Glyphosate Resistance in Sonchus oleraceus and Alternative Herbicide Options for Its Control in Southeast Australia
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Collection
2.1.1. Experiment 1. Effect of Temperature on Glyphosate Efficacy
2.1.2. Experiment 2. Performance of Different Post-Emergence Herbicides
2.1.3. Experiment 3. Effect of Sorghum Residue Amount on Efficacy of Pre-Emergence Herbicides
2.2. Statistical Analyses
3. Results and Discussion
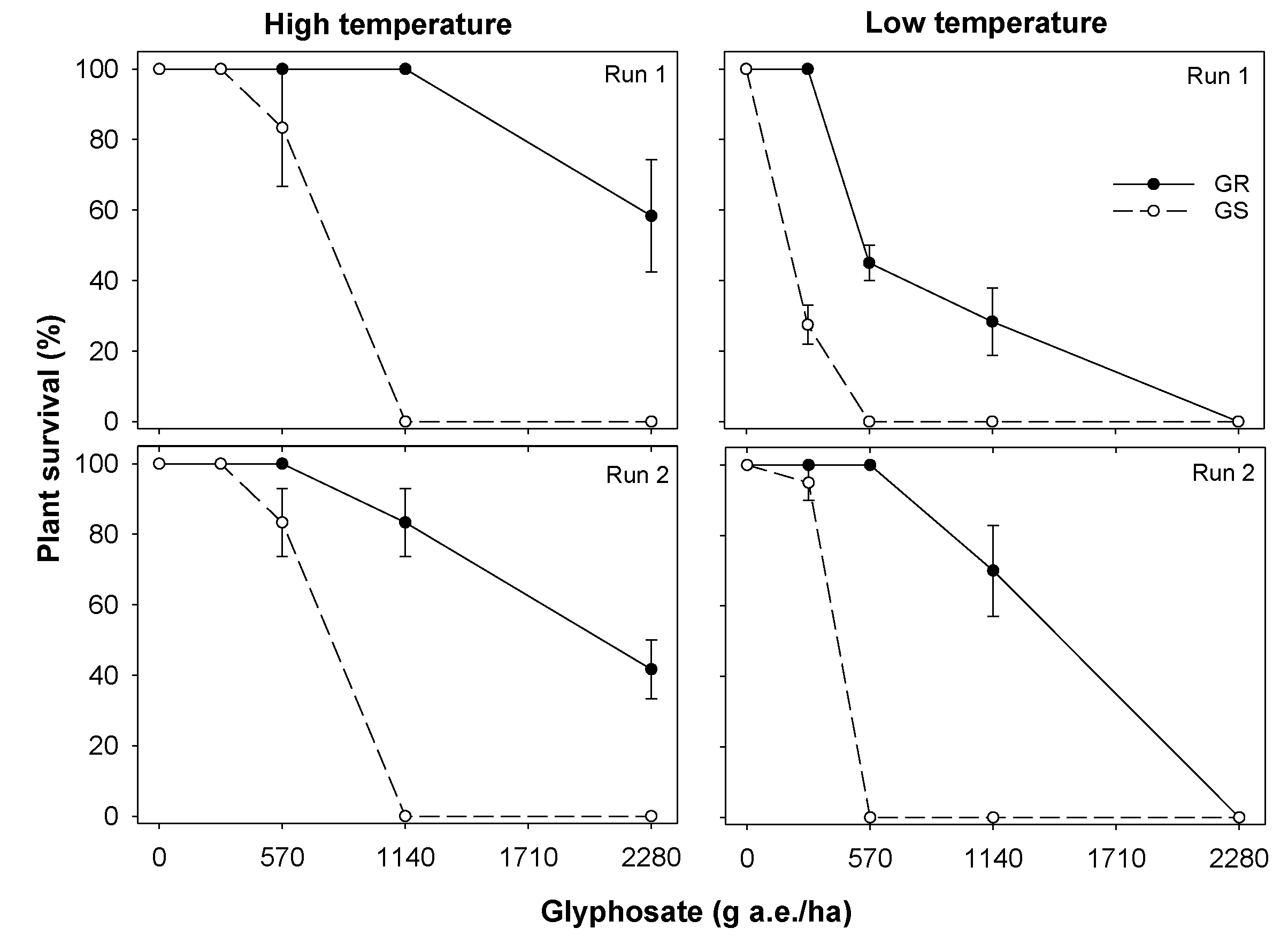
3.1. Experiment 1. Effect of Temperature on Glyphosate Efficacy
3.2. Experiment 2. Performance of Different Post-Emergence Herbicides
3.3. Experiment 3. Effect of Sorghum Residue Amount on Efficacy of Pre-Emergence Herbicides
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Llewellyn, R.; Ronning, D.; Clarke, M.; Mayfield, A.; Walker, S.; Ouzman, J. Impact of Weeds on Australian Grain Production: The Cost of Weeds to Australian Grain Growers and the Adoption of Weed Management and Tillage Practices; Report for GRDC; CSIRO: Canberra, ACT, Australia, 2016; p. 112. [Google Scholar]
- Manalil, S.; Ali, H.H.; Chauhan, B.S. Interference of annual sowthistle (Sonchus oleraceus) in wheat. Weed Sci. 2020, 68, 98–103. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Gill, G.; Preston, C. Factors affecting seed germination of annual sowthistle (Sonchus oleraceus) in southern Australia. Weed Sci. 2006, 54, 854–860. [Google Scholar] [CrossRef]
- Mobli, A.; Matloob, A.; Chauhan, B.S. The response of glyphosate-resistant and glyphosate-susceptible biotypes of annual sowthistle (Sonchus oleraceus) to mungbean density. Weed Sci. 2019, 67, 642–648. [Google Scholar] [CrossRef]
- Song, J.-S.; Kim, J.W.; Im, J.H.; Lee, K.J.; Lee, B.W.; Kim, D.S. The effects of single- and multiple-weed interference on soybean yield in the far-eastern region of Russia. Weed Sci. 2017, 65, 371–380. [Google Scholar] [CrossRef]
- Manalil, S.; Werth, J.; Jackson, R.; Chauhan, B.S.; Preston, C. An assessment of weed flora 14 years after the introduction of glyphosate-tolerant cotton in Australia. Crop Past. Sci. 2017, 68, 773–780. [Google Scholar] [CrossRef]
- Thomas, G.; Felton, W.; Radford, B. Tillage and crop residue management. In Sustainable Crop Production in the Sub-Tropics: An Australian Perspective; Clarke, A.L., Wylie, P.B., Eds.; Queensland Department of Primary Industries: Roma, QLD, Australia, 1997; pp. 195–213. [Google Scholar]
- Webb, A.; Grundy, M.; Powell, B.; Littleboy, M. The Australian sub-tropical cereal belt: Soils, climate and agriculture. In Sustainable Crop Production in the Sub-Tropics: An Australian Perspective; Clarke, A.L., Wylie, P.B., Eds.; Queensland Department of Primary Industries: Roma, QLD, Australia, 1997; pp. 8–23. [Google Scholar]
- Widderick, M.; Sindel, B.; Walker, S. Distribution, importance and management of Sonchus oleraceus (common sowthistle) in the northern cropping region of Australia. In Proceedings of the 12th Australian Weeds Conference, Hobart, TAS, Australia, 12–16 September 1999; p. 198. [Google Scholar]
- Heap, I. International Survey of Herbicide Resistant Weeds. Available online: www.weedscience.org (accessed on 1 June 2020).
- Manalil, S.; Coast, O.; Werth, J.; Chauhan, B.S. Weed management in cotton (Gossypium hirsutum L.) through weed-crop competition: A review. Crop Prot. 2017, 95, 53–59. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Malone, J.M.; Boutsalis, P.; Shirley, N.; Preston, C. Temperature influences the level of glyphosate resistance in barnyardgrass (Echinochloa colona). Pest Manag. Sci. 2016, 72, 1031–1039. [Google Scholar] [CrossRef]
- Sarangi, D.; Sandell, L.D.; Kruger, G.R.; Knezevic, S.Z.; Irmak, S.; Jhala, A.J. Comparison of herbicide programs for season-long control of glyphosate-resistant common waterhemp (Amaranthus rudis) in soybean. Weed Technol. 2017, 31, 53–66. [Google Scholar] [CrossRef]
- Harker, K.N.; Blackshaw, R.E. Influence of growth stage and broadleaf herbicides on tralkoxydim activity. Weed Sci. 1991, 39, 650–659. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Abugho, S.B. Effect of growth stage on the efficacy of postemergence herbicides on four weed species of direct-seeded rice. Sci. World J. 2012, 2012, 7. [Google Scholar] [CrossRef]
- Banks, P.A.; Robinson, E.L. The influence of straw mulch on the soil reception and persistence of metribuzin. Weed Sci. 1982, 30, 164–168. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Abugho, S.B. Interaction of rice residue and PRE herbicides on emergence and biomass of four weed species. Weed Technol. 2012, 26, 627–632. [Google Scholar] [CrossRef]
- Khalil, Y.; Flower, K.; Siddique, K.H.M.; Ward, P. Rainfall affects leaching of pre-emergent herbicide from wheat residue into the soil. PLoS ONE 2019, 14, e0210219. [Google Scholar] [CrossRef] [PubMed]
- Mobli, A.; Rinwa, A.; Sahil; Chauhan, B.S. Effects of sorghum residue in presence of pre-emergence herbicides on emergence and biomass of Echinochloa colona and Chloris virgata. PLoS ONE 2020, 15, e0229817. [Google Scholar] [CrossRef] [PubMed]
- Genstat for Windows, 20th ed.; VSN International: Hemel Hempstead, UK, 2019.
- Shrestha, A.; Budhathoki, S.; Steinhauer, K. Temperature effects on glyphosate resistance in California populations of junglerice. Agron. J. 2018, 110, 1624–1626. [Google Scholar] [CrossRef]
- Matzrafi, M.; Brunharo, C.; Tehranchian, P.; Hanson, B.D.; Jasieniuk, M. Increased temperatures and elevated CO2 levels reduce the sensitivity of Conyza canadensis and Chenopodium album to glyphosate. Sci. Rep. 2019, 9, 2228. [Google Scholar] [CrossRef]
- Ali, H.H.; Kebaso, L.; Manalil, S.; Chauhan, B.S. Emergence and germination response of Sonchus oleraceus and Rapistrum rugosum to different temperatures and moisture stress regimes. Plant Species Biol. 2020, 35, 16–23. [Google Scholar] [CrossRef]
- VanGessel, M.J.; Scott, B.A.; Johnson, Q.R.; White-Hansen, S.E. Influence Of glyphosate-resistant horseweed (Conyza canadensis) growth stage on response to glyphosate applications. Weed Technol. 2009, 23, 49–53. [Google Scholar] [CrossRef]
- Kaur, S.; Sandell, L.D.; Lindquist, J.L.; Jhala, A.J. Glyphosate-resistant giant ragweed (Ambrosia trifida) control in glufosinate-resistant soybean. Weed Technol. 2014, 28, 569–577. [Google Scholar] [CrossRef]
- Landry, R.L.; Stephenson, D.O.; Woolam, B.C. Glufosinate rate and timing for control of glyphosate-resistant rhizomatous Johnsongrass (Sorghum halepense) in glufosinate-resistant soybean. Int. J. Agron. 2016, 8040235. [Google Scholar] [CrossRef]
- Steckel, G.J.; Wax, L.M.; Simmons, F.W.; Phillips, W.H. Glufosinate efficacy on annual weeds is influenced by rate and growth stage. Weed Technol. 1997, 11, 484–488. [Google Scholar] [CrossRef]
- Eubank, T.W.; Poston, D.H.; Nandula, V.K.; Koger, C.H.; Shaw, D.R.; Reynolds, D.B. Glyphosate-resistant horseweed (Conyza canadensis) control using glyphosate-, paraquat-, and glufosinate-based herbicide programs. Weed Technol. 2008, 22, 16–21. [Google Scholar] [CrossRef]
- Yu, Q.; Cairns, A.; Powles, S.B. Paraquat resistance in a population of Lolium rigidum. Funct. Plant Biol. 2004, 31, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Odero, D.C. Response of ragweed parthenium (Parthenium hysterophorus) to saflufenacil and glyphosate. Weed Technol. 2012, 26, 443–448. [Google Scholar] [CrossRef]
- Budd, C.M.; Soltani, N.; Robinson, D.E.; Hooker, D.C.; Miller, R.T.; Sikkema, P.H. Efficacy of saflufenacil for control of glyphosate-resistant horseweed (Conyza canadensis) as affected by height, density, and time of day. Weed Technol. 2017, 65, 275–284. [Google Scholar] [CrossRef]
- Alletto, L.; Coquet, Y.; Bergheaud, V.; Benoit, P. Water pressure head and temperature impact on isoxaflutole degradation in crop residues and loamy surface soil under conventional and conservation tillage management. Chemosphere 2012, 88, 1043–1050. [Google Scholar] [CrossRef]
- Stephenson, D.O.; Bond, J.A. Evaluation of thiencarbazone-methyl- and isoxaflutole-based herbicide programs in corn. Weed Technol. 2012, 26, 37–42. [Google Scholar] [CrossRef]
- Nelson, E.A.; Penner, D. Sensitivity of selected crops to isoxaflutole in soil and irrigation water. Weed Technol. 2005, 19, 659–663. [Google Scholar] [CrossRef]
- Soni, N.; Leon, R.G.; Erickson, J.E.; Ferrell, J.A.; Silveira, M.L. Biochar decreases atrazine and pendimethalin preemergence herbicidal activity. Weed Technol. 2015, 29, 359–366. [Google Scholar] [CrossRef]
- Price, A.J.; Balkcom, K.S.; Duzy, L.M.; Kelton, J.A. Herbicide and cover crop residue integration for Amaranthus control in conservation agriculture cotton and implications for resistance management. Weed Technol. 2012, 26, 490–498. [Google Scholar] [CrossRef]

| Trial | Dates | Temperature (°C) | Assigned Details | |||
|---|---|---|---|---|---|---|
| Planting | Spray | Observation | Min | Av | ||
| 1 | 14 Feb 2019 | 25 Mar 2019 | 22 Apr 2019 | 17.3 | 28.1 | High temperature—Run 1 |
| 2 | 14 May 2019 | 20 June 2019 | 17 Jul 2019 | 8.6 | 19.0 | Low temperature—Run 1 |
| 3 | 14 Aug 2019 | 11 Sep 2019 | 10 Oct 2019 | 9.0 | 23.7 | Low temperature—Run 2 |
| 4 | 14 Nov 2019 | 4 Dec 2019 | 2 Jan 2020 | 18.2 | 30.1 | High temperature—Run 2 |
| Experiment 2: Post-Emergence | Experiment 3: Pre-Emergence | ||
|---|---|---|---|
| Herbicides | Rates (g a.i. or a.e./ha) | Herbicides | Rates (g a.i./ha) |
| 2,4-D | 700 | Isoxaflutole | 75 |
| 2,4-D | 1050 | Isoxaflutole | 150 |
| 2,4-D + picloram | 75 | Pendimethalin | 910 |
| 2,4-D + picloram | 112.5 | Pendimethalin | 1820 |
| Bromoxynil | 280 | S-metolachor | 960 |
| Bromoxynil | 420 | S-metolachlor | 1920 |
| Fluroxypyr * | 66.7 | ||
| Fluroxypyr * | 100 | ||
| Glufosinate | 500 | ||
| Glufosinate | 750 | ||
| Glyphosate | 496 | ||
| Glyphosate | 741 | ||
| Metsulfuron | 2 | ||
| Metsulfuron | 3 | ||
| Paraquat | 400 | ||
| Paraquat | 600 | ||
| Saflufenacil ** | 15.9 | ||
| Saflufenacil ** | 23.8 | ||
| Herbicide | Rates (g a.i./ha) | Seedling Survival (%) | |||
|---|---|---|---|---|---|
| 4-Leaf Stage | 6-Leaf Stage | ||||
| GR | GS | GR | GS | ||
| Control | 100 | 100 | 100 | 100 | |
| 2,4-D | 700 | 100 | 92 | 100 | 100 |
| 2,4-D | 1050 | 83 | 29 | 100 | 100 |
| 2,4-D + picloram | 75 | 0 | 0 | 0 | 100 |
| 2,4-D + picloram | 112.5 | 0 | 0 | 0 | 78 |
| Bromoxynil | 280 | 0 | 0 | 100 | 100 |
| Bromoxynil | 420 | 0 | 0 | 100 | 100 |
| Fluroxypyr | 66.7 | 100 | 100 | 100 | 100 |
| Fluroxypyr | 100 | 100 | 100 | 100 | 100 |
| Glufosinate | 500 | 0 | 0 | 0 | 0 |
| Glufosinate | 750 | 0 | 0 | 0 | 0 |
| Glyphosate | 496 | 100 | 0 | 100 | 36 |
| Glyphosate | 741 | 54 | 0 | 100 | 88 |
| Metsulfuron | 2 | 100 | 100 | 100 | 100 |
| Metsulfuron | 3 | 96 | 92 | 100 | 100 |
| Paraquat | 400 | 0 | 0 | 0 | 0 |
| Paraquat | 600 | 0 | 0 | 0 | 0 |
| Saflufenacil | 15.9 | 0 | 0 | 49 | 71 |
| Saflufenacil | 23.8 | 0 | 0 | 89 | 64 |
| LSD | 10.8 | 13.8 | 16.6 | 18.5 | |
| Herbicide | Rates (g a.i./ha) | Biomass (g/plant) | |||
|---|---|---|---|---|---|
| 4-Leaf Stage | 6-Leaf Stage | ||||
| GR | GS | GR | GS | ||
| Control | 0.34 | 0.54 | 0.27 | 0.38 | |
| 2,4-D | 700 | 0.23 (34) | 0.16 (71) | 0.18 (34) | 0.21 (44) |
| 2,4-D | 1050 | 0.12 (66) | 0.05 (91) | 0.17 (39) | 0.31 (20) |
| 2,4-D + picloram | 75 | 0 (100) | 0 (100) | 0 (100) | 0.10 (75) |
| 2,4-D + picloram | 112.5 | 0 (100) | 0 (100) | 0 (100) | 0.21 (45) |
| Bromoxynil | 280 | 0 (100) | 0 (100) | 0.20 (27) | 0.13 (66) |
| Bromoxynil | 420 | 0 (100) | 0 (100) | 0.15 (46) | 0.12 (68) |
| Fluroxypyr | 66.7 | 0.22 (37) | 0.30 (44) | 0.22 (21) | 0.30 (21) |
| Fluroxypyr | 100 | 0.22 (35) | 0.34 (37) | 0.23 (16) | 0.40 (−4) |
| Glufosinate | 500 | 0 (100) | 0 (100) | 0 (100) | 0 (100) |
| Glufosinate | 750 | 0 (100) | 0 (100) | 0 (100) | 0 (100) |
| Glyphosate | 496 | 0.05 (87) | 0 (100) | 0.14 (48) | 0.04 (90) |
| Glyphosate | 741 | 0.03 (92) | 0 (100) | 0.20 (26) | 0.01 (97) |
| Metsulfuron | 2 | 0.37 (−8) | 0.07 (87) | 0.30 (−10) | 0.19 (52) |
| Metsulfuron | 3 | 0.47 (−36) | 0.07 (88) | 0.30 (−9) | 0.17 (56) |
| Paraquat | 400 | 0 (100) | 0 (100) | 0 (100) | 0 (100) |
| Paraquat | 600 | 0 (100) | 0 (100) | 0 (100) | 0 (100) |
| Saflufenacil | 15.9 | 0 (100) | 0 (100) | 0.08 (71) | 0.05 (88) |
| Saflufenacil | 23.8 | 0 (100) | 0 (100) | 0.05 (81) | 0.06 (84) |
| LSD | 0.058 | 0.087 | 0.084 | 0.092 | |
| Herbicide | Rate (g a.i./ha) | Seedling Emergence (%) | |||||
|---|---|---|---|---|---|---|---|
| GR | GS | ||||||
| 0 t/ha | 3 t/ha | 6 t/ha | 0 t/ha | 3 t/ha | 6 t/ha | ||
| Control | - | 82 | 63 | 70 | 75 | 87 | 87 |
| Isoxaflutole | 75 | 13 | 38 | 50 | 37 | 72 | 62 |
| Isoxaflutole | 150 | 5 | 17 | 22 | 27 | 17 | 22 |
| Pendimethalin | 910 | 75 | 75 | 65 | 78 | 75 | 72 |
| Pendimethalin | 1820 | 68 | 20 | 30 | 78 | 33 | 25 |
| S-metolachor | 960 | 63 | 62 | 70 | 87 | 80 | 77 |
| S-metolachlor | 1920 | 30 | 58 | 72 | 40 | 78 | 80 |
| LSD | 21 | 19 | |||||
| Herbicide | Rate (g a.i./ha) | Biomass (g/plant) | |||||
|---|---|---|---|---|---|---|---|
| GR | GS | ||||||
| 0 t/ha | 3 t/ha | 6 t/ha | 0 t/ha | 3 t/ha | 6 t/ha | ||
| Control | - | 0.0400 | 0.0466 | 0.0285 | 0.0159 | 0.0211 | 0.0302 |
| Isoxaflutole | 75 | 0.0006 (99) | 0.0005 (99) | 0.0035 (88) | 0.0007 (96) | 0.0025 (88) | 0.0056 (81) |
| Isoxaflutole | 150 | 0.0001 (100) | 0.0067 (86) | 0.0012 (96) | 0.0016 (90) | 0.0001 (100) | 0.0001 (100) |
| Pendimethalin | 910 | 0.0074 (82) | 0.0038 (92) | 0.0028 (90) | 0.0283 (−78) | 0.0229 (−8) | 0.0210 (30) |
| Pendimethalin | 1820 | 0.0067 (83) | 0.0028 (94) | 0.0053 (81) | 0.0114 (28) | 0.0129 (39) | 0.0094 (69) |
| S-metolachor | 960 | 0.0115 (71) | 0.0239 (49) | 0.0219 (23) | 0.0180 (−13) | 0.0262 (−24) | 0.0223 (26) |
| S-metolachlor | 1920 | 0.0034 (92) | 0.0096 (79) | 0.0203 (29) | 0.0065 (59) | 0.0134 (34) | 0.0283 (6) |
| LSD | 0.0187 | 0.0106 | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chauhan, B.S.; Jha, P. Glyphosate Resistance in Sonchus oleraceus and Alternative Herbicide Options for Its Control in Southeast Australia. Sustainability 2020, 12, 8311. https://doi.org/10.3390/su12208311
Chauhan BS, Jha P. Glyphosate Resistance in Sonchus oleraceus and Alternative Herbicide Options for Its Control in Southeast Australia. Sustainability. 2020; 12(20):8311. https://doi.org/10.3390/su12208311
Chicago/Turabian StyleChauhan, Bhagirath S., and Prashant Jha. 2020. "Glyphosate Resistance in Sonchus oleraceus and Alternative Herbicide Options for Its Control in Southeast Australia" Sustainability 12, no. 20: 8311. https://doi.org/10.3390/su12208311
APA StyleChauhan, B. S., & Jha, P. (2020). Glyphosate Resistance in Sonchus oleraceus and Alternative Herbicide Options for Its Control in Southeast Australia. Sustainability, 12(20), 8311. https://doi.org/10.3390/su12208311






