Abstract
The Matāura River is the sixth largest river system in New Zealand and has long been subject to agricultural, industrial, and residential land use activities. The catchment has economic value and is of great cultural importance for local Māori, who have concerns over potential adverse impacts that anthropogenic stressors exert on the health of the river. There is a dearth of information on the impacts of these stressors towards the health of native species such as the longfin eel Anguilla dieffenbachii. This study assessed the environmental status of the Matāura River using biological and chemical methodologies incorporating A. dieffenbachii as a bioindicator species for exposure to multiple anthropogenic stressors. A range of biomarkers were measured in caged and wild-caught eels (when available) to characterize site-specific responses to anthropogenic stressors. While there was no clear indication of cumulative impacts moving from pristine headwaters to the lower reaches of the Matāura River, biomarkers of xenobiotic metabolization were induced in A. dieffenbachia and there was evidence of chemical contamination in sediment and tissue samples.
1. Introduction
River systems span a broad range of ecosystems and can transport chemicals and particles long distances before being deposited in the ocean. As headwaters progress through the landscape, they can potentially accumulate contaminants, in part due to increasing anthropogenic activity and pressure. This can impact multiple ecosystems with increasing severity as contaminants accumulate along the river, particularly in zones of reduced energy that typically predominate in lower reaches. To develop a comprehensive assessment, a “whole catchment approach” of ecosystem health can provide valuable information on the nature and severity of various aspects of river health as well as identify potentially impacted sites. An assessment of ecosystem health at multiple points along a river system can provide evidence of whether there are potential adverse effects on the environment due to anthropogenic activity, and insight into their potential sources. In this sense the Matāura River, which lies in the Murihiku (Southland) region of the South Island of New Zealand, is an ideal case study. It is New Zealand’s sixth longest river with a length of 240 km and encompasses the Southland region’s second largest catchment (5360 km2) in both area and water flow [1,2].
The Matāura River originates near Eyre Peak in the Taka Ra Haka Conservation Park (Eyre Mountains) located to the west of the Kingston arm of Lake Wakatipu in Northern Southland. The headwaters are fed predominantly by rainfall, with a significant spring snowmelt contribution to the springtime flow. The Waikaia River is the largest of the Matāura’s numerous tributaries, comprising just over half of the catchment area (1360 km2) at their confluence [1,2]. From Gore, the Matāura River flows south through a long fertile river plain where it becomes more entrenched and its gradient gradually decreases before entering the Toetoes Estuary, a small lagoon-type estuary, prior to discharging into the sea at Fortrose at the bottom of the South Island [2]. The Matāura River and its tributaries have long been valued worldwide by sports fisherman for the quality of the brown trout fishery it maintains.
Agricultural land use predominates in the surrounding catchments and is likely to be a major contribution of non-point source inputs into the river. The towns along the river are sites of significant agriculturally based industries including meat works, dairy factories and wood processing mills, all discharging wastewater into the river. These towns provide point source entry of stormwater and treated municipal wastewater. The Matāura River has significant historical and spiritual relevance for local Māori tribes, and there is growing public concern over the decline of water quality and the health of the river.
In New Zealand, there is a paucity of information regarding the effects of chemical contaminants on native species. The Australasian component of the Society of Environmental Toxicology & Chemistry (SETAC) Global Horizon Scanning Project identified the question: “What are the combined effects of very low levels of multiple contaminants?” as a priority research question for the region [3]. This highlights the need to investigate the health status of riverine systems under pressure from multiple stressors by the application of relevant test species. Tuna, the Māori name for freshwater eels in New Zealand, have been previously used in environmental studies due to their facultative catadromous nature and long life cycles facilitating interactions with contaminated sediment and prey for extended periods of time [4,5] In addition to these features, their naturally high lipid content can potentially lead to the rapid and extensive accumulation of hydrophobic organic contaminants [6], resulting in eels being recognized as useful bio-indicators of the effects of localized aquatic pollution [7]. Longfin eels (Anguilla dieffenbachii) are the only freshwater eel endemic to New Zealand and are widely distributed throughout the country’s lakes and rivers, making them an ideal species for assessing ecotoxicological hazards in aquatic ecosystems. A. dieffenbachii is a treasured (taonga) and an iconic food species of current and historical importance for the wellbeing of Māori, the indigenous people of New Zealand.
The aim of this study was to investigate the influence of anthropogenic pressures at seven sites along the Matāura River system, particularly the potential effects of non-point-source and diffuse inputs using A. dieffenbachii. The study had two components: (i) analysis of sediment and biological tissue samples for the presence of selected priority chemical contaminants and (ii) an assessment of sublethal effects towards A. dieffenbachii. To achieve this health condition, indices and a series of enzymatic exposure biomarkers were measured to assess the potential impacts of anthropogenic stressors within caged and resident eels in the Matāura River, New Zealand.
2. Materials and Methods
2.1. Study Sites
Seven sites along the length of the Matāura River catchment were selected for investigation following consultation with local authorities and environmental regulators (Figure 1). Key factors considered in the selection of these sites included their cultural relevance to Maori, recreational value (hikers, swimmers and trout fisherman), aquatic and terrestrial biodiversity, commercial value for eel and trout fishing, and urban and industrial discharges.
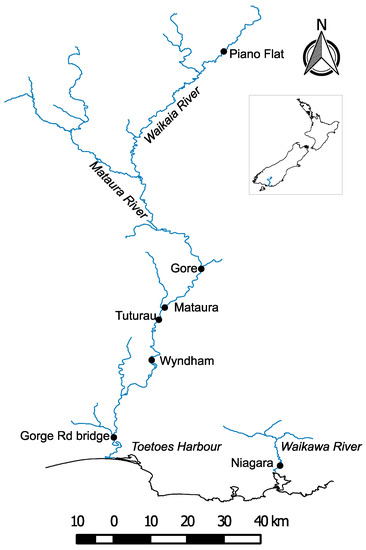
Figure 1.
Study sites of the caged and wild-caught eels on the Mataura and Waikawa Rivers.
The first site located in Piano Flat on the Waikaia River was close to the river’s headwaters, surrounded by native bush, and was the most upstream site in this study. Apart from some historical gold prospection, little anthropogenic activity has occurred in these headwaters. Five other sites were located downstream along the length of the Matāura River. The second site was located within the town boundaries of Gore and downstream from the sewage treatment plant and stormwater discharges to address concerns raised by the public about unregulated overflow discharges. The third site near the township of Matāura was located directly downstream of the discharge of treated effluent from a meat works industry that processes lamb, sheep and cattle. It is significant that the stretch of the Matāura River intersecting the township of Matāura was the first inland river site in New Zealand to gain the status of mātaitai reserve (managed by indigenous locals) [8]. The fourth site, at Tuturau, was located downstream from the discharge of the Matāura sewage treatment plant. Wyndham, the fifth site, is the location where Southland District Council discharges stormwater and septic tank effluents into the Matāura River. Gorge Road, the sixth site, was located in the lower reaches of the Matāura River where it is surrounded by agricultural land use but is also a deposition zone for sediment, making it ideal for assessing cumulative impacts from activities upstream. It is also the furthest point downstream in the Matāura River outside of tidal influence. The last site was located at Niagara in the adjacent Waikawa River away from the Matāura River catchment. The site has cultural significance and a history of being a location of indigenous temporary settlements. It is surrounded by farmland and native bush.
2.2. Field Setting
2.2.1. Eels Caging
A total of 80 non-migratory longfin eels of mixed sex, age and length (46–61 cm) were captured from Wyndham under the customary permits SI 01567 and SI 06305. Eels were maintained in flow through tanks at 10–12 °C in an eel fishery indoor tank facility for 3–4 weeks prior to caging to allow depuration of potential chemical contamination. During the first week, eels were treated with sea salt (2.5 g/L) under static conditions for a period of 24 h to remove any traces of the rock snot algae (Didymosphenia geminata) and fungal (white spot) or bacterial (fin rot) infections. No mortality or disease was observed in the eels during this period. At the end of the decontamination period, five eels were randomly allocated to one of 16 knotless nylon eel catch bags interspersed with rectangular aluminium hoops (approximately 120 × 45 × 45 cm) [9,10,11]. Each bag was placed in a stainless-steel cage (120 × 50 × 50 cm) for protection. Two cages were randomly allocated to each of the study sites. Deployment of the cages was carried out over a two-day period and began with the upstream sites followed by those further downstream.
Where feasible, cages were submerged at locations close to the riverbank where they could benefit from natural shading and protection from strong currents. The cages were secured to a stainless-steel fence post driven into the bank by a chain and secured with locks. Lengths of PVC piping were inserted into the nylon nets to provide shelter habitat for the eels during daylight hours.
Eels were deployed in the field in late spring (November 2007) and late summer (March 2008) for a period of 21 days. Previous research demonstrated this to be a sufficient time for triggering a range of physiological responses resulting from acute (short term) or sub-chronic (medium term) exposures to environmental pollutants [12]. The eel cages were physically and visually checked weekly for damage or fouling due to river debris, and for evidence of abnormal fish behaviour.
2.2.2. Capture of Resident Eels
Eels naturally residing at each of the sampling sites—resident eels—were caught for comparison with the caged eels. Fyke nets were set at each site a week prior to retrieving the cages and placed within 200 m either up or down stream of each cage site. Resident eels were captured using raw eggs as an attractant placed inside plastic containers and secured inside 25 mm stretch mesh fyke nets. These resident eels were caught and harvested using customary permits SI 01567 and SI 06305.
2.3. Sampling
2.3.1. Sediment Sampling
Samples were collected using a stainless-steel auger pushed to a depth of 10 cm, working downstream to upstream from the cage sites. Approximately 500 g of sediment was collected as a composite of subsamples collected over an area of about 1 m2. Sediment was subsequently sub-sampled into pre-cleaned 250 mL glass jars (100 g for metal analysis and 100 g for semi-volatile organic compounds) and stored in the dark at 4 °C for transportation to the lab. Samples were then frozen at −20 °C until analysis [13].
2.3.2. Tissue Sampling
After 21 days of exposure, the caged eels were retrieved from the field sites. Rain events in summer produced high river flows that damaged some of the cages, allowing the escape of some eels and potentially adding additional stress to the fish. The eels were anesthetized using clove oil [14] to minimize effects on blood chemistry [15], before being measured (weight and length) and killed by spinal severance. Otoliths were collected to determine the age of the fish. The sex was not recorded because non-migratory eels remain in a state of sexual immaturity [16,17] and reproductive organs are regressed, making it difficult to distinguish testis from ovary tissue. A sample of fillet (60 g) from the true right of the animal was removed, split into two and frozen at −20 °C for further chemical analysis. Liver and gill samples collected for biomarker analysis were snap frozen and kept in liquid nitrogen until use.
2.4. Chemical Analysis
2.4.1. Sediment Analysis
Chemical analysis of total recoverable metals in sediments was carried out by R J Hill Laboratories Ltd. (Hamilton, New Zealand) [18].
For organic contaminant analysis, sediments were thoroughly mixed and stored at −18 °C until further analysis. A 10 g sample was dried at 50 °C for 24 h to determine sediment dry weight. Organochlorine pesticides (OCs) and polychlorinated biphenyls (PCBs) in the sediment were analysed using a single extraction and a three-stage clean-up method. A 40 g aliquot of sediment was spiked with 50 ng of aldrin and α-BHC as internal recovery standards. The sediment was extracted by sonication and shaking with a mixture of hexane/acetone solvent. The extract was filtered and partitioned with water and a subsample of the upper hexane layer removed and concentrated. The resulting crude extract was subjected to gel permeation chromatography (GPC) to remove lipids and other high molecular weight co-extracted substances and isolate the fraction containing the OCs and PCBs. This isolated fraction was concentrated and further purified and fractionated using Florisil® column chromatography to produce two fractions: (i) fraction A, containing all the PCBs, several OCs such as dichloro-diphenyl-trichloroethane (DDT) metabolites (p.p. dichloro-diphenyl-dichloroethane (DDE)) and the internal recovery standard aldrin, and (ii) fraction B containing the chlordanes, the remainder of the OCs including dieldrin and the internal recovery standard α BHC.
Quantitative analysis was performed using high resolution gas chromatography with ion trap MS/MS detection using a Varian Star 3400CX Gas Chromatograph fitted with a Varian SPI temperature programmable injector and Varian Saturn 2000 ion-trap mass spectrometer. Retention times and spectral matches were used to identify compounds. Calibration standard solutions were prepared from pure analytical standards. Chlordanes were quantitated using standards of the cis-and trans-chlordane isomers supplied by the United States Environmental Protection Agency (US-EPA). A multiplication factor of 2.33 was applied to the sum of the isomers to derive equivalent technical chlordane levels.
Polycyclic aromatic hydrocarbons (PAHs) and chlorophenols were analysed by removing aliquots of the same crude hexane solvent extract. The aliquot of hexane for PAHs analysis was concentrated and subjected to GPC followed by Alumina column chromatography clean-up. PAHs in the purified sample extracts were analysed by high performance liquid chromatography (HPLC) with fluorescence detection and quantitated against a mixed PAH standard (US-EPA). The sub-sample of the raw hexane extract for chlorophenol analysis was subjected to acid/base partitioning before the chlorophenols were acetylated with acetic anhydride. Quantitative analysis was carried out by high resolution gas chromatography with ion trap MS/MS detection using a Varian Star 3400CX Gas Chromatograph fitted with a Varian SPI temperature programmable injector and a Varian Saturn 2000 ion-trap mass spectrometer. Retention times and spectral matches were used to identify compounds. Calibration standard solutions were prepared from pure analytical standards.
The detection limits for OCs, PCBs, PAHs and chlorophenols were 0.05, 0.05, 0.3 and 0.5 ng/g dry weight (dw), respectively. Limits of quantitation (LOQ) were set at three times the detection limit (Table A1 in Appendix A). Results less than the LOQ are reported but the levels are in the region of uncertainty. Where compounds were not detected, or below the limit, “N.D” was used (Analytical method detection limits in Tables S1–S4). Recovery experiments were carried out for most compounds by spiking sub-samples of the sediment at two levels (0.3 and 3.2 ng/g dw) of each compound and analysed by the method described above.
For total organic carbon content (TOC), sediments were pre-treated with acid to remove inorganic carbonates and dried to a constant weight at 105 °C. TOC concentrations were determined by elemental analysis (Elementar Combustion Analyser).
2.4.2. Tissue Analysis
Chemical analyses were carried out on composite samples of eel fillets prepared from animals contained in the cage from each sample site together with the harvested resident eels. The first frozen portion of fillet was analysed for total trace metals (mercury, lead, cadmium, copper, zinc, arsenic and chromium) by Hill Laboratories (Hamilton, New Zealand) [18]. The second portion of the sample was sent to HortResearch (Hamilton, New Zealand) and analysed for organic contaminants (polychlorinated bi-phenyls, DDT, polycyclic aromatic hydrocarbons, organochlorine pesticides) using the methods described above. Chemical analyses of PCBs, organochlorines and PAHs were not carried out in eel sampled during summer since no chemical was detected in sediments.
2.5. Biological Analysis
2.5.1. Health Condition Indices and Age Determination
Health between individuals was assessed using the hepato-somatic index (HSI) [19]. Sagittal otoliths were prepared using the crack-and-burn method [20]. Otolith fragments were mounted in silicone rubber sealant on microscope slides and observed under a binocular microscope using reflected light. Age was estimated by counting the number of black winter rings [21].
2.5.2. Biomarker Analysis
Frozen tissues (gills and liver) of each animal were weighted and homogenized in phosphate buffer (pH 7.4, 100 mM) (1:3 w:v) with a PYREX® Potter–Elvehjem homogenizer. Homogenates were centrifuged for 30 min (4 °C) at 9000 g [22]. The sub-mitochondrial fractions (S9) were aliquoted and stored at −80 °C before use. Four biomarkers of exposure were measured both in liver and gills using the procedure described in [23]: the ethoxyresorufin-O-deethylase (EROD) [24], following the modified protocol for the microplate reader [25], glutathione-s-transferase (GST) [26] and catalase [27] activities and the concentration of endogenous malondialdehyde expressing the level of lipid peroxidation were assessed through the measurement of thiobarbituric acid reactive species (TBARs) [28]. All data were expressed in relation to protein concentration determined using the fluorescamine method [29]. Results were analysed by comparing biomarker responses between resident and caged eels as well as differences between test sites.
2.6. Statistical Analyses
Statistical analyses were carried out using R [30]. A multiple comparison of biomarker values between sites was carried out using a nonparametric Kruskal–Wallis test followed by Dunn’s test adjusted for multiple comparisons using a Bonferroni adjustment (“dunn.test” package [31]) with a statistical difference determined at p < 0.05.
3. Results
On both field sampling trips, the Murihiku region received significant rainfall prior to the recovery of the caged eels. While care was taken to locate the cages in locations away from the main current flows, the contribution from these rain events produced high river flows, which resulted in some cage damage where animals escaped and could not be recovered (Table 1).

Table 1.
Number of caged eels recovered and number of resident eels caught.
3.1. Chemical Analyses
3.1.1. Sediments
The concentration of metals in sediment sampled in spring did not exhibit a consistent trend in concentration between the different sampling sites, but in general the concentration was higher in sediment downstream at Wyndham compared to that in the headwaters at Piano Flat (Table 2). The concentration of Cu and Pb in sediment from the Wyndham and Niagara sites was higher than the other sites and Hg was only detected in sediment from the Wyndham site. In summer, the concentrations of Pb and Zn generally increased in sediment at all the sampled sites compared to those in spring. Notably, Hg was detected in sediment at all sites sampled in summer (concentrations of trace metals in sediment samples in Table S5).

Table 2.
Concentrations of trace metals in sediment (in mg/kg dry weight) in spring and summer samples.
Recoveries of the α BHC and aldrin surrogate recovery standards added to each sample were consistent with mean recoveries of 88 ± 13% and 62 ± 14%, respectively, at the 95% confidence level, and CVs were within 21% (Table A1). The results are not corrected for recovery.
Resides of PCBs were not detected in any of the sediment samples and are not discussed further. In comparison, residues of DDT were detected in all sediment samples and, together with other organochlorine pesticides, their concentration in sediment increased going downstream (Table 3), with the highest concentrations at Gorge Rd. Residues of chlorophenols were only detected in sediment at the Tuturau site and organochlorine pesticides in sediment from the Matāura and Tuturau sampling sites. Interestingly, the sediment from the Niagara sampling site on the Waikawa River had a similar concentration of DDT residues to sediment from the Wyndham site on the Matāura River (concentrations of residues in sediment in Tables S7 and S8).

Table 3.
Total concentration of chlorophenol, DDT and other organochlorines residues in sediment samples (µg/kg dry weight) in spring.
The concentration of PAHs (Table 4) were higher in sediment sampled in spring than in summer. The concentration of PAHs in sediment from Gorge Road in the lower Mataura River was one to two orders of magnitude higher in concentration than sediment from the other sites in spring, and three orders of magnitude greater than sediment sampled from the same site in summer (concentrations of residues in sediment in Table S9).

Table 4.
Sum A of polycyclic aromatic hydrocarbons (PAHs) residues in sediments in spring and summer (µg/kg dry weight).
3.1.2. Eels
Copper (Cu) and zinc (Zn) were the metals most frequently detected in the flesh of eel and at higher concentrations than Cadmium (Cd), lead (Pb) and mercury (Hg) (Table 5). Trace metal residues in caged (average ± standard deviation) and resident eels’ fillet (mg/kg dry weight) in spring and summer are shown. Lead was found in caged eels’ fillets in spring but was not detected in summer (trace metal concentrations in caged and resident eels are summarised in Table S10).

Table 5.
Residue of trace metals in caged (average ± standard deviation) and resident eels’ fillet (mg/kg dry weight) in spring and summer.
No residues of PCBs, chlorophenols or PAHs were detected above the reporting limit in the tissue of eel at any of the sampling sites during the spring or summer sampling. In comparison, residues of the organochlorine pesticide DDT were measured in eel samples from both sampling times (Table 6). This reflects continued exposure of eels in Southland and other regions of New Zealand to historical residues of DDT that were applied to pasture to control grass grub and other grass- and crop-eating insect pests (residue concentrations in eels are summarised in Tables S11 and S12).

Table 6.
Concentration of DDT pesticide residues (sum of DDT congeners) in eels’ fillet (μg/kg dry weight).
3.2. Biological Analysis
3.2.1. Age Determination and Health Condition Index
The age, length and weight range of caged eels were similar within and between the two sampling times (Table 7); so were the residents together. Bigger resident eels were captured upstream of Matāura. Hepatosomatic indices were similar within and between the two sampling times except for the resident eels at Piano Flat in summer, which were statistically different (p < 0.05) from the others (age and condition indices are summarised in Tables S13 and S14).

Table 7.
Average (±standard deviation) of age, length, weight and hepatosomatic (HSI) index for caged and resident eels in spring and summer. (n/a: animals not available). Asterisk (*) indicates a significant difference (p < 0.05) from other sites at the same season.
3.2.2. Biomarkers
GST and TBARs measured in eels’ livers in spring did not show significant differences between the seven sampling sites. Measurement of the EROD in the livers of caged eels in Wyndham and Niagara was significantly higher when compared to that in eel from the most upstream site (Piano Flat) (Figure 2B). In comparison, hepatic EROD activitiesin resident eels were statistically different between the most upstream site (Piano Flat) and the first township downstream site at Gore. The EROD activity in eels’ livers from Gore was statistically higher than that in eel liver from Tuturau about 20 km downstream (Figure 2B). GST activity in the gills from caged eels in Piano Flat (Figure 2D) was significantly higher than values measured in Gore, Tuturau, and Gorge Road (Figure 2D). GST, catalase and TBARs measured in gills of resident eels showed a significant difference between Gore and the further downstream sites of Matāura, Tuturau and Niagara (Figure 2D–F).
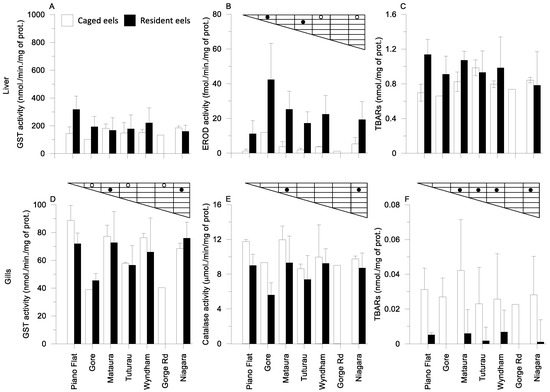
Figure 2.
Biomarkers (A,D): GST; (B): EROD; (C,F): TBAR; (E): catalase measured in caged and resident eels’ livers and gills in spring at the seven sites. (Statistical significance (p < 0.05) between sites is indicated for ○ caged and ● resident eels.).
In summer, a statistical difference was observed in EROD activity in the liver of caged eels from the upper catchment site at Piano Flat and the most downstream site at Gorge Road (Figure 3B). EROD activity in resident eels’ livers at the Matāura site was significantly lower than that at the Gore (about 15 km upstream), and Wyndham (about 20 km downstream) sites (Figure 3B).
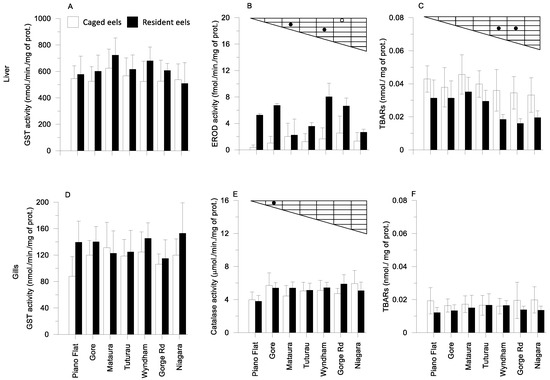
Figure 3.
Biomarkers (A,D): GST; (B): EROD; (C,F): TBAR; (E): catalase measured in caged and resident eels’ livers and gills in summer at the seven sites. (Statistical significance (p < 0.05) between sites is indicated for ○ caged and ● resident eels.).
In spring and summer, the EROD activity is always higher in resident eels than in caged eels. The EROD activity along the sampling sites between the two seasons seems to follow a similar pattern with the lowest activity observed in eels from Piano Flat, followed by an increase in Gore and a progressive decrease going downstream.
The TBARs in liver of resident eels from the two most downstream sites on the Mataura River (Wyndham and Gorge Rd) were significantly lower than those from the Tuturau site (about 15 km upstream) (Figure 3C). With respect to gill biomarkers, only catalase from resident eels at the Gore site were significantly different from those at the most upstream site (Piano Flat) (Figure 3E).
Qualitatively, GST activity in the liver and gills (slightly) of eel were elevated in summer compared those in spring. In contrast, EROD, catalase and TBARS were notably lower in summer compared to spring (biomarkers in eels in spring and summer in Tables S13 and S14).
4. Discussion
This study was a first health assessment of the Matāura River that integrated physicochemical and biological-effect data to assess the potential adverse effects of anthropogenic stressors derived from land use activities in that catchment. The concentration of recognized anthropogenic contaminants (metals, organochlorines, PAHs) in the analysed sediment samples generally fell below the Australian and New Zealand Environment and Conservation Council (ANZECC) sediment default guideline values [32], with the exception of the Gorge Road site. Sediment from the Gorge Road site was the only one in which contaminants exceeded the ANZECC sediment guideline default values for zinc in summer (200 mg/kg of dry sediment), and for Total DDT in spring (5 µg/kg of dry sediment). While the concentration of PAHs in Gorge road sediment sampled in spring did not exceed the ANZECC sediment default guideline value, it was an order of magnitude higher than sediments at any other site. The absence of residues of PCBs, together with the infrequent detection of residues of chlorophenols and organochlorine pesticides other than DDT in sediment from the Matāura River, is explained by the absence of their production and use within the catchment. In comparison, the presence of DDT residues in all the analysed sediment samples reflects the previous widespread historical use of DDT on pasture within the Southland region and the continuing leakage of soil residues by erosion and their subsequent migration into riverine catchments in Southland.
When comparing the metal concentrations in the sediment samples, they varied between the spring and summer seasons, most notably for zinc at the Tuturau and Wyndham sites. While the concentration of Zn remained under guideline values, it suggests a seasonal effect with respect to potential anthropogenic inputs, which could pose a risk to these ecosystems. The concentrations of persistent organic contaminants in sediment at each site were consistent with those for metals, with their concentrations falling below ANZECC guidelines. Regardless, the elevated concentration of PAHs in sediment from the Gorge Road site is of concern as there is strong evidence demonstrating that the PAHs in sediments are a main contributor to toxicity [33]. Overall, the risk posed by these sediment-borne contaminants to aquatic organisms is low based on their respective guideline values.
Variations of chemicals’ concentrations in sediments between spring and summer can be explained by the physical characteristics of the river. The riverbed of the Matāura River contains schistose sediment and weakly weathered gravel [34]. The region often experiences intense rain events resulting in high river flows and flash floods that wash out and strip deposited sediment and accumulated contaminants from the hard river bedrock further downstream and into the Mataura River estuary at Fortrose.
However, measuring endpoints at differing levels of biological organisation in a sentinel animal species (biological receptors) integrates realistic exposure to multiple, or mixtures of, contaminants.
All the tissue trace metal concentrations measured in eel were lower than those reported in other overseas eel species [4,35]. In comparison to previous overseas studies, no residues of PCBs, chlorophenols or PAHs were detected in the tissues of eel in this study [35,36]. DDT residues were measured in all the eel tissue samples at comparable concentrations to previous measurements. The results are particularly interesting as the elevated concentration of PAHs and DDT in sediment from the Gorge Road site suggests that A. dieffenbachii would similarly accumulate elevated residues of PAHs and DDTs. This is not supported by the tissue data, which suggest that contemporary sediment concentrations of these contaminants are not high enough to lead to elevated biological accumulation, and/or that A. dieffenbachii is able to detoxify and depurate these contaminants. There was no correlation between the sum of DDT congeners and the EROD response, which suggests that the fish may be exposed to other inducers likely found in the water column. It is important to use both bioaccumulation and biomarker response data in environmental risk assessment frameworks [37].
This interpretation is supported by the biological results. Previous biomarker studies in the European eel Anguilla anguilla [38] have demonstrated limited effects on GST and catalase response following exposure to PAHs. However, EROD activity is a biomarker that generally responds to PAHs exposures in European eels and other fish species [37,39]. Sublethal effects measured through enzymatic biomarkers in A. dieffenbachii showed no clear evidence adverse effects of anthropogenic effects and natural variations [40,41]. Using contaminant tissue residues to assess sublethal impacts of pollution is ineffective as effects will only emerge when fat is metabolized [42]. The accumulation of residues is likely linked to historical rather than contemporary environmental conditions. Further investigations assessing the potential long-term implications of anthropogenic contaminants within the Matāura River catchment may require the use of alternative bioindicator species, and/or biomarkers of exposure.
5. Conclusions
This study using A. dieffenbachii suggests that despite an increase in anthropogenic activity and inputs down the length of the river, there is limited evidence of increasing negative effects and, therefore, negligible adverse outcomes occurring in the Matāura River system as it progresses towards the sea. Despite this lack of evidence, future studies may be necessary to fully characterize the health status of the Matāura River using alternative bioindicator species across trophic levels. For example, alternative fish species like Gobiomorphus cotidianus (common bully), which are in more immediate contact with prey habituating sediment, may have a greater exposure to, and exhibit a heightened sensitivity to, the low levels of contamination that were present in the sediment of the Matāura River.
Supplementary Materials
The following are available online at https://www.mdpi.com/2071-1050/12/20/8412/s1. Table S1. Analytical method detection limits (MDL) in mg.kg−1 (ppm) for organochlorine pesticides; Table S2. Analytical method detection limits (MDL) in mg.kg−1 (ppm) for chlorinated phenols; Table S3. Analytical method detection limits (MDL) in mg.kg−1 (ppm) for polychlorinated biphenyls; Table S4. Analytical method detection limits (MDL) in mg.kg−1 (ppm) for total recoverable trace metals; Table S5. Concentration of trace metals in sediment samples from the Matāura River (mg.kg−1 (ppm) dry weight); Table S6. Concentrations of chlorophenol residues in sediment samples from the Matāura River (µg.kg−1 (ppb) dry weight of sample); Table S7. Concentrations of organochlorine pesticide residues in sediment samples from the Matāura River (µg.kg−1 (ppb) dry weight of sample); Table S8. Concentrations of polychlorinated biphenyls residues in sediment samples from the Matāura River (µg.kg−1 (ppb) dry weight of sample); Table S9. Polycyclic aromatic hydrocarbons residues in spring sediment samples from the Matāura River (µg.kg−1 (ppb) dry weight of sample); Table S10. Trace metal concentration in caged and resident eels (mg.kg−1 (ppm) dry weight of sample); Table S11. Concentrations of organochlorine pesticide residues in eels (µg.kg−1 (ppb) dry weight of sample); Table S12. Concentrations of polycyclic aromatic hydrocarbons (PAHs) residues in eels (µg.kg−1 (ppb) dry weight of sample); Table S13. Size, condition index and biomarker values for caged and resident eels in spring; Table S14. Size, condition index and biomarker values for caged and resident eels in summer.
Author Contributions
Conceptualization, J.M.A., L.A.T. and G.L.N.; methodology, J.M.A., G.L.N. and L.A.T.; formal analysis, O.C.; investigation, J.M.A., O.C., G.K., G.L.N.; resources, J.M.A.; data curation, G.L.N. and O.C.; writing—original draft preparation, O.C., G.L.N. and A.B.; writing—review and editing, A.B., J.M.A., L.A.T., G.L.N.; visualization, O.C.; supervision, J.M.A. and L.T.; project administration, J.M.A.; funding acquisition, J.M.A. and L.A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Nga Pae o Te Maramatanga “He pūau awa—he ūngututanga mātauranga: Environmental reporting for a riverine system—a bicultural approach” (06-RF1-22) and co-funded by NZ Ministry of Business Innovation and Employment grants (MBIE C03X0902 and CAWX1708). G.K. was supported by a Japan Society for the Promotion of Science Overseas Research Fellowships.
Acknowledgments
The authors wish to thank Manaaki Whenua (Landcare Research Ltd.), Te Ao Mārama Inc. (TAMI), Henare Makoare, site manager at the Mataura Industrial Estate for access to facilities to process samples, Hokonui Rūnanga and Environment Southland staff for their support, help and collaboration and Gretchen Rash for editing. The caging experiment was carried out under the Landcare Research Animal Ethic Committee approval no. 07/09/04. We thank Gretchen Rasch (Cawthron) for editorial services.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A

Table A1.
Analytical method detection limits (mg/kg) for metals and organic compounds.
Table A1.
Analytical method detection limits (mg/kg) for metals and organic compounds.
| Sediment | Biota | |
|---|---|---|
| Total recoverable metals | ||
| Cadmium | 0.010 | 0.020 |
| Chromium | 0.20 | N/A |
| Copper | 0.20 | 0.050 |
| Lead | 0.040 | 0.010 |
| Mercury | 0.010 | 0.010 |
| Zinc | 0.40 | 0.50 |
| Organic compounds | ||
| Organochlorine pesticides | 0.001 | 0.001 |
| Equivalent technical chlordane | 0.002 | 0.003 |
| Chlorinated phenols | 0.001 | N/A |
| Polychlorinated biphenyls (PCBs) | 0.001 | 0.001 |
| Sum of 35 PCB congeners | 0.04 | 0.04 |
| Polycyclic aromatic hydrocarbons (PAHs) | 0.001 | 0.001 |
| Sum of 16 PAHs | 0.02 | 0.02 |
References
- Witherow, W.D.; Scott, D. The Mataura Trout Fishery; Acclimatisation Societies of Southland and Otago: Invercargill, New Zealand, 1984; p. 71. [Google Scholar]
- Ryder, G. Matāura Catchment: Water Quality Review; The Southland Regional Council: Invercargill, New Zealand, 1995; p. 250.
- Gaw, S.; Harford, A.; Pettigrove, V.; Sevicke-Jones, G.; Manning, T.; Ataria, J.; Cresswell, T.; Dafforn, K.A.; Leusch, F.D.; Moggridge, B.; et al. Towards sustainable environmental quality: Priority research questions for the australasian region of Oceania. Integr. Environ. Assess. Manage. 2019, 15, 917–935. [Google Scholar] [CrossRef] [PubMed]
- Maes, G.E.; Raeymaekers, J.A.M.; Pampoulie, C.; Seynaeve, A.; Goemans, G.; Belpaire, C.; Volckaert, F.A.M. The catadromous European eel Anguilla anguilla (L.) as a model for freshwater evolutionary ecotoxicology: Relationship between heavy metal bioaccumulation, condition and genetic variability. Aquat. Toxicol. 2005, 73, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Patey, G.; Couillard, C.M.; Pierron, F.; Baudrimont, M.; Couture, P. Biotransformation, antioxidant and histopathological biomarker responses to contaminants in European and American yellow eels from the Gironde and St. Lawrence estuaries. Chemosphere 2017, 188, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Robinet, T.T.; Feunteun, E.E. Sublethal effects of exposure to chemical compounds: A cause for the decline in Atlantic eels? Ecotoxicology 2002, 11, 265–277. [Google Scholar] [CrossRef]
- Belpaire, C.; Goemans, G. The European eel Anguilla anguilla, a rapporteur of the chemical status for the Water Framework Directive. In Proceedings of the GIS GRISAM EEL Meeting (Perpignan 2006-04-24), Laboratoire Arago, Université Pierre et Marie Curie, Paris, France, 24 April 2006; Volume 57, pp. 235–252. [Google Scholar]
- NZ Fisheries. South Island Customary Fishing, Regulations 1999—Declaration of Mātaitai Reserve at Mataura River and Appointment of Tangata Tiaki/Kaitiaki—Notice 2005-go5185 (No. F329). 2005; Issue number 123, 3010p. Available online: https://gazette.govt.nz/notice/id/2005-go5185 (accessed on 23 March 2020).
- Fenet, H.; Casellas, C.; Bontoux, J. Laboratory and field-caging studies on hepatic enzymatic activities in European eel and rainbow trout. Ecotoxicol. Environ. Saf. 1998, 40, 137–143. [Google Scholar] [CrossRef]
- Fenet, H.; Gomez, E.; Rosain, D.; Casellas, C. Polycyclic aromatic hydrocarbon metabolites and 7-ethoxyresorufin O-deethylase activity in caged European eels. Arch. Environ. Contam. Toxicol. 2006, 51, 232–236. [Google Scholar] [CrossRef]
- van den Heuvel, M.R.; Landman, M.J.; Tremblay, L.A. Responses of shortfin eel (Anguilla australis) exposed in situ to pulp and paper effluent. J. Toxicol. Environ. Health A 2006, 69, 1763–1779. [Google Scholar] [CrossRef]
- Oikari, A. Caging techniques for field exposures of fish to chemical contaminants. Aquat. Toxicol. 2006, 78, 370–381. [Google Scholar] [CrossRef]
- Buckland, S. Sampling Protocols and Analytical Methods for Determining Petroleum Products in Soil and Water. Report Prepared by the Oil Industry Environmental Working Group; 1999; p. 59. Available online: https://www.mfe.govt.nz/sites/default/files/sampling-protocols-oil-may99.pdf (accessed on 29 March 2020).
- Walsh, C.T.; Pease, B.C. The use of clove oil as an anaesthetic for the longfinned eel, Anguilla reinhardtii (Steindachner). Aquacult. Res. 2002, 33, 627–635. [Google Scholar] [CrossRef]
- Altun, T.; Hunt, A.O.; Usta, F. Effects of clove oil and eugenol on anaesthesia and some hematological parameters of European eel Anguilla anguilla, L., 1758. J. Appl. Anim. Res. 2006, 30, 171–176. [Google Scholar] [CrossRef]
- Lokman, P.M.; Young, G. Gonad histology and plasma steroid profiles in wild New Zealand freshwater eels (Anguilla dieffenbachii and A. australis) before and at the onset of the natural spawning migration. II. Males. Fish Physiol. Biochem. 1998, 19, 339–347. [Google Scholar] [CrossRef]
- Haro, A. Downstream migration of silver-phase anguillid eels. In Eel Biology; Aida, K., Tsukamoto, K., Yamauchi, K., Eds.; Springer: Tokyo, Japan, 2003; pp. 215–222. [Google Scholar]
- US EPA. Method 200.2, Revision 2.8: Sample Preparation Procedure for Spectrochemical Determination of Total Recoverable Elements; US EPA: Washington, DC, USA, 1994; p. 13.
- Ricker, W.E. Computation and inter-pretation of biological statistics of fish populations. J. Fish. Res. Board Can. 1975, 191, 1–382. [Google Scholar]
- Hu, L.C.; Todd, P.R. An improved technique for preparing eel otoliths for aging. N. Z. J. Mar. Freshwat. Res. 1981, 15, 445–446. [Google Scholar] [CrossRef]
- Graynoth, E. Improved otolith preparation, ageing and back-calculation techniques for New Zealand freshwater eels. Fish. Res. 1999, 42, 137–146. [Google Scholar] [CrossRef]
- Aarab, N.; Champeau, O.; Mora, P.; Daubèze, M.; Garrigues, P.; Narbonne, J.-F. Scoring approach based on fish biomarkers applied to French river monitoring. Biomarkers 2004, 9, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Labrot, F.; Ribera, D.; Denis, M.S.; Narbonne, J.F. In vitro and in vivo studies of potential biomarkers of lead and uranium contamination: Lipid peroxidation, acetylcholinesterase, catalase and glutathione peroxidase activities in three non-mammalian species. Biomarkers 1996, 1, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Pohl, R.J.; Fouts, J.R. A rapid method for assaying the metabolism of 7-ethoxyresorufin by microsomal subcellular fractions. Anal. Biochem. 1980, 107, 150–155. [Google Scholar] [CrossRef]
- Kennedy, S.W.; Jones, S.P. Simultaneous measurement of cytochrome P4501A catalytic activity and total protein concentration with a fluorescence plate reader. Anal. Biochem. 1994, 222, 217–223. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferase. The first step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar]
- Clairborne, A. Catalase activity. In Handbook of Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 283–284. [Google Scholar]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar]
- Lorenzen, A.K.S.W. A fluorescence-based protein assay for use with a microplate reader. Anal. Biochem. 1993, 214, 346–348. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Dinno, A. Dunn’s Test of Multiple Comparisons Usig Rank Sums. 2017. Available online: https://cran.r-project.org/web/packages/dunn.test/dunn.test.pdf (accessed on 20 February 2020).
- ANZECC. Australian and New Zealand Guidelines for Fresh and Marine Water Quality. Available online: https://www.waterquality.gov.au/anz-guidelines/guideline-values/default/sediment-quality-toxicants (accessed on 12 October 2020).
- McGrath, J.A.; Joshua, N.; Bess, A.S.; Parkerton, T.F. Review of polycyclic aromatic hydrocarbons (PAHs) sediment quality guidelines for the protection of benthic life. Integr. Environ. Assess. Manage. 2019, 15, 505–518. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, P.D.; Eden, D.N.; Burgham, S.J. Quaternary deposits and landscape evolution in northeast Southland, New Zealand. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1990, 81, 95–113. [Google Scholar] [CrossRef]
- Oliveira Ribeiro, C.A.; Vollaire, Y.; Sanchez-Chardi, A.; Roche, H. Bioaccumulation and the effects of organochlorine pesticides, PAH and heavy metals in the Eel (Anguilla anguilla) at the Camargue Nature Reserve, France. Aquat. Toxicol. 2005, 74, 53–69. [Google Scholar] [CrossRef]
- De Meyer, J.; Belpaire, C.; Boeckx, P.; Bervoets, L.; Covaci, A.; Malarvannan, G.; De Kegel, B.; Adriaens, D. Head shape disparity impacts pollutant accumulation in European eel. Environ. Pollut. 2018, 240, 378–386. [Google Scholar] [CrossRef] [PubMed]
- van der Oost, R.; Beyer, J.; Vermeulen, N.P.E. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. [Google Scholar] [CrossRef]
- van der Oost, R.; Goksøyr, A.; Celander, M.; Heida, H.; Vermeulen, N.P.E. Biomonitoring of aquatic pollution with feral eel (Anguilla anguilla) II. Biomarkers: Pollution-induced biochemical responses. Aquat. Toxicol. 1996, 36, 189–222. [Google Scholar] [CrossRef]
- Pacheco, M.; Santos, M.A. Induction of EROD activity and genotoxic effects by polycyclic aromatic hydrocarbons and resin acids on the juvenile eel (Anguilla anguilla L.). Ecotoxicol. Environ. Saf. 1997, 38, 252–259. [Google Scholar] [CrossRef]
- Barrick, A.; Châtel, A.; Marion, J.M.; Perrein-Ettajani, H.; Bruneau, M.; Mouneyrac, C. A novel methodology for the determination of biomarker baseline levels in the marine polychaete Hediste diversicolor. Mar. Pollut. Bull. 2016, 108, 275–280. [Google Scholar] [CrossRef]
- Burgeot, T.; Gagné, F.; Forget-Leray, J.; Bocquené, G. Acethylcholinesterase: Methodology Development of A Biomarker and Challenges of Its Application for Biomonitoring; The International Council for the Exploration of the Sea (ICES) CM Code: Copenhagen, Denmark, 2010; p. 15. [Google Scholar]
- Belpaire, C.; Pujolar, J.M.; Geeraerts, C.; Maes, G.E. Contaminants in eels and their role in the collapse of the eel stocks. In Biology and Ecology of Anguillid Eels; Arai, T., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 225–250. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).