The Recovery of Ca and Zn from the Municipal Solid Waste Incinerator Fly Ash
Abstract
1. Introduction
2. Materials and Methods
2.1. MSWI Fly Ash
2.2. Water Washing
2.3. Ca Recovery by Ion Exchange
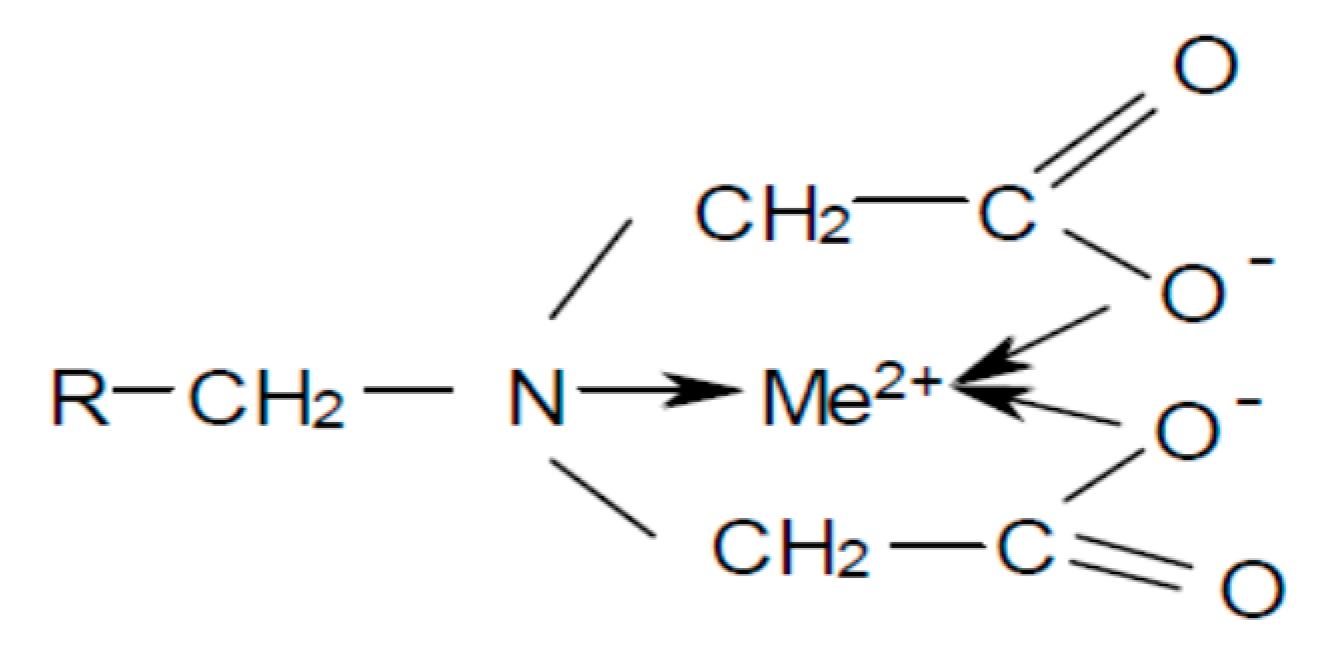
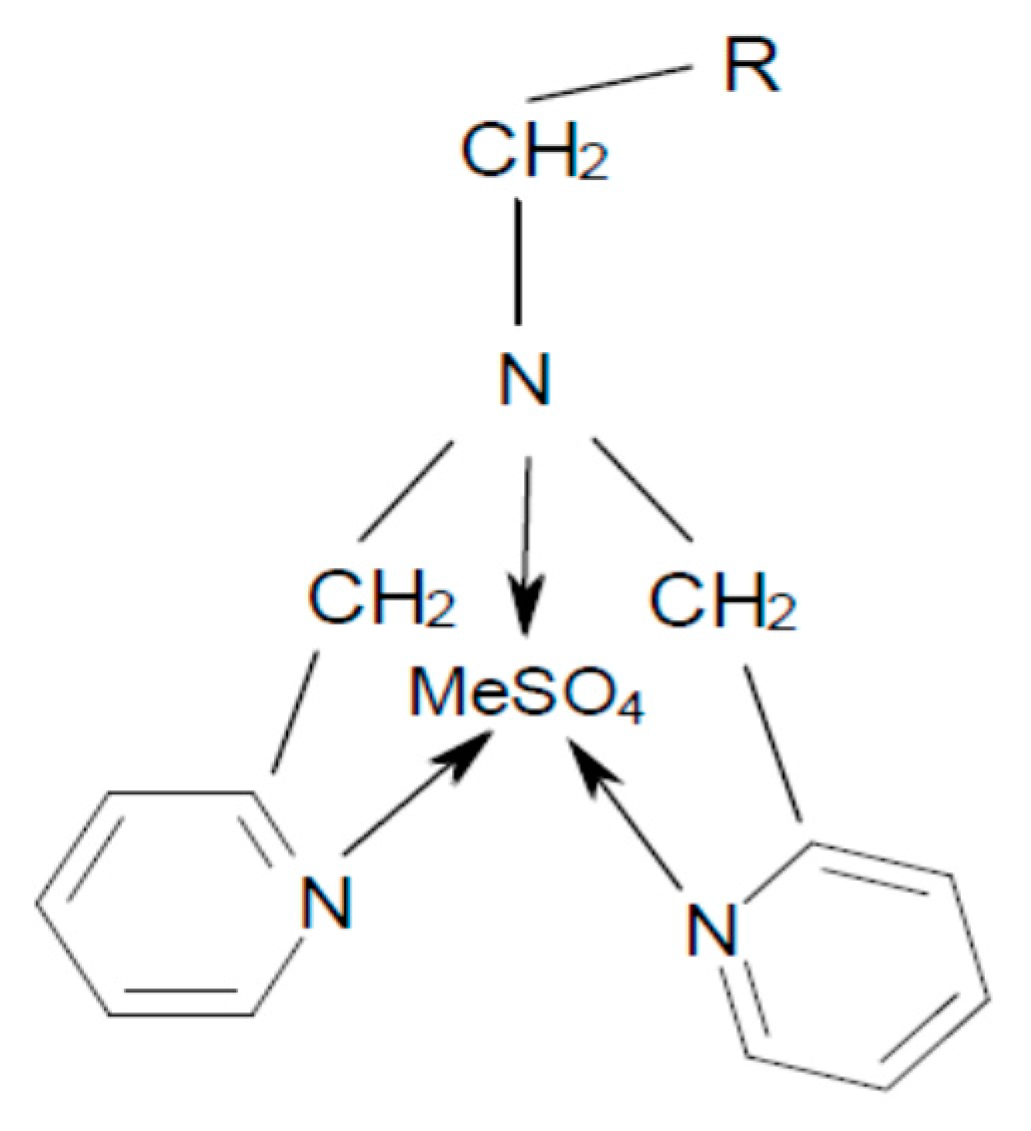
2.3.1. Resin
2.3.2. Column Experiments
2.4. Acid Leaching
2.5. Zn Recovery by Ion Exchange
2.5.1. Resin
2.5.2. Column Experiments
3. Results
3.1. Characteristics of Fly Ashes
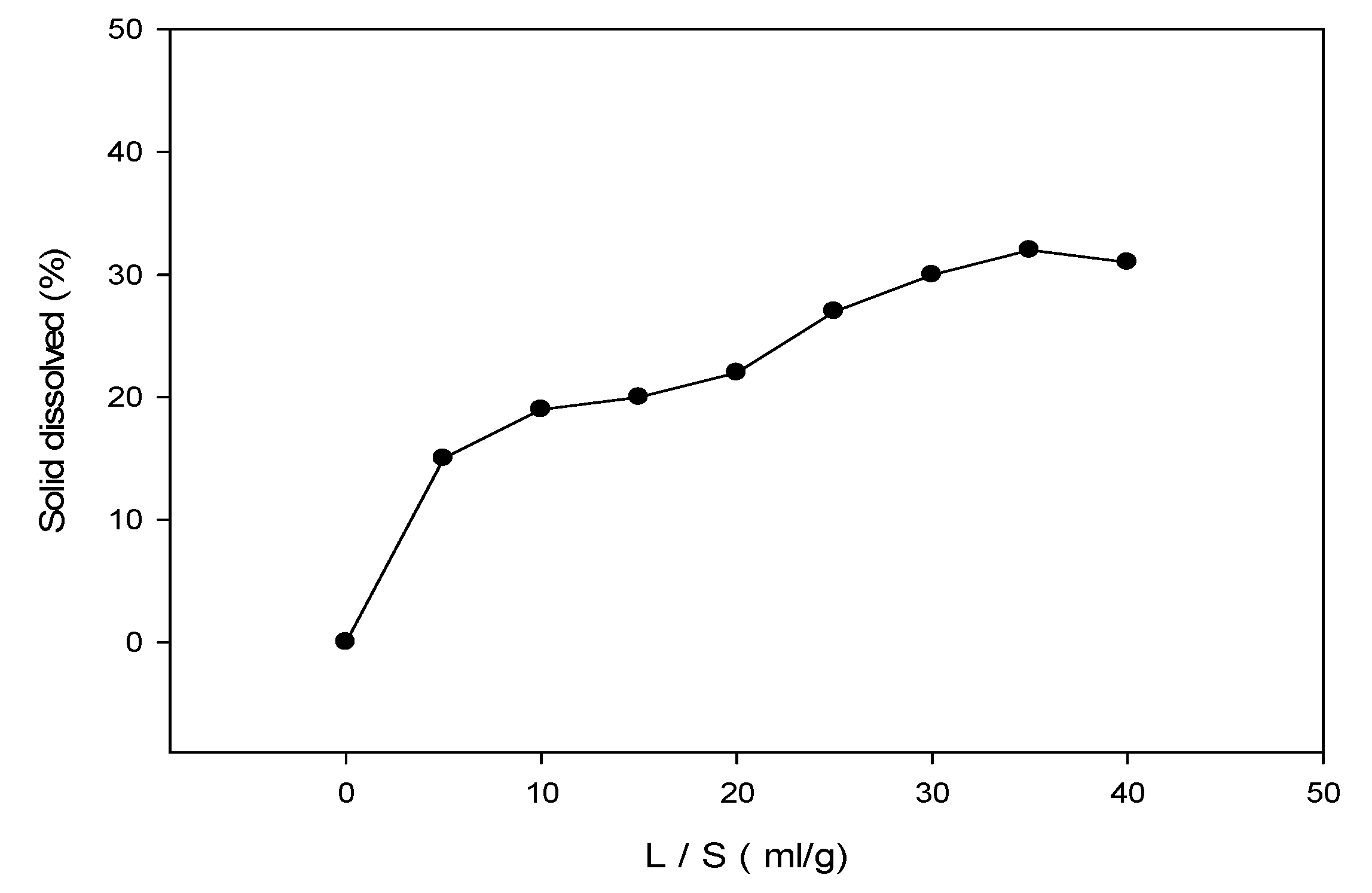
3.2. Water Washing
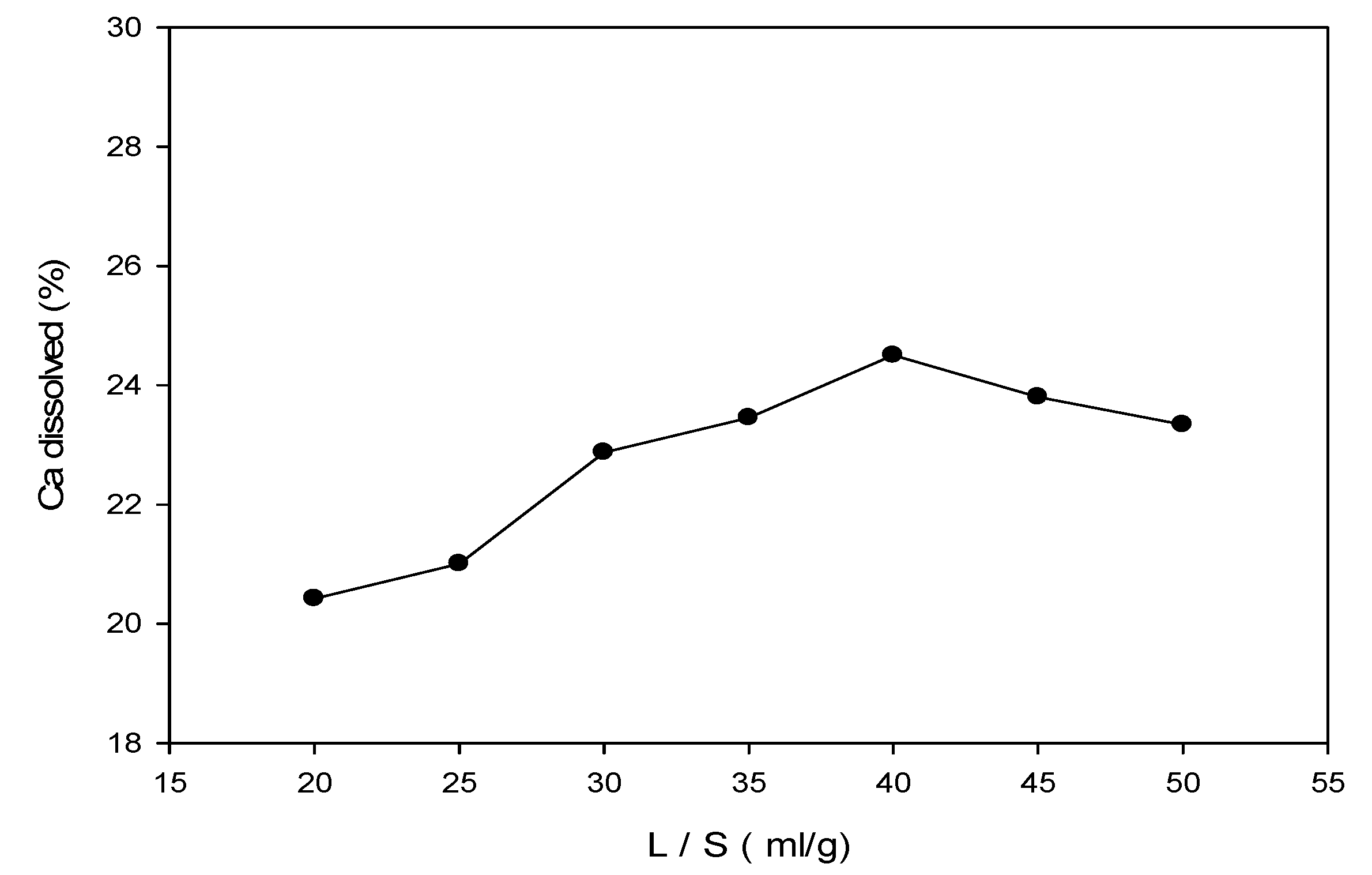
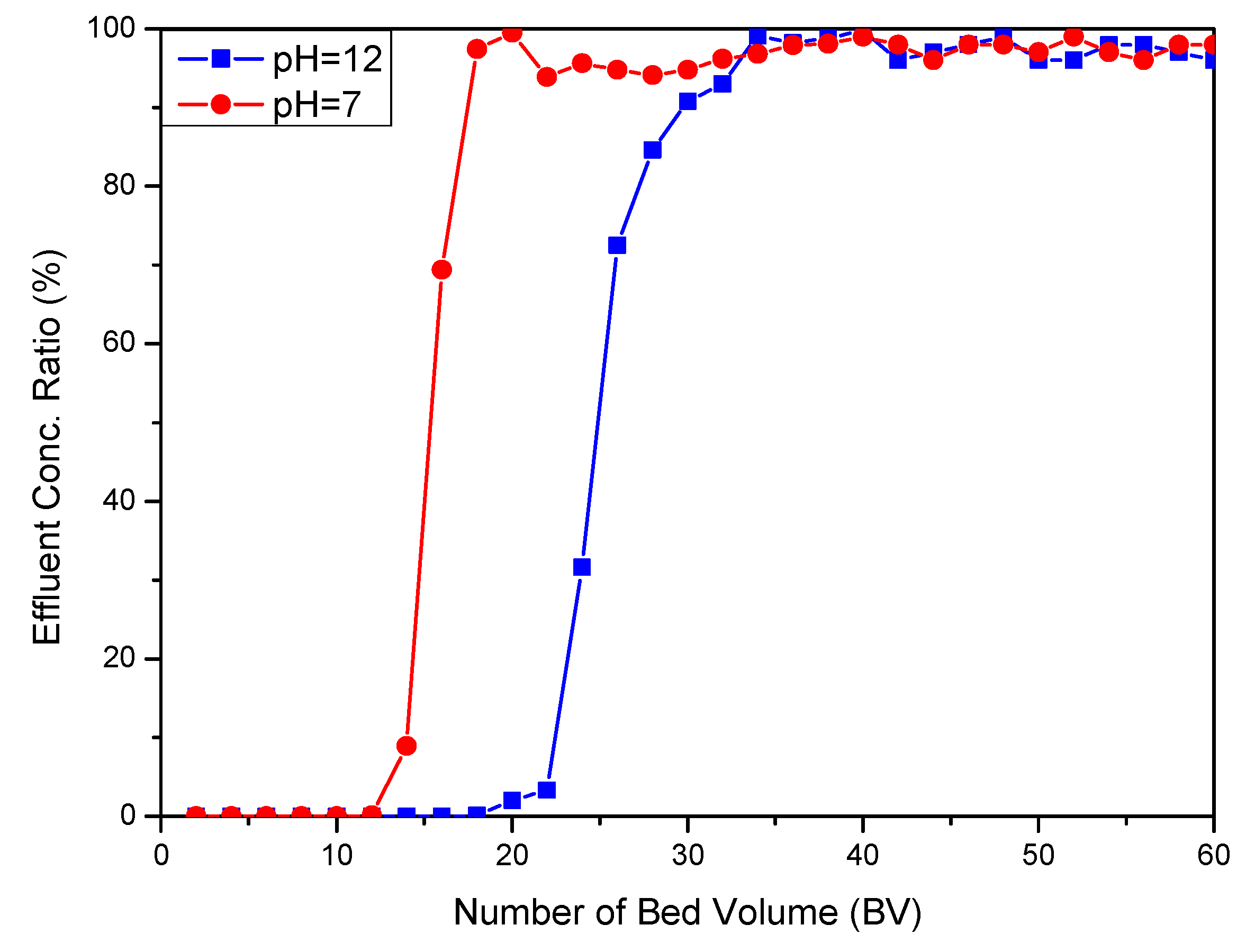
3.3. Ca Recovery Using Ion Exchange
3.4. Acid Leaching
3.5. Zn Recovery Using Ion Exchange
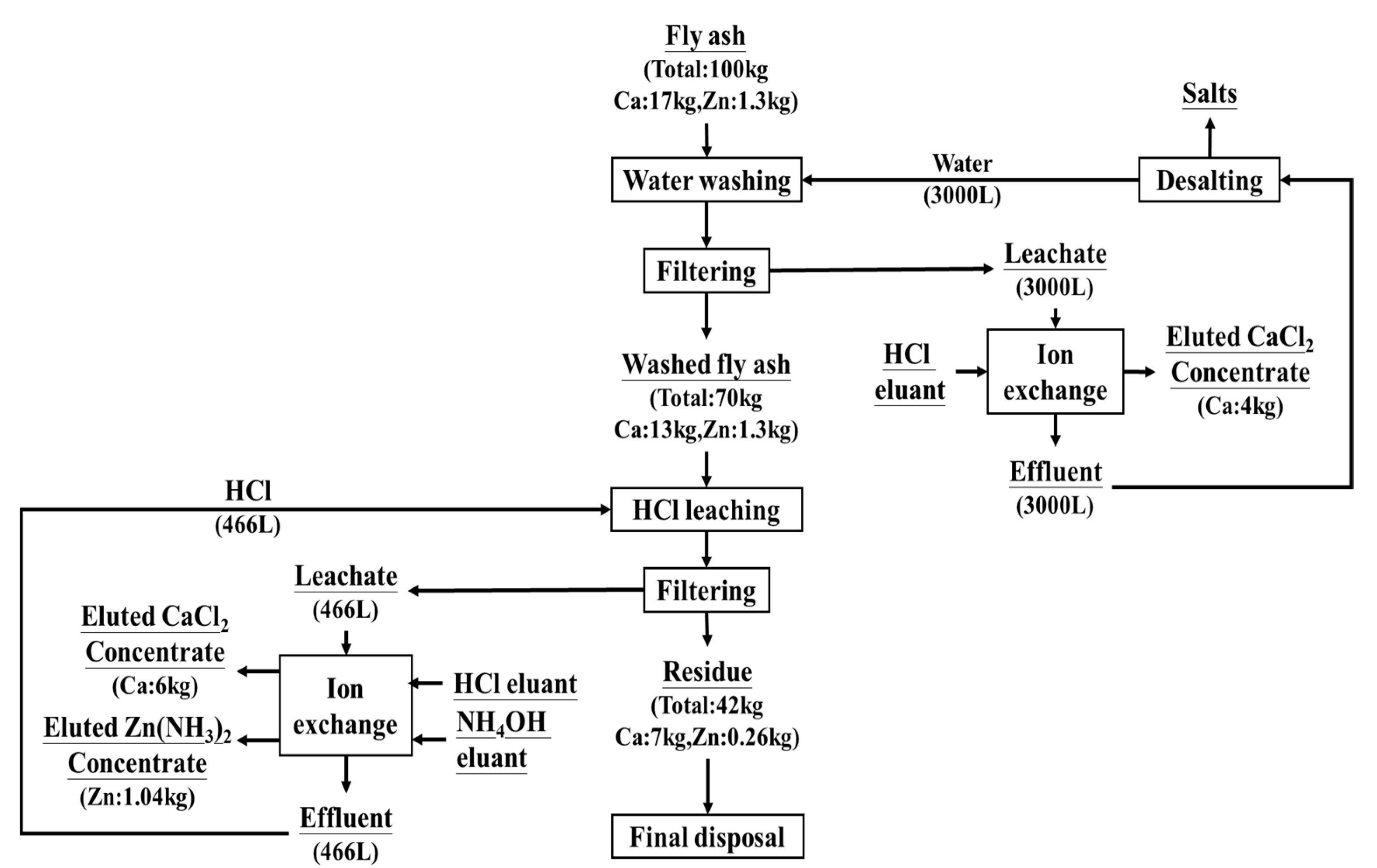
3.6. Recommended Process for Recovery of Ca and Zn
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Taiwan Environmental Protection Administration (EPA). Year Book of Environmental Protection Statistics; Taiwan EPA: Taipei, China, 2019.
- Kirby, C.S.; Rimstidt, J.D. Mineralogy and surface properties of municipal solid waste ash. Environ. Sci. Technol. 1993, 27, 652–660. [Google Scholar] [CrossRef]
- Hjelmar, O. Disposal strategies for municipal solid waste incineration residues. J. Hazard. Mater. 1996, 47, 345–368. [Google Scholar] [CrossRef]
- Rosen, C.J.; Bierman, P.M.; Olson, D. Swiss chard and alfalfa responses to soil amended with municipal solid waste incinerator ash: Growth and elemental composition. J. Agric. Food Chem. 1994, 42, 1361–1368. [Google Scholar] [CrossRef]
- Bierman, P.M.; Rosen, C.J. Phosphate and trace metal availability from sewage sludge incineration ash. J. Environ. Qual. 1994, 23, 822–830. [Google Scholar] [CrossRef]
- Nagib, S.; Inoue, K. Recovery of lead and zinc from fly ash generated from municipal incineration plants by means of acid and/or alkaline leaching. Hydrometallurgy 2000, 56, 269–292. [Google Scholar] [CrossRef]
- Zhao, L.J.; Zhang, F.S.; Wang, K.S.; Zhu, J.X. Chemical properties of heavy metals in typical hospital waste incinerator ashes in China. Waste Manag. 2008, 29, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, H.-Q.; Wei, G.-X.; Zhang, R.; Zeng, T.-T.; Liu, G.-S.; Zhou, J.-H. Characteristics and treatment methods of medical waste incinerator fly ash: A review. Processes 2018, 6, 173. [Google Scholar] [CrossRef]
- Sakai, S.; Hiraoka, M. Municipal solid waste incinerator residue recycling by thermal process. Waste Manag. 2000, 20, 249–258. [Google Scholar] [CrossRef]
- Wey, M.Y.; Liu, K.Y.; Tsai, T.H.; Chou, J.T. The thermal treatment of the fly ash from municipal solid waste incinerator with rotary kiln. J. Hazard. Mater. 2006, 137, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.W.; Ueng, T.H.; Chen, Y.S.; Chiu, J.P. Production of glass-ceramic from incinerator fly ash. Ceram. Int. 2002, 28, 779–783. [Google Scholar] [CrossRef]
- Aloisi, M.; Karamanov, A.; Pelino, M. Sintered glass-ceramic from municipal solid waste incinerator ashes. J. Non-Cryst. Solids 2004, 345–346, 192–196. [Google Scholar] [CrossRef]
- Mangialardi, T. Sintering of MSW fly ash for reuse as a concrete aggregate. J. Hazard. Mater. 2001, 87, 225–239. [Google Scholar] [CrossRef]
- Wang, K.S.; Chiang, K.Y.; Perng, J.K.; Sun, C.J. The characteristics study on sintering of municipal solid waste incinerator ashes. J. Hazard. Mater. 1998, 59, 201–210. [Google Scholar] [CrossRef]
- Hong, K.J.; Tokunaga, S.; Ishigami, Y.; Kajiguchi, T. Extraction of heavy metals from MSW incinerator fly ash using saponins. Chemosphere 2000, 14, 345–352. [Google Scholar] [CrossRef]
- Katsuura, H.; Inoue, T.; Hiraoka, M.; Sakai, S. Full-scale plant study on fly ash treatment by the acid extraction process. Waste Manag. 1996, 16, 491–499. [Google Scholar] [CrossRef]
- Zhang, F.; Itoh, H. Extraction of metals from municipal solid waste incinerator fly ash by hydrothermal process. J. Hazard. Mater. 2006, 136, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Itoh, H. A novel process utilizing subcritical water and nitrilotriacetic acid to extract hazardous elements from MSW incinerator fly ash. Sci. Total Environ. 2006, 369, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Ishigaki, T.; Nakanishi, A.; Tateda, M.; Ike, M.; Fujita, M. Bioleaching of metal from municipal waste incineration fly ash using a mixed culture of sulfur-oxidizing and iron-oxidizing bacteria. Chemosphere 2005, 60, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.-H.; Liu, C.-J.; Tsai, T.-Y.; Shen, Y.-H. A process for the recovery of gallium from gallium arsenide scrap. Processes 2019, 7, 921. [Google Scholar] [CrossRef]
- Li, X.B.; Ye, J.J.; Liu, Z.H.; Qiu, Y.Q.; Li, L.J.; Mao, S.; Wang, X.C.; Zhang, Q. Microwave digestion and alkali fusion assisted hydrothermal synthesis of zeolite from coal fly ash for enhanced adsorption of Cd(II) in aqueous solution. J. Cent. South Univ. 2018, 25, 9–20. [Google Scholar] [CrossRef]








| Composition | Wt.% |
|---|---|
| Ca | 17.14 |
| Na | 6.01 |
| K | 3.22 |
| Mg | 1.74 |
| Zn | 1.30 |
| Pb | 0.31 |
| Cu | 0.08 |
| Cd | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, C.-P.; Zhou, S.-Y.; Shen, Y.-H. The Recovery of Ca and Zn from the Municipal Solid Waste Incinerator Fly Ash. Sustainability 2020, 12, 9086. https://doi.org/10.3390/su12219086
Yen C-P, Zhou S-Y, Shen Y-H. The Recovery of Ca and Zn from the Municipal Solid Waste Incinerator Fly Ash. Sustainability. 2020; 12(21):9086. https://doi.org/10.3390/su12219086
Chicago/Turabian StyleYen, Chen-Piao, Song-Yan Zhou, and Yun-Hwei Shen. 2020. "The Recovery of Ca and Zn from the Municipal Solid Waste Incinerator Fly Ash" Sustainability 12, no. 21: 9086. https://doi.org/10.3390/su12219086
APA StyleYen, C.-P., Zhou, S.-Y., & Shen, Y.-H. (2020). The Recovery of Ca and Zn from the Municipal Solid Waste Incinerator Fly Ash. Sustainability, 12(21), 9086. https://doi.org/10.3390/su12219086





