A Sustainable Approach to the Low-Cost Recycling of Waste Glass Fibres Composites towards Circular Economy
Abstract
1. Introduction
2. Experimental
2.1. Materials
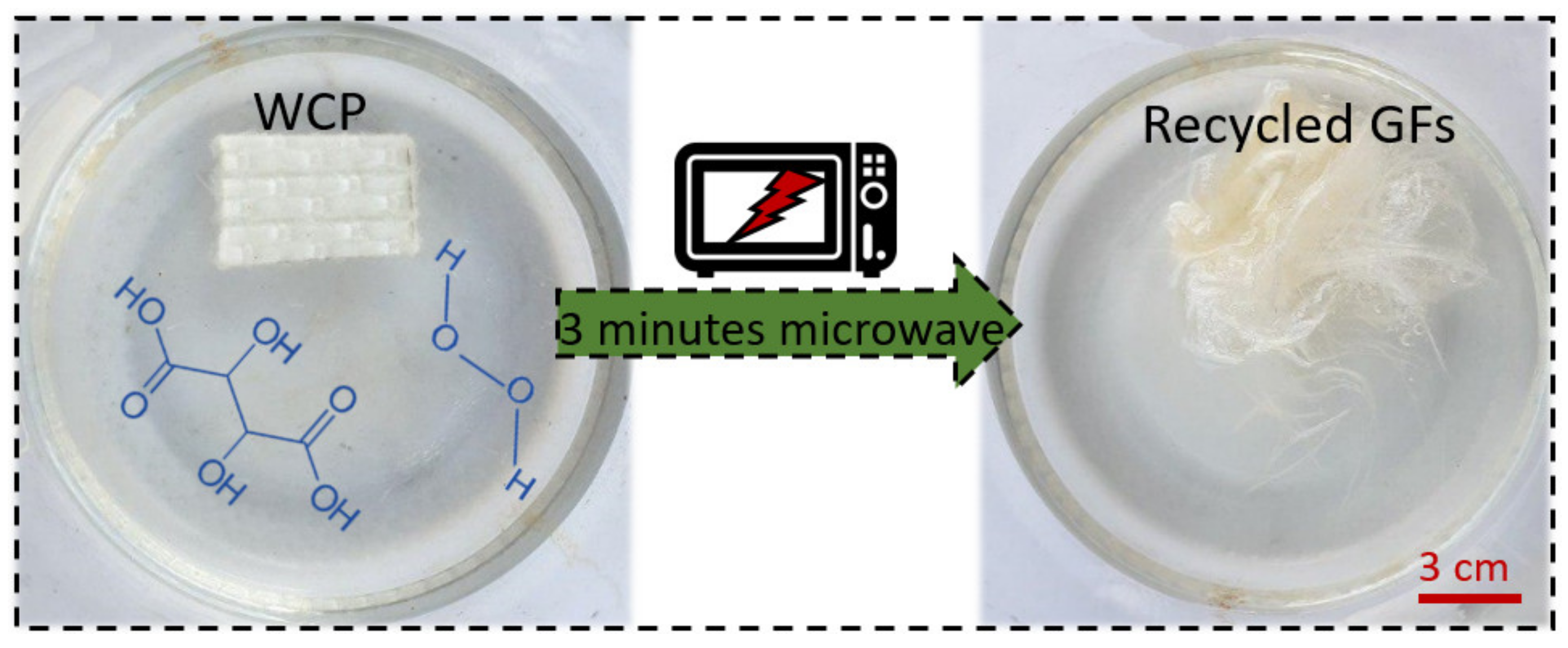
2.2. Recycling Process
2.3. Characterizations
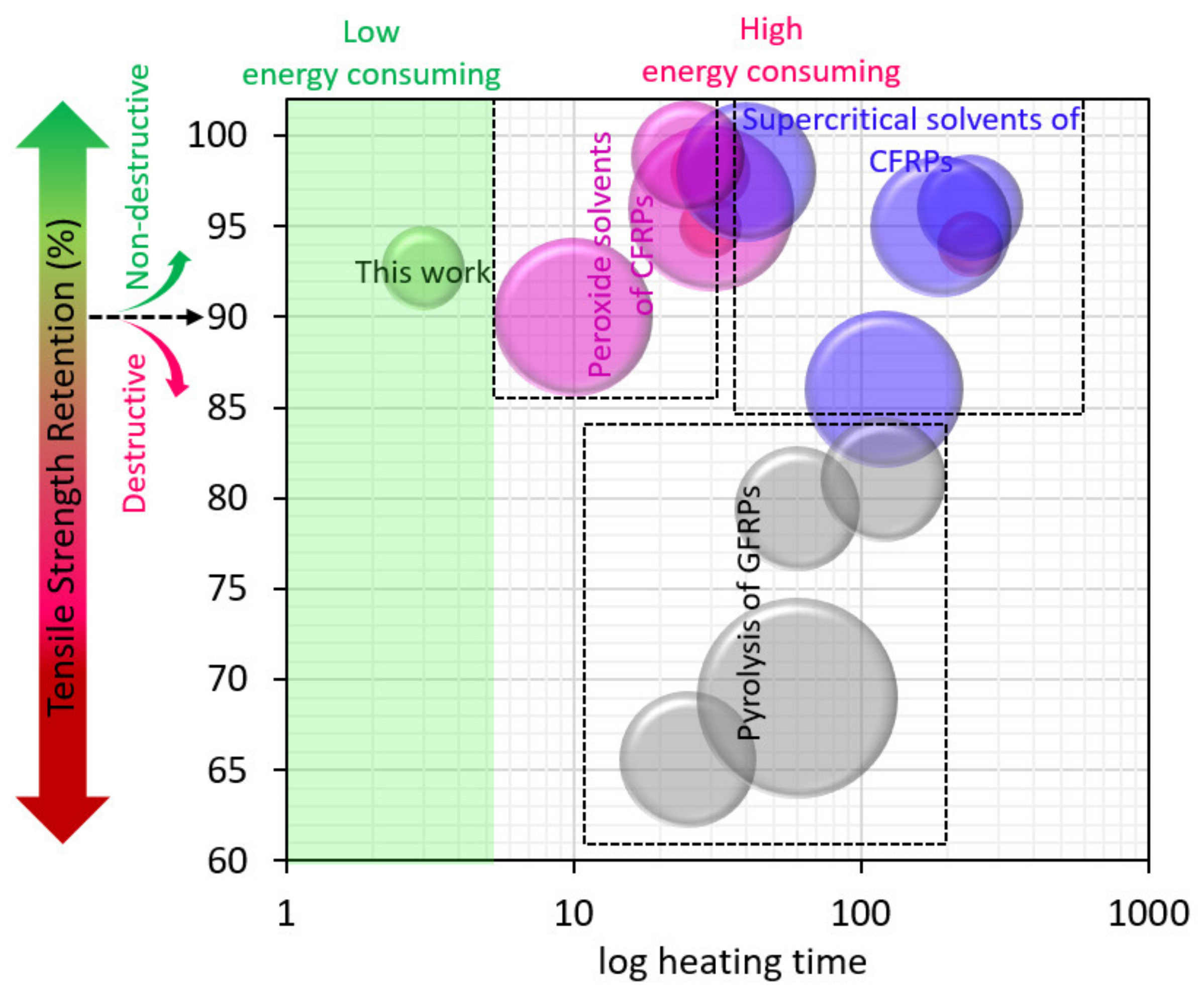
3. Results and Discussion
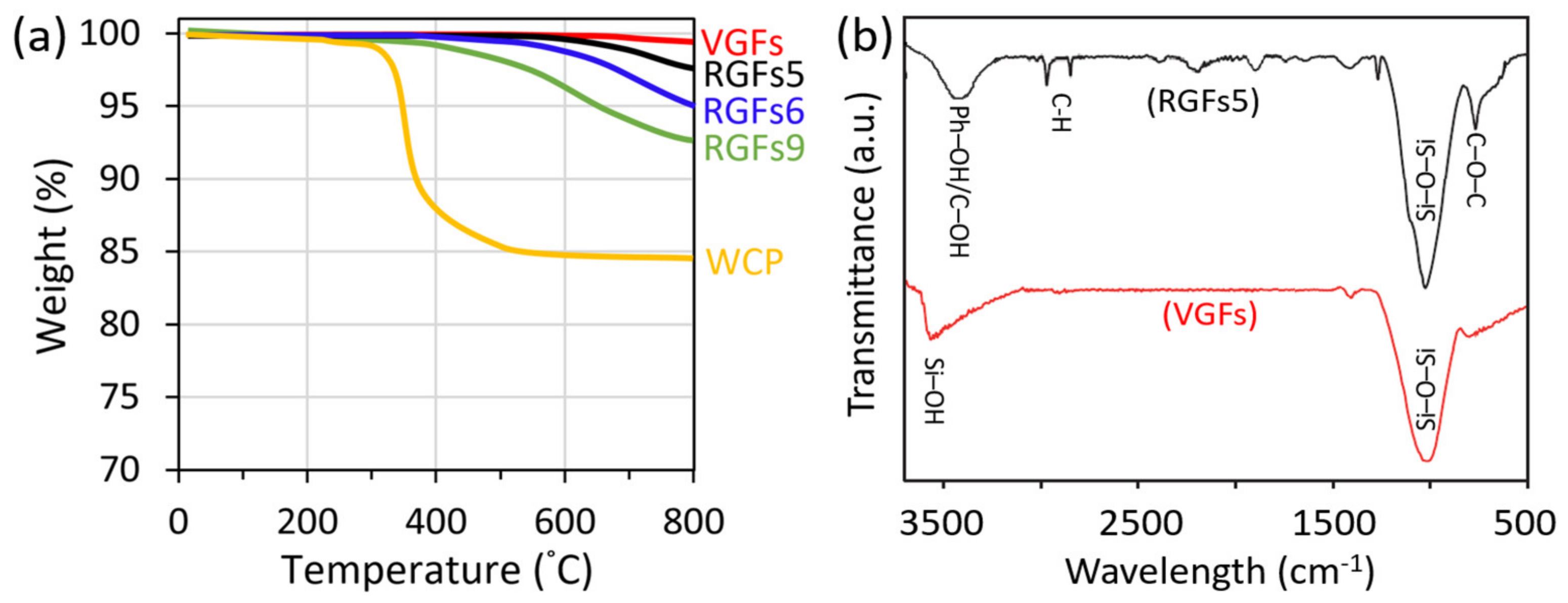
3.1. Recycling Process Optimization
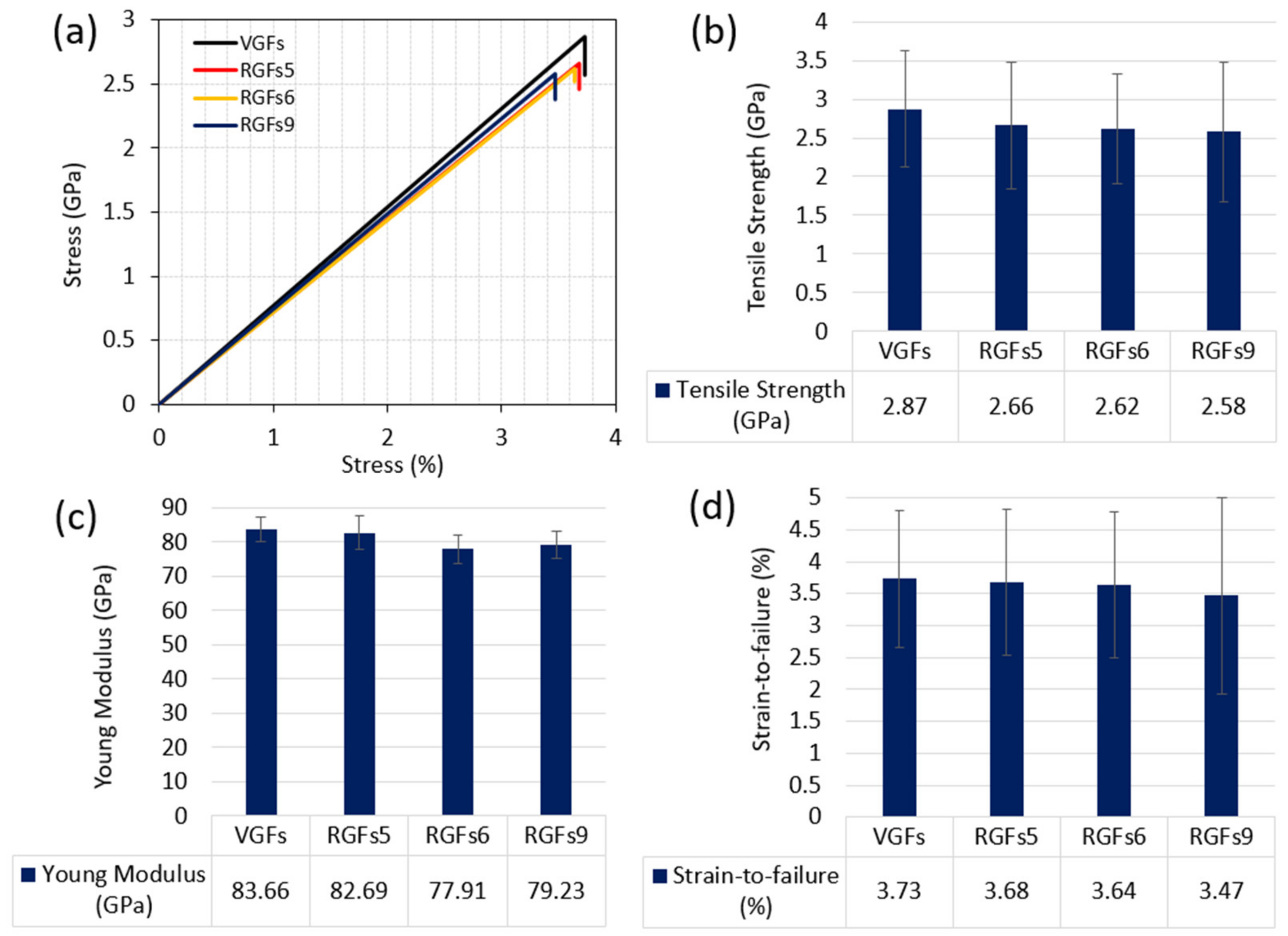
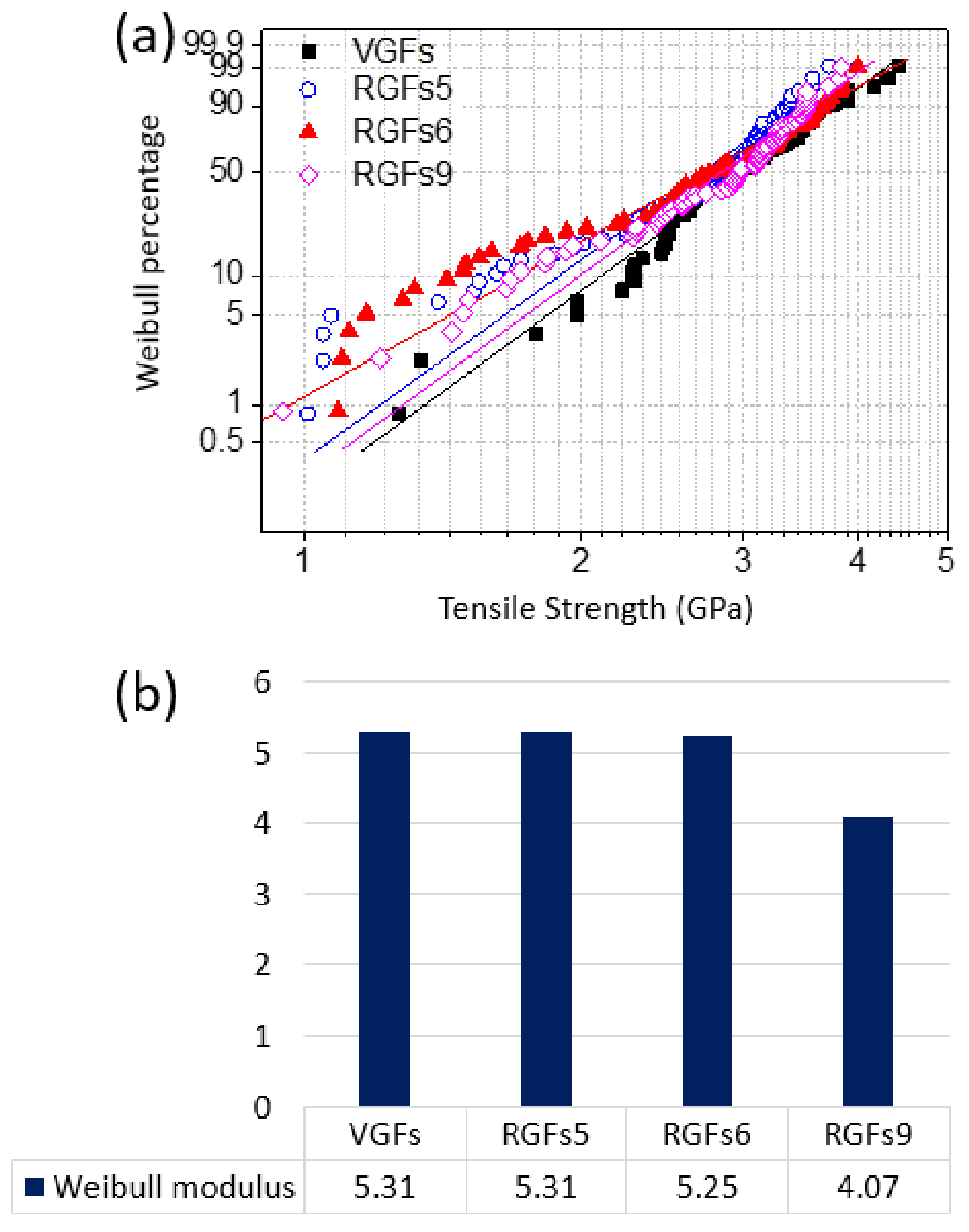
3.2. Mechanical Properties of Recycled GFs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, P.; Meng, F.; Barlow, C.Y. Wind turbine blade end-of-life options: An eco-audit comparison. J. Clean. Prod. 2019, 212, 1268–1281. [Google Scholar] [CrossRef]
- Khayyam, H.; Jazar, R.N.; Nunna, S.; Golkarnarenji, G.; Badii, K.; Mousa Fakhrhoseini, S.; Kumar, S.; Naebe, M. PAN Precursor Fabrication, Applications and Thermal Stabilization Process in Carbon Fiber Production: Experimental and Mathematical Modelling. Prog. Mater. Sci. 2019, 107, 100575. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Lemanski, S.; Nezhad, H.Y.; Ayre, D. Experimental and numerical study of process-induced defects and their effect on fatigue debonding in composite joints. Int. J. Fatigue 2019, 125, 47–57. [Google Scholar] [CrossRef]
- An, D.; Lotfian, S.; Mesbah, D.; Ayre, D.; Yoosefinejad, A.; Thakur, V.K.; Yazdani Nezhad, H. Ultra-thin electrospun nanofibers for development of damage-tolerant composite laminates. Mater. Today Chem. 2019, 14, 100202. [Google Scholar] [CrossRef]
- Zabihi, O.; Ahmadi, M.; Nikafshar, S.; Chandrakumar Preyeswary, K.; Naebe, M. A technical review on epoxy-clay nanocomposites: Structure, properties, and their applications in fiber reinforced composites. Compos. Part B: Eng. 2018, 135, 1–24. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Prabhakara, H.M.; Bramer, E.A.; Dierkes, W.; Akkerman, R.; Brem, G. A critical review on recycling of end-of-life carbon fibre/glass fibre reinforced composites waste using pyrolysis towards a circular economy. Resour. Conserv. Recycl. 2018, 136, 118–129. [Google Scholar] [CrossRef]
- Verma, S.; Balasubramaniam, B.; Gupta, R.K. Recycling, reclamation and re-manufacturing of carbon fibres. Curr. Opin. Green Sustain. Chem. 2018, 13, 86–90. [Google Scholar] [CrossRef]
- Rahimizadeh, A.; Kalman, J.; Fayazbakhsh, K.; Lessard, L. Recycling of fiberglass wind turbine blades into reinforced filaments for use in Additive Manufacturing. Compos. Part B: Eng. 2019, 175, 107101. [Google Scholar] [CrossRef]
- Zaidi, Z.; Crosky, A. Unidirectional Rubber-Toughened Green Composites Based on PHBV. Sustainability 2019, 11, 2411. [Google Scholar] [CrossRef]
- Li, Y.-F.; Hsu, T.-H.; Hsieh, F.-C. A Study on Improving the Mechanical Behaviors of the Pultruded GFRP Composite Material Members. Sustainability 2019, 11, 577. [Google Scholar] [CrossRef]
- Lefeuvre, A.; Garnier, S.; Jacquemin, L.; Pillain, B.; Sonnemann, G. Anticipating in-use stocks of carbon fiber reinforced polymers and related waste flows generated by the commercial aeronautical sector until 2050. Resour. Conserv. Recycl. 2017, 125, 264–272. [Google Scholar] [CrossRef]
- Lefeuvre, A.; Garnier, S.; Jacquemin, L.; Pillain, B.; Sonnemann, G. Anticipating in-use stocks of carbon fibre reinforced polymers and related waste generated by the wind power sector until 2050. Resour. Conserv. Recycl. 2019, 141, 30–39. [Google Scholar] [CrossRef]
- Hagnell, M.K.; Åkermo, M. The economic and mechanical potential of closed loop material usage and recycling of fibre-reinforced composite materials. J. Clean. Prod. 2019, 223, 957–968. [Google Scholar] [CrossRef]
- Knappich, F.; Klotz, M.; Schlummer, M.; Wölling, J.; Mäurer, A. Recycling process for carbon fiber reinforced plastics with polyamide 6, polyurethane and epoxy matrix by gentle solvent treatment. Waste Manag. 2019, 85, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Zabihi, O.; Ahmadi, M.; Shafei, S.; Seraji, S.M.; Oroumei, A.; Naebe, M. One-step amino-functionalization of milled carbon fibre for enhancement of thermo-physical properties of epoxy composites. Compos. Part A: Appl. Sci. Manuf. 2016, 88, 243–252. [Google Scholar] [CrossRef]
- Howarth, J.; Mareddy, S.S.R.; Mativenga, P.T. Energy intensity and environmental analysis of mechanical recycling of carbon fibre composite. J. Clean. Prod. 2014, 81, 46–50. [Google Scholar] [CrossRef]
- Pimenta, S.; Pinho, S.T. Recycling carbon fibre reinforced polymers for structural applications: Technology review and market outlook. Waste Manag. 2011, 31, 378–392. [Google Scholar] [CrossRef]
- Oliveux, G.; Dandy, L.O.; Leeke, G.A. Current status of recycling of fibre reinforced polymers: Review of technologies, reuse and resulting properties. Prog. Mater. Sci. 2015, 72, 61–99. [Google Scholar] [CrossRef]
- Jiang, G.; Pickering, S.J.; Lester, E.H.; Turner, T.A.; Wong, K.H.; Warrior, N.A. Characterisation of carbon fibres recycled from carbon fibre/epoxy resin composites using supercritical n-propanol. Compos. Sci. Technol. 2009, 69, 192–198. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, W.; Jin, X.; Liang, X.; Sui, G.; Yang, X. Efficient reclamation of carbon fibers from epoxy composite waste through catalytic pyrolysis in molten ZnCl2. RSC Adv. 2019, 9, 377–388. [Google Scholar] [CrossRef]
- Yu, K.; Shi, Q.; Dunn, M.L.; Wang, T.; Qi, H.J. Carbon Fiber Reinforced Thermoset Composite with Near 100% Recyclability. Adv. Funct. Mater. 2016, 26, 6098–6106. [Google Scholar] [CrossRef]
- Jiang, J.; Deng, G.; Chen, X.; Gao, X.; Guo, Q.; Xu, C.; Zhou, L. On the successful chemical recycling of carbon fiber/epoxy resin composites under the mild condition. Compos. Sci. Technol. 2017, 151, 243–251. [Google Scholar] [CrossRef]
- Li, J.; Xu, P.-L.; Zhu, Y.-K.; Ding, J.-P.; Xue, L.-X.; Wang, Y.-Z. A promising strategy for chemical recycling of carbon fiber/thermoset composites: Self-accelerating decomposition in a mild oxidative system. Green Chem. 2012, 14, 3260–3263. [Google Scholar] [CrossRef]
- Zhu, J.-H.; Chen P-y Su M-n Pei, C.; Xing, F. Recycling of carbon fibre reinforced plastics by electrically driven heterogeneous catalytic degradation of epoxy resin. Green Chem. 2019, 21, 1635–1647. [Google Scholar] [CrossRef]
- Sun, H.; Guo, G.; Memon, S.A.; Xu, W.; Zhang, Q.; Zhu, J.-H.; Xing, F. Recycling of carbon fibers from carbon fiber reinforced polymer using electrochemical method. Compos. Part A: Appl. Sci. Manuf. 2015, 78, 10–17. [Google Scholar] [CrossRef]
- Nikafshar, S.; Zabihi, O.; Moradi, Y.; Ahmadi, M.; Amiri, S.; Naebe, M. Catalyzed Synthesis and Characterization of a Novel Lignin-Based Curing Agent for the Curing of High-Performance Epoxy Resin. Polymers 2017, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Radkar, S.S.; Amiri, A.; Ulven, A.C. Tensile Behavior and Diffusion of Moisture through Flax Fibers by Desorption Method. Sustainability 2019, 11, 3558. [Google Scholar] [CrossRef]
- Zabihi, O.; Shafei, S.; Fakhrhoseini, S.M.; Ahmadi, M.; Ajdari Nazarloo, H.; Stanger, R.; Anh Tran, Q.; Lucas, J.; Wall, T.; Naebe, M. Low-Cost Carbon Fibre Derived from Sustainable Coal Tar Pitch and Polyacrylonitrile: Fabrication and Characterisation. Materials 2019, 12, 1281. [Google Scholar] [CrossRef]
- Zabihi, O.; Ahmadi, M.; Ghandehari Ferdowsi, M.R.; Li, Q.; Fakhrhoseini, S.M.; Mahmoodi, A.V.; Naebe, M. Natural bauxite nanosheets: A multifunctional and sustainable 2D nano-reinforcement for high performance polymer nanocomposites. Compos. Sci. Technol. 2019, 184, 107868. [Google Scholar] [CrossRef]
- Zabihi, O.; Ahmadi, M.; Li, Q.; Ferdowsi, M.R.G.; Mahmoodi, R.; Kalali, E.N.; Wang, D.; Naebe, M. A sustainable approach to scalable production of a graphene based flame retardant using waste fish deoxyribonucleic acid. J. Clean. Prod. 2020, 247, 119150. [Google Scholar] [CrossRef]
- Kim, Y.N.; Kim, Y.-O.; Kim, S.Y.; Park, M.; Yang, B.; Kim, J.; Jung, Y.C. Application of supercritical water for green recycling of epoxy-based carbon fiber reinforced plastic. Compos. Sci. Technol. 2019, 173, 66–72. [Google Scholar] [CrossRef]
- Okajima, I.; Sako, T. Recycling fiber-reinforced plastic using supercritical acetone. Polym. Degrad. Stab. 2019, 163, 1–6. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, X.; Ge, H.; Yang, Y.; Wang, Y.; Zhang, C.; Li, J.; Deng, T.; Qin, Z.; Hou, X. Chemical Recycling of Carbon Fiber Reinforced Epoxy Resin Composites via Selective Cleavage of the Carbon–Nitrogen Bond. ACS Sustain. Chem. Eng. 2015, 3, 3332–3337. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Z.; Feng, L. Chemical recycling of carbon fibers reinforced epoxy resin composites in oxygen in supercritical water. Mater. Des. 2010, 31, 999–1002. [Google Scholar] [CrossRef]
- Boulanghien, M.; Mili, M.; Bernhart, G.; Berthet, F.; Soudais, Y. Mechanical Characterization of Carbon Fibres Recycled by Steam Thermolysis: A Statistical Approach. J. Adv. Mater. Sci. Eng. 2018, 2018, 10. [Google Scholar] [CrossRef]
- Kuang, X.; Zhou, Y.; Shi, Q.; Wang, T.; Qi, H.J. Recycling of Epoxy Thermoset and Composites via Good Solvent Assisted and Small Molecules Participated Exchange Reactions. ACS Sustain. Chem. Eng. 2018, 6, 9189–9197. [Google Scholar] [CrossRef]
- Xu, P.; Li, J.; Ding, J. Chemical recycling of carbon fibre/epoxy composites in a mixed solution of peroxide hydrogen and N.,N-dimethylformamide. Compos. Sci. Technol. 2013, 82, 54–59. [Google Scholar] [CrossRef]
- Das, M.; Chacko, R.; Varughese, S. An Efficient Method of Recycling of CFRP Waste Using Peracetic Acid. ACS Sustain. Chem. Eng. 2018, 6, 1564–1571. [Google Scholar] [CrossRef]
- Zhu, Y.-J.; Chen, F. Microwave-Assisted Preparation of Inorganic Nanostructures in Liquid Phase. Chem. Rev. 2014, 114, 6462–6555. [Google Scholar] [CrossRef]
- Bassyouni, F.A.; Abu-Bakr, S.M.; Rehim, M.A. Evolution of microwave irradiation and its application in green chemistry and biosciences. Res. Chem. Intermed. 2012, 38, 283–322. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, X.-L.; Tian, F.; An, W.-L.; Xu, S.; Wang, Y.-Z. A fast and mild closed-loop recycling of anhydride-cured epoxy through microwave-assisted catalytic degradation by trifunctional amine and subsequent reuse without separation. Green Chem. 2019, 21, 2487–2493. [Google Scholar] [CrossRef]
- Anastas, P.T.; Zimmerman, J.B. Peer Reviewed: Design Through the 12 Principles of Green Engineering. Environ. Sci. Technol. 2003, 37, 94A–101A. [Google Scholar] [CrossRef] [PubMed]
- Naito, K.; Tanaka, Y.; Yang, J.-M.; Kagawa, Y. Tensile properties of ultrahigh strength PAN-based, ultrahigh modulus pitch-based and high ductility pitch-based carbon fibers. Carbon 2008, 46, 189–195. [Google Scholar] [CrossRef]
- Zabihi, O.; Ahmadi, M.; Li, Q.; Shafei, S.; Huson, M.G.; Naebe, M. Carbon fibre surface modification using functionalized nanoclay: A hierarchical interphase for fibre-reinforced polymer composites. Compos. Sci. Technol. 2017, 148, 49–58. [Google Scholar] [CrossRef]
- Keating, K.B.; Rozner, A.G. Decomposition of Hydrogen Peroxide on Glass. J. Phys. Chem. 1965, 69, 3658–3660. [Google Scholar] [CrossRef]
- Zabihi, O.; Aghaie, M.; Aghaie, H.; Zare, K.; Saghapour, Y. Description of phenomenological process during thermal formation of an epoxy system in presence of metal nanoparticles using advanced kinetics analysis. J. Therm. Anal. Calorim. 2014, 117, 53–61. [Google Scholar] [CrossRef]
- Zabihi, O.; Khodabandeh, A.; Mostafavi, S.M. Preparation, optimization and thermal characterization of a novel conductive thermoset nanocomposite containing polythiophene nanoparticles using dynamic thermal analysis. Polym. Degrad. Stab. 2012, 97, 3–13. [Google Scholar] [CrossRef]
- Van der Zwaag, S. The Concept of Filament Strength and the Weibull Modulus. J. Test. Eval. 1989, 17, 292–298. [Google Scholar]

| Sample | H2O2/TA (v/w) | Time (min) | Dy (%) |
|---|---|---|---|
| RGFs1 | 1 | 1.5 | 64 |
| RGFs2 | 1 | 3 | 73 |
| RGFs3 | 1 | 4.5 | 81 |
| RGFs4 | 2 | 1.5 | 84 |
| RGFs5 | 2 | 3 | 90 |
| RGFs6 | 2 | 4.5 | 92 |
| RGFs7 | 3 | 1.5 | 85 |
| RGFs8 | 3 | 3 | 92 |
| RGFs9 | 3 | 4.5 | 93 |
| RGFs10 | 3 | 6 | 93 |
| RGFs11 | H2O2 | 4.5 | <3 |
| RGFs12 | TA (5M) | 4.5 | <3 |
| RGFs13 | 3 | 360 * | 67 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zabihi, O.; Ahmadi, M.; Liu, C.; Mahmoodi, R.; Li, Q.; Ghandehari Ferdowsi, M.R.; Naebe, M. A Sustainable Approach to the Low-Cost Recycling of Waste Glass Fibres Composites towards Circular Economy. Sustainability 2020, 12, 641. https://doi.org/10.3390/su12020641
Zabihi O, Ahmadi M, Liu C, Mahmoodi R, Li Q, Ghandehari Ferdowsi MR, Naebe M. A Sustainable Approach to the Low-Cost Recycling of Waste Glass Fibres Composites towards Circular Economy. Sustainability. 2020; 12(2):641. https://doi.org/10.3390/su12020641
Chicago/Turabian StyleZabihi, Omid, Mojtaba Ahmadi, Chao Liu, Roya Mahmoodi, Quanxiang Li, Mahmoud Reza Ghandehari Ferdowsi, and Minoo Naebe. 2020. "A Sustainable Approach to the Low-Cost Recycling of Waste Glass Fibres Composites towards Circular Economy" Sustainability 12, no. 2: 641. https://doi.org/10.3390/su12020641
APA StyleZabihi, O., Ahmadi, M., Liu, C., Mahmoodi, R., Li, Q., Ghandehari Ferdowsi, M. R., & Naebe, M. (2020). A Sustainable Approach to the Low-Cost Recycling of Waste Glass Fibres Composites towards Circular Economy. Sustainability, 12(2), 641. https://doi.org/10.3390/su12020641







