Alpinia oceanica Burkill | Zingiberaceae | Leaf | 3 |
| Torch ginger | Etlingera elatior (Jack) R.M.Sm. | Zingiberaceae | Leaf | 3 |
| Ryuunou-giku | Chrysanthemum makinoi Matsum. & Nakai | Asteraceae | Leaf | 2 |
| Marian thistle | Silybum marianum (L.) Gaertn. | Asteraceae | Leaf | 2 |
| Ao-tsuzura-fuji (Kamiebi) | Cocculus trilobus (Thunb.) DC. | Menispermaceae | Leaf | 2 |
| Kusano-ou | Chelidonium majus L. var. asiaticum H.Hara | Papaveraceae | Leaf | 2 |
| Japanese umbrella pine | Sciadopitys verticillata (Thunb.) Siebold & Zucc. | Sciadopityaceae | Leaf | 2 |
| Sosnowskyi’s hogweed | Heracleum sosnowskyi Manden | Apiaceae | Leaf | 0 |
| Japanese spurge | Pachysandra terminalis Siebold & Zucc. | Buxaceae | Leaf | 0 |
| Japanese honeysuckle | Lonicera japonica Thunb. | Caprifoliaceae | Leaf | 0 |
| Garlic | Allium sativum L. | Amaryllidaceae | Leaf | 0 |
| Urn orchid | Bletilla striata (Thunb.) Rchb. f. | Orchidaceae | Leaf | 0 |
| Chinese moonseed | Sinomenium acutum (Thunb.) Rehder & E.H.Wilson | Menispermaceae | Leaf | −2 |
| Botan | Paeonia suffruticosa Andrews | Paeoniaceae | Leaf | −2 |
| Wild betel | Piper sarmentosum Roxb. | Piperaceae | Leaf | −2 |
| Lovage root | Ligusticum sinense Oliv. | Apiaceae | Leaf | −2 |
| Beach carrot | Glehnia littoralis F.Schmidt ex Miq. | Apiaceae | Leaf | −3 |
| Tatarian aster | Aster tataricus L.f. | Asteraceae | Leaf | −3 |
| Joint-pine | Ephedra gerardiana Wall. ex Stapf | Ephedraceae | Leaf | −3 |
| Kiwi | Actinidia chinensis Planch. | Actinidiaceae | Leaf | −5 |
| Bittersweet | Celastrus orbiculatus Thunb. | Celastraceae | Leaf | −5 |
| Tiger-lily | Lilium lancifolium Thumb. | Liliaceae | Leaf | −5 |
| Victory onion | Allium victorialis L. var. platyphyllum (Hultén) Makino | Amaryllidaceae | Leaf | −6 |
| Giant butterbur | Petasites japonicus (Siebold & Zucc.) Maxim. | Asteraceae | Leaf | −6 |
| Sugar leaf | Stevia rebaudiana (Bertoni) Bertoni | Asteraceae | Leaf | −6 |
| Japanese pagodatree | Styphnolobium japonicum (L.) Schott | Leguminosae | Leaf | −6 |
| Red sage | Salvia miltiorhiza Bunge | Lamiaceae | Leaf | −6 |
| Angular solomon’s seal | Polygonatum odoratum (Mill.) Druce var. pluriflorum (Miq.) Ohwi | Asparagaceae | Leaf | −6 |
| Chinese peony | Paeonia lactiflora Pall. | Paeoniaceae | Leaf | −6 |
| Great burnet | Sanguisorba officinalis L. | Rosaceae | Leaf | −6 |
| Eagle fern | Pteridium aquilinum Kuhn var. latiusculum (Desv.) Underw. ex A. Heller | Dennstaedtiaceae | Leaf | −9 |
| Authentic Compound | Concentration, %, v/v | |||||
|---|---|---|---|---|---|---|
| 10 | 5.0 | 2.5 | 1.7 | 1.3 | 1 | |
| Octanal | 92.2 | 66.8 | 44.1 | 41.9 | 28.8 | 26.4 |
| Octanol | 8.6 | 8.0 | 7.7 | 5.1 | 2.9 | 2.3 |
| Trans-2-hexenal | 137.0 | 94.6 | 51.7 | 33.1 | 14.3 | 18.9 |
| Authentic Compound | Fusarium oxysporum f.sp. lycopersici | |
|---|---|---|
| Race 1 | Race 2 | |
| Octanol | 8.1 | 9.3 |
| Octanal | 57.0 | 51.0 |
| Trans-2-hexenal | 26.0 | 8.6 |
| Treatment | DSI (%) | |
|---|---|---|
| Race 1 | Race 2 | |
| Control + | 95 | 95 |
| Control − | 0 | 0 |
| Octanal | 92 | 16 |
| Octanol | 8 | 8 |
| trans-2-hexenal | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hpoo, M.K.; Mishyna, M.; Prokhorov, V.; Arie, T.; Takano, A.; Oikawa, Y.; Fujii, Y. Potential of Octanol and Octanal from Heracleum sosnowskyi Fruits for the Control of Fusarium oxysporum f. sp. lycopersici. Sustainability 2020, 12, 9334. https://doi.org/10.3390/su12229334
Hpoo MK, Mishyna M, Prokhorov V, Arie T, Takano A, Oikawa Y, Fujii Y. Potential of Octanol and Octanal from Heracleum sosnowskyi Fruits for the Control of Fusarium oxysporum f. sp. lycopersici. Sustainability. 2020; 12(22):9334. https://doi.org/10.3390/su12229334
Chicago/Turabian StyleHpoo, May Khaing, Maryia Mishyna, Valery Prokhorov, Tsutomu Arie, Akihito Takano, Yosei Oikawa, and Yoshiharu Fujii. 2020. "Potential of Octanol and Octanal from Heracleum sosnowskyi Fruits for the Control of Fusarium oxysporum f. sp. lycopersici" Sustainability 12, no. 22: 9334. https://doi.org/10.3390/su12229334
APA StyleHpoo, M. K., Mishyna, M., Prokhorov, V., Arie, T., Takano, A., Oikawa, Y., & Fujii, Y. (2020). Potential of Octanol and Octanal from Heracleum sosnowskyi Fruits for the Control of Fusarium oxysporum f. sp. lycopersici. Sustainability, 12(22), 9334. https://doi.org/10.3390/su12229334






