Influence of Different Types of Land Use on the Contents of Potentially Toxic Elements and De-icing Salts in Roadside Soils and Trees in Urban Areas
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Sampling
2.2. Preparation and Chemical Analyses
2.3. Soil and Plant Potentially Toxic Elements and De-icing Salts Analyses
2.4. Statistical Analysis
3. Results and Discussion
3.1. pH, Electronic Conductivity in Roadside Soils
3.2. Potentially Toxic Elements and De-icing Salt in Roadside Soils
3.3. Potentially Toxic Elements and De-icing Salt in Roadside Tree Leaves (Ginkgo biloba)
3.4. Potentially Toxic Elements and De-icing Salts Mobility from Roadside Soil to Tree
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pourret, O.; Hursthouse, A. It’s time to replace the term “Heavy Metals” with “Potentially Toxic Elements” when reporting environmental research. Int. J. Environ. Res. Public Health 2019, 16, 4446. [Google Scholar] [CrossRef] [PubMed]
- Pourret, O.; Bollinger, J.C. “Heavy metal”-What to do now: To use or not to use? Sci. Total Environ. 2018, 610–611, 419–420. [Google Scholar] [CrossRef] [PubMed]
- Apeagyei, E.; Bank, M.S.; Spengler, J.D. Distribution of heavy metals in road dust along an urban-rural gradient in Massachusetts. Atmos. Environ. 2011, 45, 2310–2323. [Google Scholar] [CrossRef]
- Snell-Rood, E.C.; Espeset, A.; Boser, C.J.; White, W.A.; Smykalski, R. Anthropogenic changes in sodium affect neural and muscle development in butterflies. Proc. Natl. Acad. Sci. USA 2014, 111, 10221–10226. [Google Scholar] [CrossRef] [PubMed]
- Norrström, A.C.; Jacks, G. Concentration and fractionation of heavy metals in roadside soils receving de-icing salts. Sci. Total Environ. 1998, 218, 161–174. [Google Scholar] [CrossRef]
- Kakuturu, S.P.; Clark, S.E. Effects of de-icing salts on the clogging of stormwater filter media and on the media chemistry. J. Environ. Eng. 2015, 141, 4015020. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Wang, Z.; Ding, M.; Jiang, Y.; Xie, Z. Traffic-related metal (loid) status and uptake by dominant plants growing naturally in roadside soils in the Tibetan plateau, China. Sci. Total Environ. 2016, 573, 915–923. [Google Scholar] [CrossRef]
- Phillips, B.B.; Bullock, J.M.; Osborne, J.L.; Gaston, K.J. Ecosystem service provision by road verges. J. Appl. Ecol. 2020, 57, 488–501. [Google Scholar] [CrossRef]
- Karger, M.; Jutras, P.; Clark, O.G.; Hendershot, W.H.; Prasher, S.O. Macro-nutrient availability in surface soil of urban tree pits influenced by land use, soil age, and soil organic matter content. Urban Ecosyst. 2015, 18, 321–936. [Google Scholar] [CrossRef]
- Gürcan, G.; Arslan, H.; Celik, C.; Guer, S.; Kendall, M. Heavy metal content of plant species along Nilufer stream in industrialized Bursa City, Turkey. Water Air Soil Pollut. 2008, 195, 275–284. [Google Scholar]
- Tromp, K.; Lima, A.T.; Barendregt, A.; Verboeven, J.T.A. Retention of heavy metals and poly aromatic hydrocarbons from road water in an constructed wetland and the effect of de-icing. J. Hazard. Mater. 2012, 203–204, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Norrström, A.C. Metal mobility by de-icing salt from an infiltration trench for highway runoff. Appl. Geochem. 2005, 20, 1907–1919. [Google Scholar] [CrossRef]
- Ryu, H.J.; Park, I.K.; Chun, B.S.; Chang, S.I. Spatial statistical analysis of the effects of urban form indicators in road-traffic noise exposure of a city in South Korea. Appl. Acoust. 2017, 115, 93–100. [Google Scholar] [CrossRef]
- Huber, M.; Hilbig, H.; Badenberg, S.C.; Fassnacht, J.; Drewes, J.E.; Helmreich, B. Heavy metal removal mechanisms of sorptive filter materials for road runoff treatment and remobilization under de-icing salt application. Water Res. 2016, 102, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Hyun, B.K.; Sonn, Y.K.; Jeon, S.H.; Yun, S.G.; cho, H.J.; Jung, S.J.; Choi, J.W.; Lee, D.S. Revised soil survey of Chungju-Si Chunchangbukdo. Korean J. Soil Sci. Fert. 2018, 51, 143–158. [Google Scholar]
- Scott, T.; Craig, S.; Zhu, W. The effects of simulated nitrate and salt deposition on nitrogen mineralization and soil chemistry in a roadside ecosystem. Bull. N. J. Acad. Sci. 2011, 56, 9–11. [Google Scholar]
- Backstrom, M.; Karlsson, S.; Backman, L.; Folkeson, L.; Lind, B. Mobilization of heavy metals by de-icing salts in roadside environment. Water Res. 2004, 38, 720–732. [Google Scholar] [CrossRef]
- Ju, J.H.; Choi, E.Y.; Yoon, Y.H. A pilot study to determine the substrate threshold for heavy metal toxicity in groundcover plants used in urban landscape. Appl. Ecol. Environ. Res. 2016, 14, 59–70. [Google Scholar] [CrossRef]
- Vesna, G.C.; Vera, R.; Milorad, V.; Mara, T.T.; Dragica, V. Influence of heavy metals on seed germination and growth of Picea abies L. Karst. Pol. J. Environ. Stud. 2012, 21, 355–361. [Google Scholar]
- Maksymiec, W. Signaling responses in plants to heavy metal stress. Acta Physiol. Plant. 2007, 29, 177–187. [Google Scholar] [CrossRef]
- Jiang, B.; Jiang, G.B.; Liu, C.J.; Ma, K.; Jin, L.M.; Gao, J.M. Determination of metals in Ginkgo biloba leaves by atomic absorption spectrometry with microwave digestion. Spectrosc. Spectr. Anal. 2010, 30, 812–815. [Google Scholar]
- Ghavir, S.V.; Bauddh, K.; Kumar, S.; Singh, R.P. Bioaccumulation and translocations potential of Na+ and K+ in native weeds grown on industrially contaminated soil. Int. J. Chem. Tech. Res. 2013, 5, 1867–1875. [Google Scholar]
- Ascosta, J.A.; Jansen, B.; Kalbitz, K.; Faz, A.; Martinez-Martinez, S. Salinity increase mobility of heavy metals in soils. Chemosphere 2011, 85, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
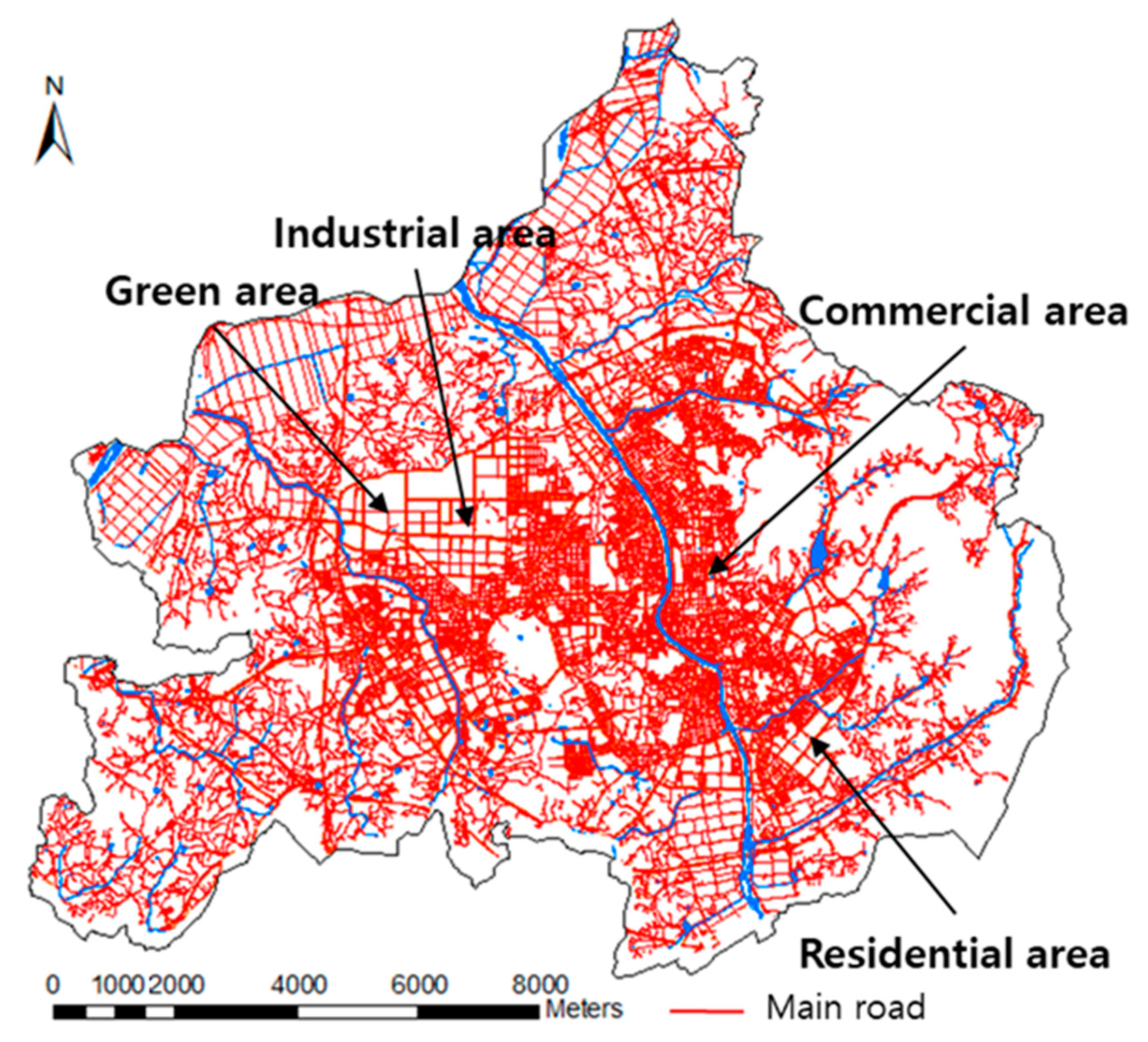
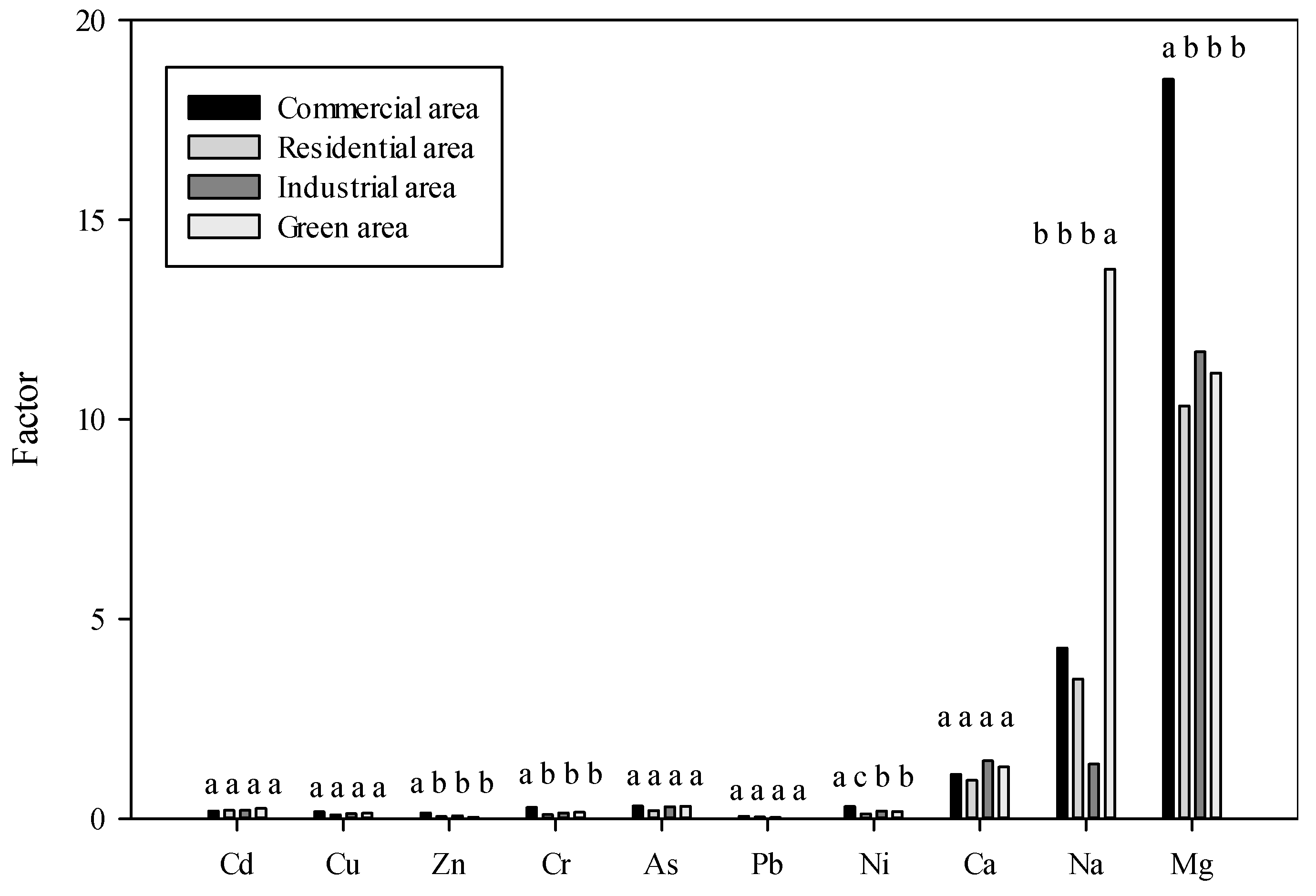
| Land Use | pH | EC (ɥS/cm) | Salinity (psu) |
|---|---|---|---|
| Commercial area | 8.30 a * | 0.25 b | 0.29 a |
| Residential area | 7.66 a | 0.53 a | 0.26 a |
| Industrial area | 7.64 a | 0.44 ab | 0.21 a |
| Green area | 6.58 b | 0.56 a | 0.27 a |
| Land Use | Potentially Toxic Elements (mg/kg) | De-icing Salts (cmol/kg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cd | Cu | Zn | Cr | As | Pb | Ni | Na | Ca | Mg | |
| Commercial area | 0.552 b * | 21.474 a | 102.324 b | 18.052 b | 8.521 c | 10.482 b | 9.958 b | 0.568 a | 7.497 b | 0.877 b |
| Residential area | 0.753 a | 35.014 a | 206.774 ab | 49.391 a | 16.617 a | 18.951 b | 24.881 a | 0.172 c | 11.622 a | 1.544 a |
| Industrial area | 0.709 a | 28.895 a | 174.084 b | 43.533 a | 12.664 b | 20.887 b | 18.528 a | 0.385 b | 9.489 ab | 1.384 a |
| Green area | 0.720 a | 31.073 a | 335.874 a | 37.684 a | 10.937 bc | 42.221 a | 19.729 a | 0.071 c | 7.940 ab | 1.263 a |
| Land Use | Potentially Toxic Elements (mg/kg) | De-icing Salts (cmol/kg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cd | Cu | Zn | Cr | As | Pb | Ni | Na | Ca | Mg | |
| Commercial area | 0.104 a * | 3.755 a | 14.663 a | 5.044 a | 2.721 a | 0.637 a | 3.035 b | 2.426 a | 8.321 c | 16.253 a |
| Residential area | 0.159 a | 3.295 a | 10.711 b | 5.223 a | 3.455 a | 0.790 a | 3.023 a | 0.601 b | 11.162 a b | 15.966 a |
| Industrial area | 0.152 a | 3.597 a | 12.064 a b | 6.315 a | 3.732 a | 0.701 a | 3.552 a | 0.527 b | 13.713 a | 16.186 a |
| Green area | 0.185 a | 4.281 a | 12.371 a b | 6.155 a | 3.411 a | 0.792 a | 3.535 a | 0.970 b | 10.268 bc | 14.098 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, J.-H.; Park, J.-Y.; Yoon, Y.-H. Influence of Different Types of Land Use on the Contents of Potentially Toxic Elements and De-icing Salts in Roadside Soils and Trees in Urban Areas. Sustainability 2020, 12, 8985. https://doi.org/10.3390/su12218985
Ju J-H, Park J-Y, Yoon Y-H. Influence of Different Types of Land Use on the Contents of Potentially Toxic Elements and De-icing Salts in Roadside Soils and Trees in Urban Areas. Sustainability. 2020; 12(21):8985. https://doi.org/10.3390/su12218985
Chicago/Turabian StyleJu, Jin-Hee, Ju-Young Park, and Yong-Han Yoon. 2020. "Influence of Different Types of Land Use on the Contents of Potentially Toxic Elements and De-icing Salts in Roadside Soils and Trees in Urban Areas" Sustainability 12, no. 21: 8985. https://doi.org/10.3390/su12218985
APA StyleJu, J.-H., Park, J.-Y., & Yoon, Y.-H. (2020). Influence of Different Types of Land Use on the Contents of Potentially Toxic Elements and De-icing Salts in Roadside Soils and Trees in Urban Areas. Sustainability, 12(21), 8985. https://doi.org/10.3390/su12218985




