The Effect of Visitors on the Properties of Vegetation of Calcareous Grasslands in the Context of Width and Distances from Tourist Trails
Abstract
1. Introduction
2. Materials and Methods
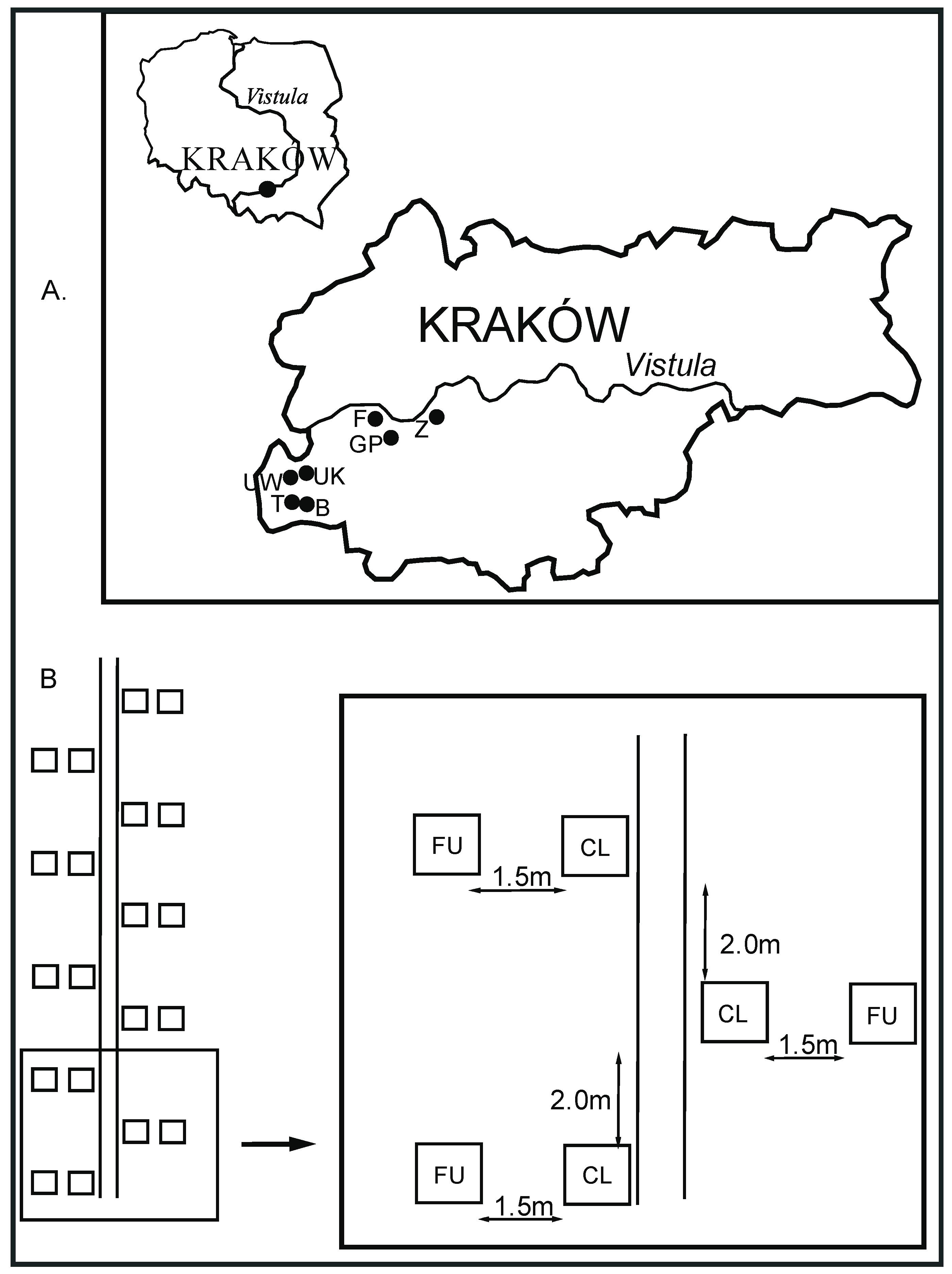
2.1. Location of the Study Sites
2.2. The Overview of the Study Design and Characteristics of the Study Plots
2.3. The Field Trial
- -“+”- species covers less than 1% of the studied area,
- -“1”- species covers 1–5% of the studied area,
- -“2”- species covers 6–25% of the studied area,
- -“3”- species covers 26–50% of the studied area,
- -“4”- species covers 51–75% of the studied area,
- -“5”- species covers 76–100% of the studied area.
2.4. The Species Groups
2.5. The Data Analysis
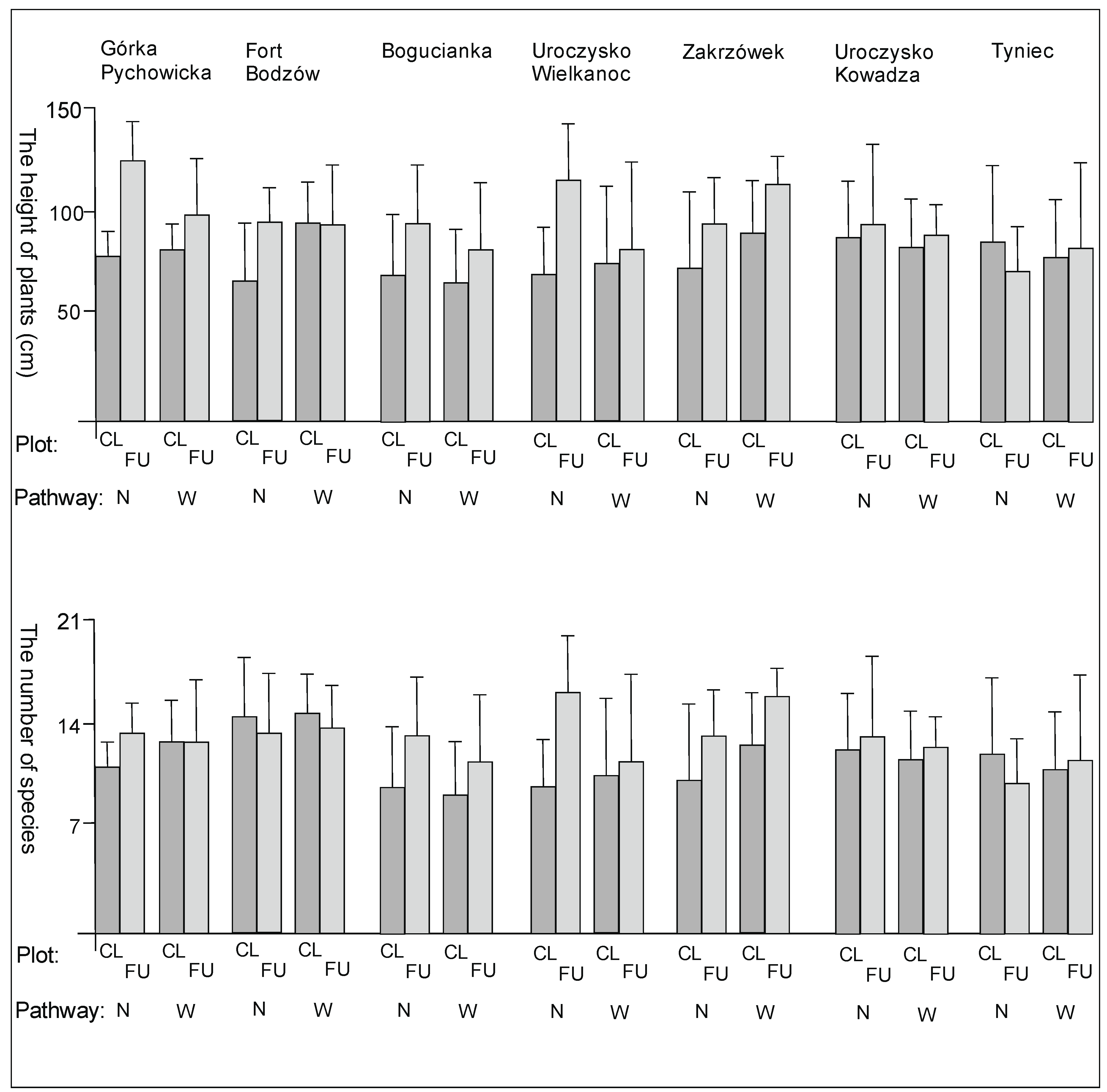
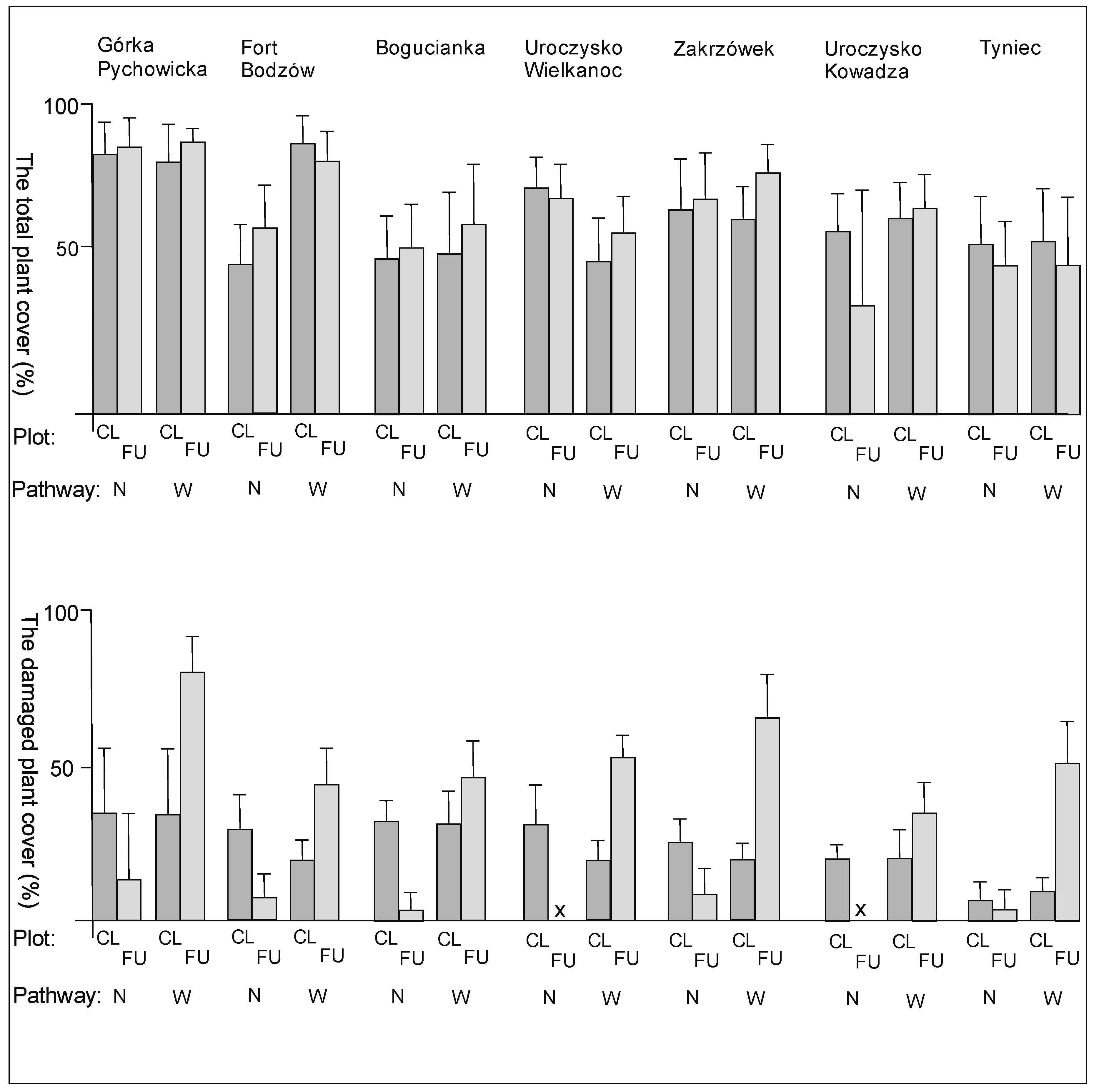
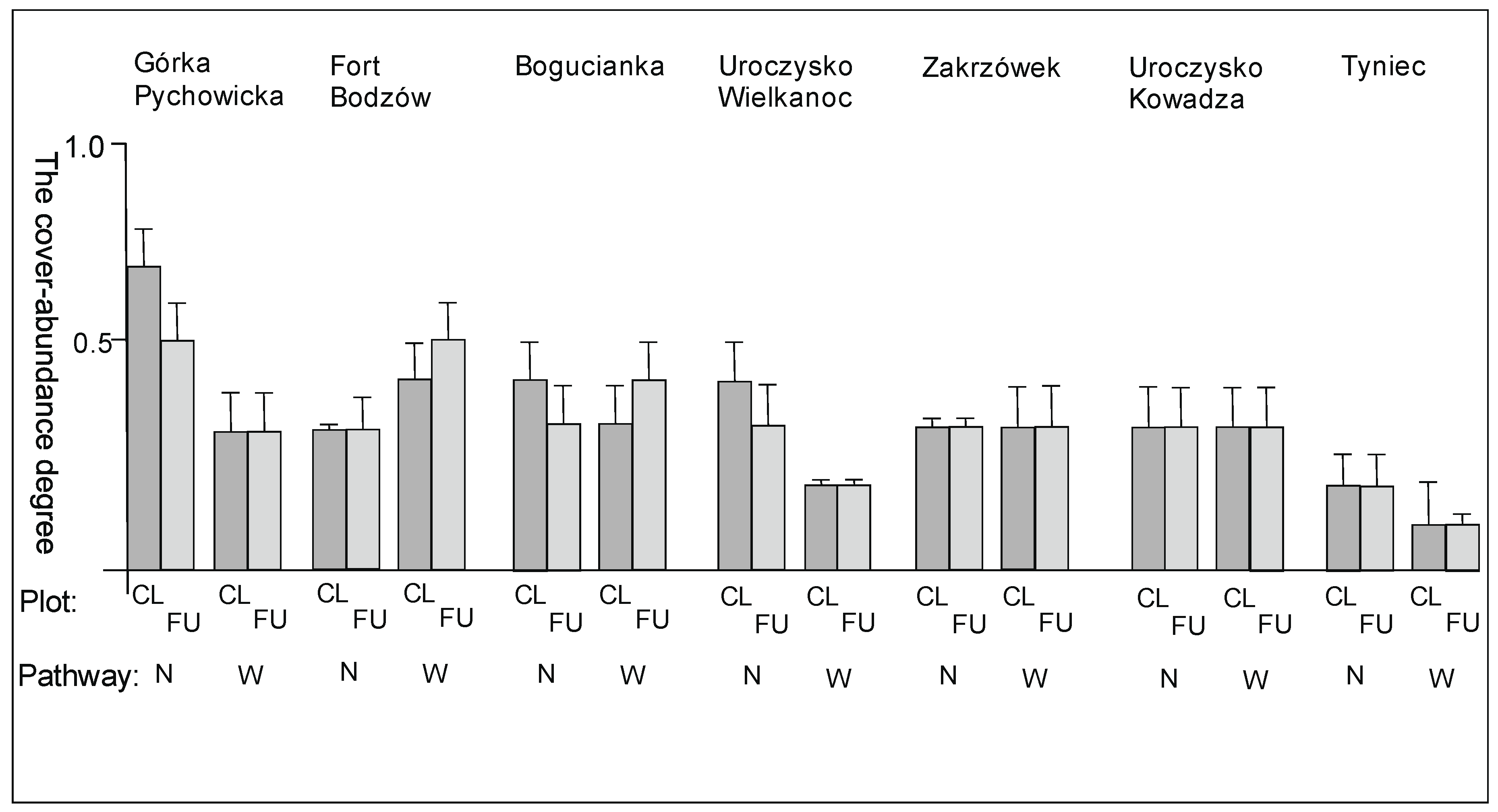
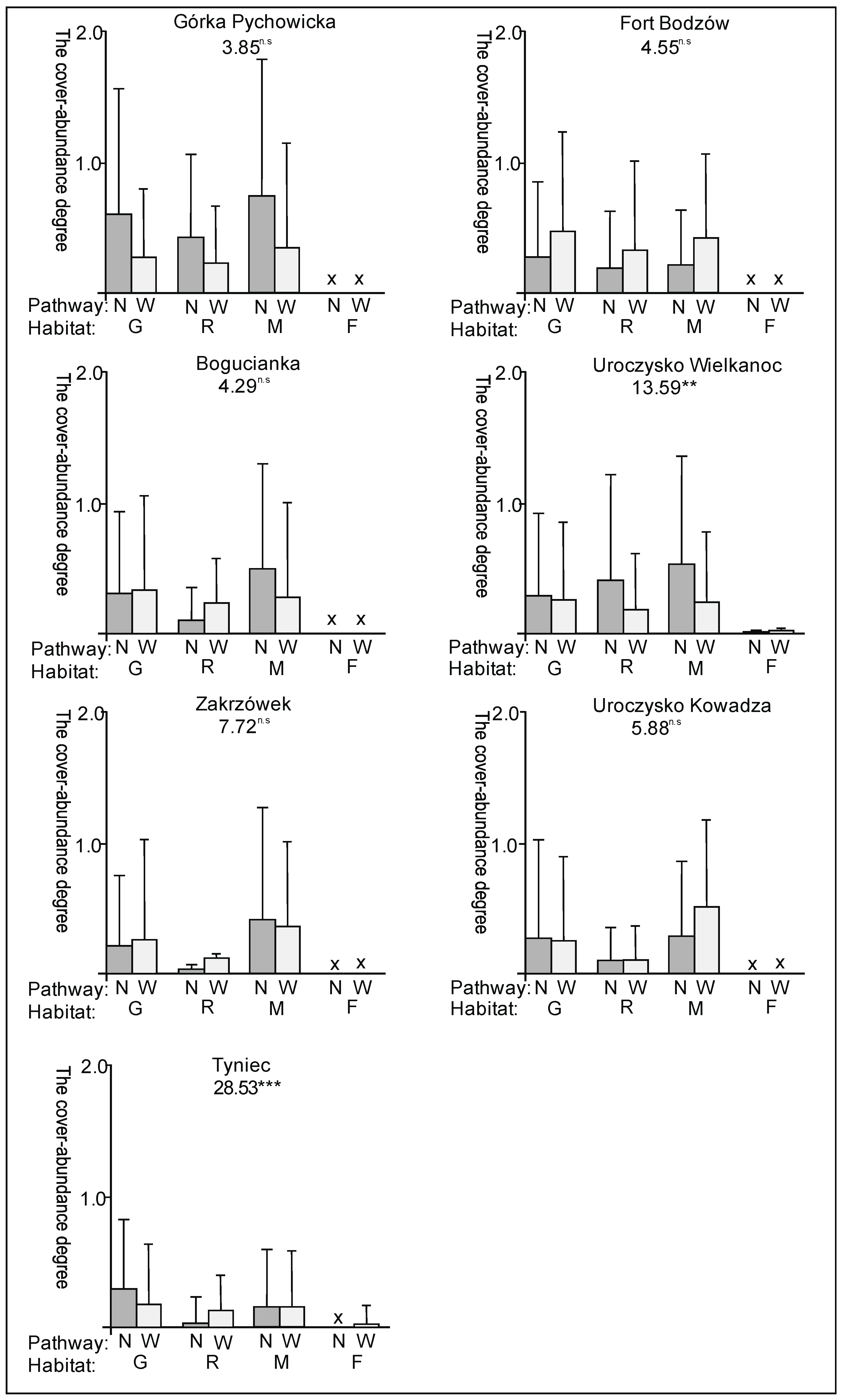
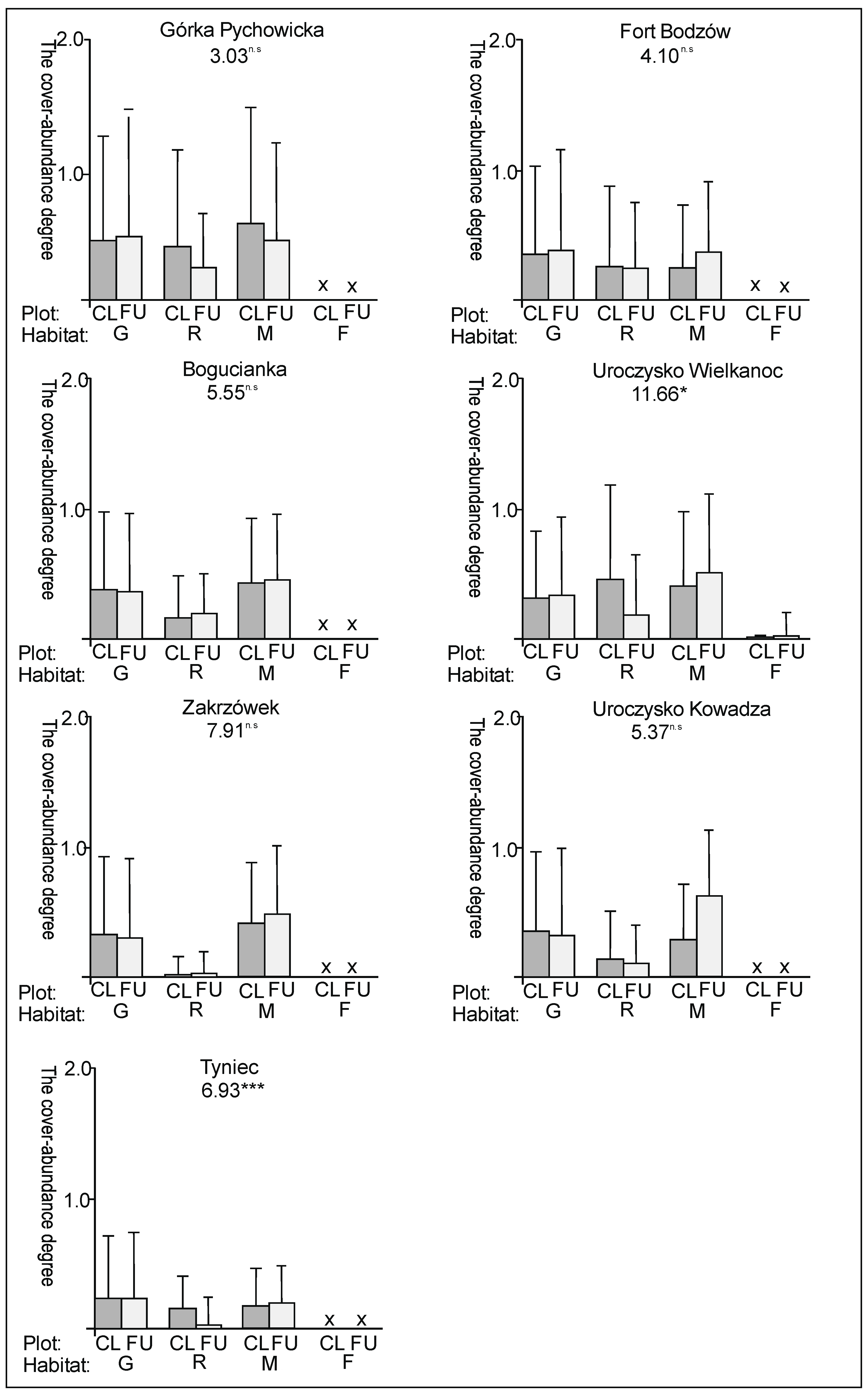
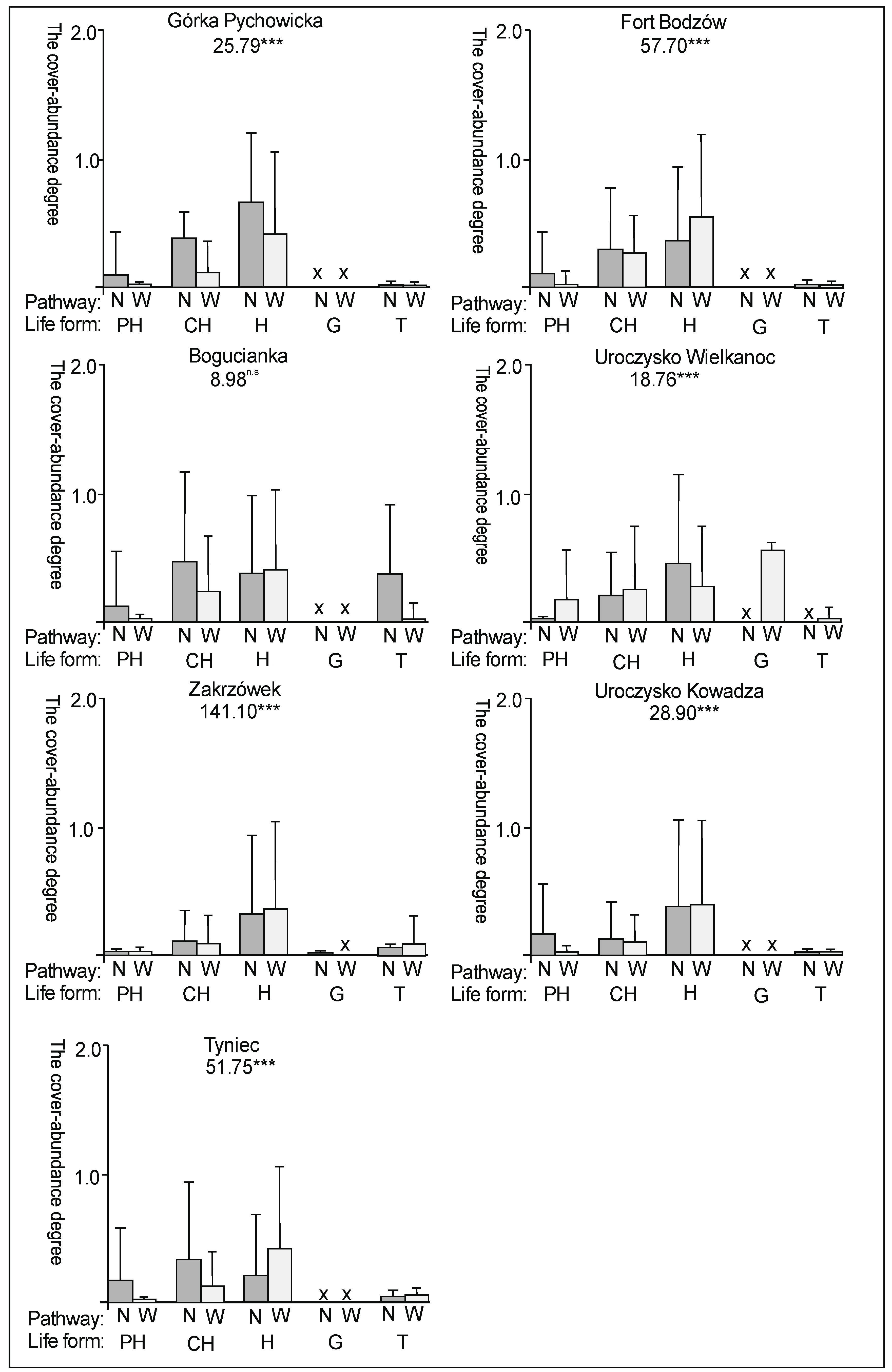
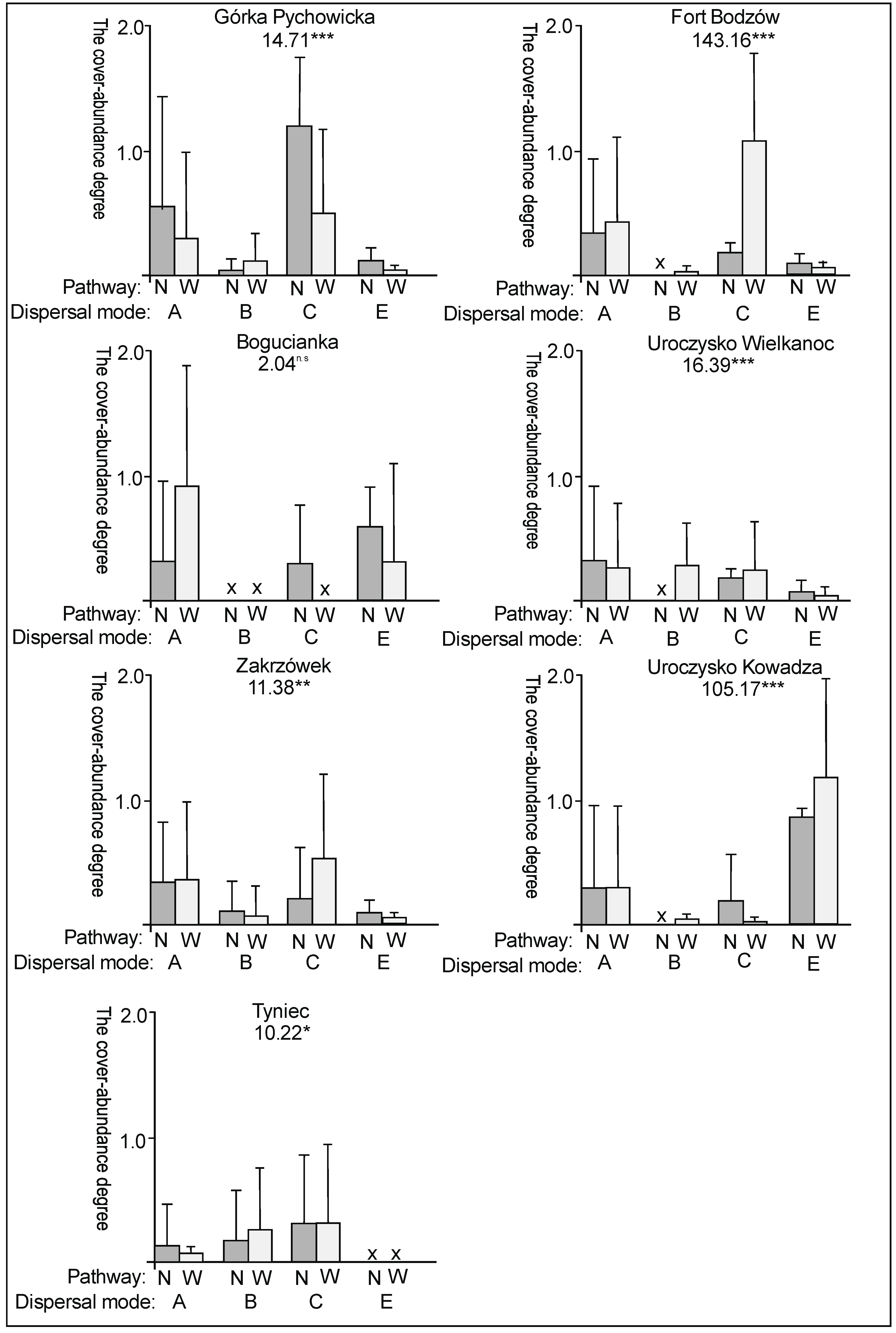
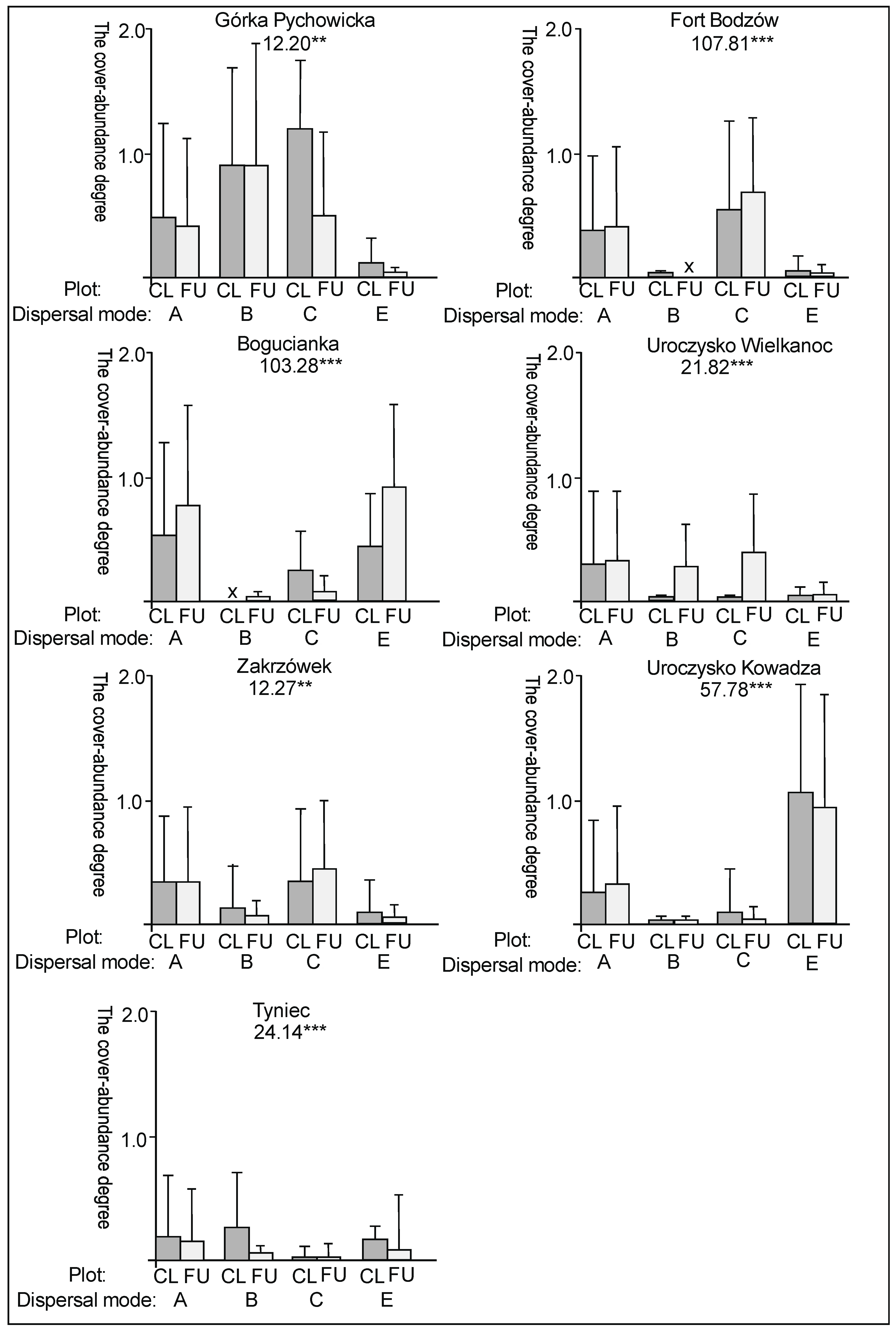
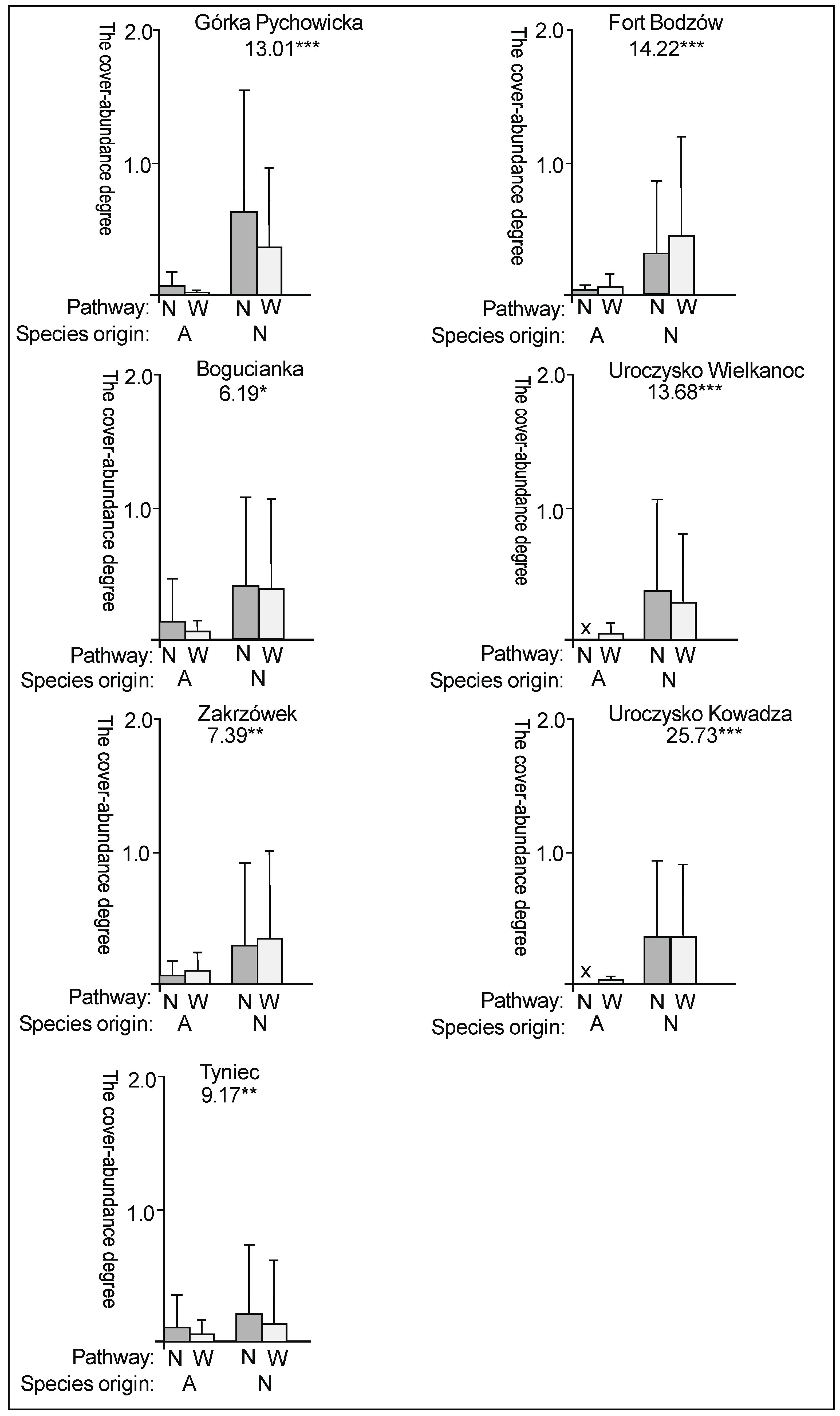
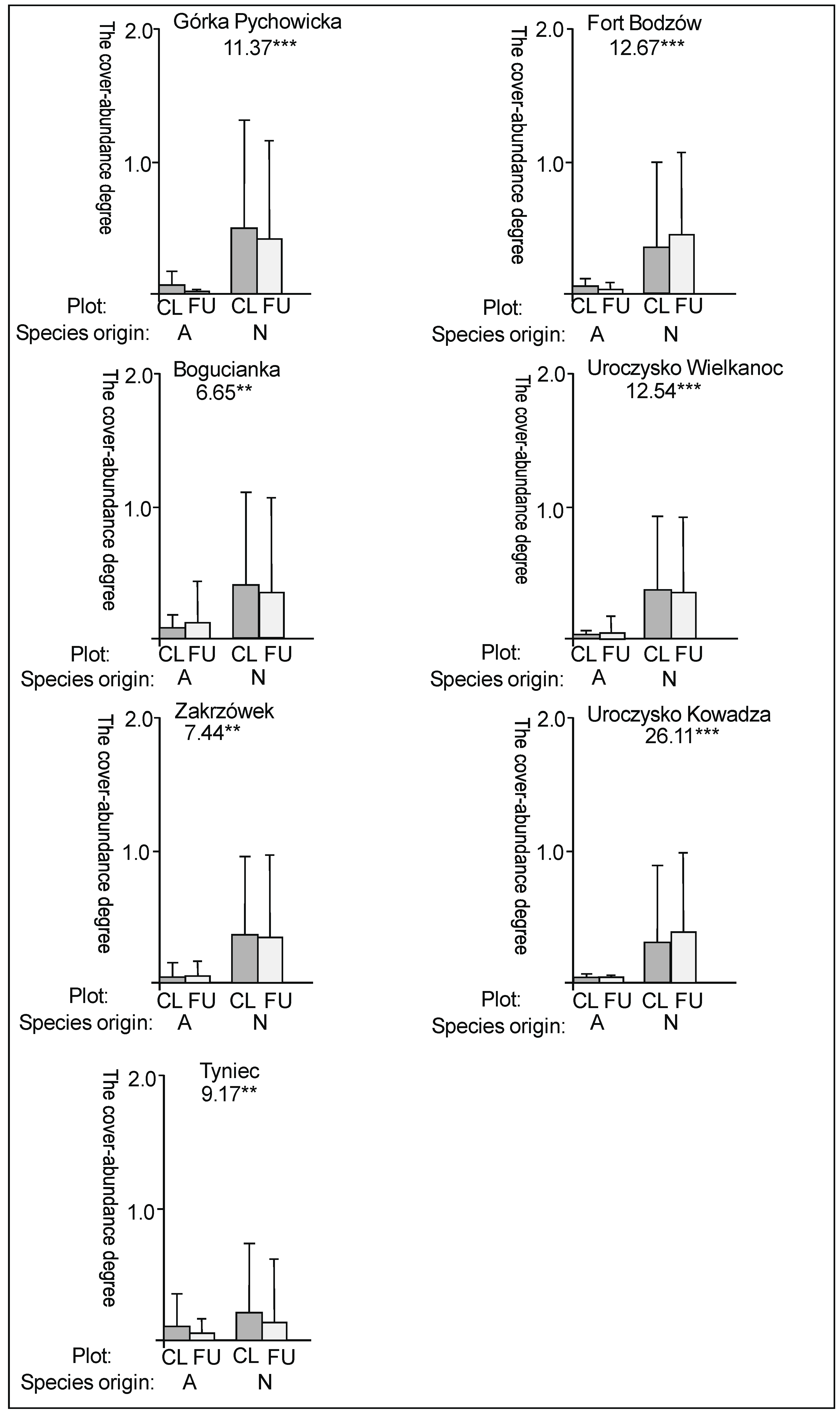
3. Results
3.1. The Plant Cover and Richness
3.2. The Species Groups Characteristics
4. Discussion
4.1. The Plant Cover Characteristics
4.2. The Species Groups Characteristics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Taxon | Habitat | Life Form | Dispersal Type | Origin |
|---|---|---|---|---|
| Acer platanoides L. | Forest | Phanerophyte | Epilobium | Native |
| Acer pseudoplatanus L. | Forest | Phanerophyte | Epilobium | Native |
| Achilleamillefolium L. | Meadow | Chamaephyte | Allium | Native |
| Acinosarvensis (Lam.) Dandy | Grassland | Therophyte | Allium | Native |
| Aegopodium podagraria L. | Forest | Hemicryptophyte | Allium | Native |
| Agrimonia eupatoria L. | Grassland | Hemicryptophyte | Bidens | Native |
| Agrostis capillaris L. | Grassland | Hemicryptophyte | Allium | Native |
| Ajuga genevensis L. | Grassland | Hemicryptophyte | Allium | Native |
| Allium montanum F. W. Schmidt | Grassland | Geophyte | Allium | Native |
| Alyssum alyssoides L. | Grassland | Therophyte | Allium | Native |
| Anchusa officinalis L. | Ruderal | Hemicryptophyte | Allium | Alien |
| Anthyllis vulneraria L. | Grassland | Hemicryptophyte | Allium | Native |
| Anthoxanthum odoratum L. | Meadow | Hemicryptophyte | Allium | Native |
| Arabidopsis thaliana (L.) Heynh. | Ruderal | Therophyte | Allium | Native |
| Arabis hirsuta (L.) Scop. | Grassland | Hemicryptophyte | Allium | Native |
| Arenaria serpyllifolia L. | Grassland | Therophyte | Allium | Native |
| Arrhenatherum elatius (L.) P. Beauv. ex J. & C. Presl | Meadow | Hemicryptophyte | Allium | Native |
| Artemisia campestris L. | Grassland | Chamaephyte | Allium | Native |
| Artemisia vulgaris L. | Ruderal | Hemicryptophyte | Allium | Native |
| Asperula cynanchica L. | Grassland | Hemicryptophyte | Allium | Native |
| Astragalus glycyphyllos L. | Grassland | Hemicryptophyte | Allium | Native |
| Avenula pratensis (L.) Dumort. | Grassland | Hemicryptophyte | Allium | Native |
| Avenula pubescens (Huds.) Dumort. | Meadow | Hemicryptophyte | Allium | Native |
| Briza media L. | Meadow | Hemicryptophyte | Allium | Native |
| Bromus erectus Huds. | Grassland | Hemicryptophyte | Allium | Native |
| Bromus hordeaceus L. | Meadow | Hemicryptophyte | Allium | Native |
| Bromus sterilis L. | Ruderal | Therophyte | Allium | Alien |
| Calamagrostis epigejos (L.) Roth. | Ruderal | Hemicryptophyte | Epilobium | Native |
| Calystegia sepium (L.) R. Br. | Ruderal | Hemicryptophyte | Allium | Native |
| Carduus acanthoides L. | Ruderal | Hemicryptophyte | Epilobium | Alien |
| Carex caryophyllea L. | Grassland | Hemicryptophyte | Allium | Native |
| Carex hirta L. | Meadow | Hemicryptophyte | Allium | Native |
| Carex ovalis Gooden. | Grassland | Hemicryptophyte | Allium | Native |
| Carex praecox Schreb. | Grassland | Hemicryptophyte | Allium | Native |
| Carex sp. | - | - | - | - |
| Carlina acaulis L. | Grassland | Hemicryptophyte | Epilobium | Native |
| Centaurea stoebe Tausch | Grassland | Hemicryptophyte | Allium | Native |
| Cerastium arvense L. | Grassland | Chamaephyte | Allium | Native |
| Cerasus sp. | - | - | - | - |
| Cerasus vulgaris Mill. | - | Phanerophyte | Cornus | Alien |
| Cerinthe minor L. | Ruderal | Hemicryptophyte | Allium | Native |
| Cichorium intybus L. | Ruderal | Hemicryptophyte | Allium | Alien |
| Convolvulus arvensis L. | Ruderal | Hemicryptophyte | Allium | Native |
| Cornus sanguinea L. | Grassland | Phanerophyte | Cornus | Native |
| Coronilla varia L. | Grassland | Hemicryptophyte | Allium | Native |
| Crataegus sp. | - | - | - | - |
| Cuscuta epithymum L. | Grassland | Therophyte | Allium | Native |
| Dactylis glomerata L. | Meadow | Hemicryptophyte | Allium | Native |
| Daucus carota L. | Meadow | Hemicryptophyte | Bidens | Native |
| Deschampsia caespitosa (L.) P. B. | Meadow | Hemicryptophyte | Allium | Native |
| Dianthus carthusianorum L. | Grassland | Chamaephyte | Allium | Native |
| Echium vulgare L. | Ruderal | Hemicryptophyte | Allium | Native |
| Elymus hispidus (Opiz) Melderis | Ruderal | Hemicryptophyte | Allium | Native |
| Elymus repens (L.) Gould | Ruderal | Hemicryptophyte | Allium | Native |
| Erigeron acris ssp. serotinus (Weihe) Greuter | Grassland | Hemicryptophyte | Epilobium | Native |
| Erigeron annuus (L.) Desf. | Ruderal | Hemicryptophyte | Epilobium | Alien |
| Erigeron canadensis L. | Ruderal | Therophyte | Epilobium | Alien |
| Euonymus europaeus L. | Forest | Phanerophyte | Cornus | Native |
| Euphorbia cyparissias L. | Grassland | Hemicryptophyte | Allium | Native |
| Euphrasia stricta J. P. Wolff. ex Lehmann | Grassland | Therophyte | Allium | Native |
| Fallopia convolvulus (L.) Á. Löve | Ruderal | Therophyte | Allium | Alien |
| Festuca pratensis Huds. | Meadow | Hemicryptophyte | Allium | Native |
| Festuca rubra L. | Meadow | Hemicryptophyte | Allium | Native |
| Festuca rupicola Heuff. | Grassland | Hemicryptophyte | Allium | Native |
| Festuca sp. | - | - | - | - |
| Fragaria viridis Weston | Grassland | Hemicryptophyte | Cornus | Native |
| Galium mollugo L. | Meadow | Hemicryptophyte | Allium | Native |
| Galium verum L. | Grassland | Hemicryptophyte | Allium | Native |
| Geranium pratense L. | Ruderal | Hemicryptophyte | Allium | Native |
| Geum urbanum L. | Meadow | Hemicryptophyte | Bidens | Native |
| Helianthemum nummularium (L.) Mill. | Grassland | Chamaephyte | Allium | Native |
| Hieracium pilosella L. | Grassland | Hemicryptophyte | Epilobium | Native |
| Holcus lanatus L. | Meadow | Hemicryptophyte | Allium | Native |
| Hypericum perforatum L. | Ruderal | Hemicryptophyte | Allium | Native |
| Knautia arvensis (L.) J. M. Coult. | Meadow | Hemicryptophyte | Allium | Native |
| Koeleria macrantha (Ledeb.) Schult. | Grassland | Hemicryptophyte | Allium | Native |
| Leontodon autumnalis L. | Meadow | Hemicryptophyte | Epilobium | Native |
| Leontodon hispidus L. | Meadow | Hemicryptophyte | Epilobium | Native |
| Leucanthemum vulgare Lam. | Meadow | Hemicryptophyte | Allium | Native |
| Ligustrum vulgare L. | Grassland | Phanerophyte | Cornus | Native |
| Linaria vulgaris Mill. | Ruderal | Hemicryptophyte | Allium | Native |
| Linum catharticum L. | Meadow | Hemicryptophyte | Allium | Native |
| Lolium perenne L. | Meadow | Hemicryptophyte | Allium | Native |
| Lotus corniculatus L. | Meadow | Hemicryptophyte | Allium | Native |
| Malus domestica Borkh. | - | Phanerophyte | Cornus | Alien |
| Medicago falcata L. | Grassland | Hemicryptophyte | Allium | Native |
| Medicago lupulina L. | Ruderal | Hemicryptophyte | Allium | Native |
| Medicago sativa L. | Ruderal | Hemicryptophyte | Allium | Alien |
| Pastinaca sativa L. | Meadow | Hemicryptophyte | Allium | Alien |
| Peucedanum oreoselinum (L.) Moench | Grassland | Hemicryptophyte | Allium | Native |
| Phleum phleoides (L.) H. Karst. | Grassland | Hemicryptophyte | Allium | Native |
| Phleum pratense L. | Meadow | Hemicryptophyte | Allium | Native |
| Picris hieracioides L. | Ruderal | Hemicryptophyte | Epilobium | Native |
| Pimpinella saxifraga L. | Meadow | Hemicryptophyte | Allium | Native |
| Plantago lanceolata L. | Grassland | Hemicryptophyte | Allium | Native |
| Plantago major L. | Meadow | Hemicryptophyte | Allium | Native |
| Plantago media L. | Grassland | Hemicryptophyte | Allium | Native |
| Poa compressa L. | Ruderal | Hemicryptophyte | Allium | Native |
| Poa pratensis L. | Ruderal | Hemicryptophyte | Allium | Native |
| Polygonum aviculare L. | Ruderal | Therophyte | Allium | Native |
| Populus tremula L. | Ruderal | Phanerophyte | Epilobium | Native |
| Potentilla arenaria Borkh. | Grassland | Hemicryptophyte | Allium | Native |
| Potentilla argentea L. | Grassland | Hemicryptophyte | Allium | Native |
| Potentilla reptans L. | Meadow | Hemicryptophyte | Allium | Native |
| Prunella vulgaris L. | Meadow | Hemicryptophyte | Allium | Native |
| Prunus sp. | - | - | - | - |
| Prunus spinosa L. | Grassland | Phanerophyte | Cornus | Native |
| Rhamnus cathartica L. | Grassland | Phanerophyte | Cornus | Native |
| Ranunculus bulbosus L. | Grassland | Hemicryptophyte | Allium | Native |
| Robinia pseudoacacia L. | Forest | Phanerophyte | Allium | Alien |
| Rosa canina L. | Grassland | Phanerophyte | Cornus | Native |
| Rubus caesius L. | Grassland | Phanerophyte | Cornus | Native |
| Rumex crispus L. | Ruderal | Hemicryptophyte | Allium | Native |
| Rumex obtusifolius L. | Ruderal | Hemicryptophyte | Allium | Native |
| Rumex thyrsiflorus Fingerh. | Meadow | Hemicryptophyte | Allium | Native |
| Salvia pratensis L. | Grassland | Hemicryptophyte | Allium | Native |
| Salvia verticillata L. | Grassland | Hemicryptophyte | Allium | Native |
| Sanguisorba minor Scop. | Grassland | Hemicryptophyte | Allium | Native |
| Sarothamnus scoparius(L.) Wimm. | Grassland | Phanerophyte | Allium | Native |
| Scabiosa ochroleuca L. | Grassland | Hemicryptophyte | Allium | Native |
| Sedum acre L. | Grassland | Chamaephyte | Allium | Native |
| Sedum sexangulare L. | Grassland | Chamaephyte | Allium | Native |
| Senecio jacobaea L. | Ruderal | Hemicryptophyte | Epilobium | Native |
| Seseli annuum L. | Grassland | Hemicryptophyte | Allium | Native |
| Setaria viridis (L.) P. Beauv. | Ruderal | Therophyte | Bidens | Alien |
| Silene otites (L.) Wibel | Grassland | Hemicryptophyte | Allium | Native |
| Solidago canadensis L. | Ruderal | Hemicryptophyte | Epilobium | Alien |
| Solidago gigantea Aiton | Ruderal | Hemicryptophyte | Epilobium | Alien |
| Solidago virgaurea L. | Grassland | Hemicryptophyte | Epilobium | Native |
| Sonchus oleraceus L. | Ruderal | Hemicryptophyte | Epilobium | Alien |
| Stachys recta L. | Grassland | Hemicryptophyte | Allium | Native |
| Taraxacum sp. | - | - | - | - |
| Thymus austriacus Bernh. | Grassland | Chamaephyte | Allium | Native |
| Thymus glabrescens Willd. | Grassland | Chamaephyte | Allium | Native |
| Thymus pulegioides L. | Grassland | Chamaephyte | Allium | Native |
| Tragopogon pratensis L. | Meadow | Hemicryptophyte | Epilobium | Native |
| Trifolium arvense L. | Grassland | Therophyte | Allium | Native |
| Trifolium campestre Schreb. | Grassland | Therophyte | Allium | Native |
| Trifolium montanum L. | Meadow | Hemicryptophyte | Allium | Native |
| Trifolium pratense L. | Meadow | Hemicryptophyte | Allium | Native |
| Trifolium repens L. | Meadow | Hemicryptophyte | Allium | Native |
| Trisetum flavescens (L.) P. Beauv. | Grassland | Hemicryptophyte | Allium | Native |
| Verbascum lychnitis L. | Grassland | Hemicryptophyte | Allium | Native |
| Verbascum thapsus L. | Ruderal | Hemicryptophyte | Allium | Native |
| Veronica arvensis L. | Ruderal | Therophyte | Allium | Alien |
| Veronica austriaca L. | Grassland | Chamaephyte | Allium | Native |
| Veronica chamaedrys L. | Ruderal | Chamaephyte | Allium | Native |
| Veronica spicata L. | Grassland | Chamaephyte | Allium | Native |
| Vicia cracca L. | Meadow | Hemicryptophyte | Allium | Native |
| Vicia grandiflora Scop. | Ruderal | Therophyte | Allium | Alien |
| Vicia hirsuta (L.) S. F. Gray | Ruderal | Therophyte | Allium | Alien |
| Vicia tetrasperma (L.) Schreb. | Ruderal | Therophyte | Allium | Alien |
| Vincetoxicum hirundinaria Medik. | Grassland | Hemicryptophyte | Epilobium | Native |
| Viola hirta L. | Grassland | Hemicryptophyte | Allium | Native |
| Viola odorata L. | Ruderal | Hemicryptophyte | Allium | Native |
References
- Kruczek, Z. Tourists vs. Residents. The Influence of Excessive Tourist Attendance on the Process of Gentrification of Historic Cities on the Example of Kraków. Tur. Kult. 2018, 3, 21–49. [Google Scholar]
- Martín Martín, J.M.; GuaitaMartínez, J.M.; SalinasFernández, J.A. An Analysis of the Factors behind the Citizen’s Attitude of Rejection towards Tourism in a Context of Overtourism and Economic Dependence on This Activity. Sustainability 2018, 10, 2851. [Google Scholar] [CrossRef]
- Hospers, G.-J. Overtourism in Euro pean cities: From challenges to coping strategies. CESifo Forum 2019, 20, 20–24. [Google Scholar]
- Milano, C.; Novelli, M.; Cheer, J.M. Overtourism and degrowth: A social movements perspective. J. Sustain. Tour. 2019, 27, 1857–1875. [Google Scholar] [CrossRef]
- Capocchi, A.; Vallone, C.; Pierotti, M.; Amaduzzi, A. Overtourism: A Literature Review to Assess Implications and Future Perspectives. Sustainability 2019, 11, 3303. [Google Scholar] [CrossRef]
- Koens, K.; Postma, A.; Papp, B. Isovertourism overused? Understanding the impact of tourism in a city context. Sustainability 2018, 10, 4384. [Google Scholar] [CrossRef]
- Kruczek, Z. Overtourism”-aroundthedefinition. Encyclopedia 2019, 1. Available online: https://encyclopedia.pub/163 (accessed on 6 December 2019).
- Witkowski, Z. Wpływ turystyki na ochronę przyrody. In Integralna Ochrona Przyrody; Grzegorczyk, M., Ed.; Institute of Nature Conservation PAS: Kraków, Poland, 2007; pp. 187–189. [Google Scholar]
- Witkowski, Z.; Adamski, P.; Mroczka, A.; Ciapała, S. Limits of tourism and recreation interference in land areas of national parks and nature reserves. Prądnik 2010, 20, 427–440. [Google Scholar]
- Partyka, J. Tourist traffic in Polish national parks. Folia Tur. 2010, 22, 9–23. [Google Scholar]
- Fidelus, J.; Rogowski, M. Geomorphological effects of tourist usage of the mountain ridges on the example oftourist footpaths in the Western Tatra Mountains (Poland) and the Bucegi Mountains (Romania). Landf. Anal. 2012, 19, 29–40. [Google Scholar]
- Duda, T. Zrównoważona turystyka kulturowa na obszarach przyrodniczo cennych. Studium przypadku Drawieńskiego Parku Narodowego. Tur. Kult. 2018, 7, 102–108. [Google Scholar]
- Kapera, I. Sustainable Development of Tourism. Environmental, Social, and Economic Issues on the Example of Poland, 1st ed.; Andrzej Frycz Modrzewski Krakow University: Kraków, Poland, 2018; pp. 87–102. [Google Scholar]
- Kruczek, Z. Tourist traffic in national parks and consequences of excessive frequency of visitors. In National Parks and Socio-Economic Environment. Condemned to Dialogue, 1st ed.; Nocoń, M., Pasierbek, T., Sobczuk, J., Walas, B., Eds.; The University College of Tourism and Ecology: Sucha Beskidzka, Poland, 2019; pp. 160–177. [Google Scholar]
- Balmford, A.; Green, J.M.H.; Anderson, M.; Beresford, J.; Huang, C.; Naidoo, R.; Walpole, M.; Manica, A. Walk on the wild side: Estimating the global magnitude of visits to protected areas. PLoS Biol. 2015, 13, 1002074. [Google Scholar] [CrossRef] [PubMed]
- Kiszka, K. Degradation of tourist routes in the Pieniny Mts. caused by hiking. Pienin—Przyr. Człowiek 2016, 14, 145–165. [Google Scholar]
- Leung, Y.-F.; Marion, J.L. Recreation impacts and management in wilderness: A state-of-knowledge review. In Proceedings of the Wilderness Science in a Time of Change Conference—Vol. 5, Wilderness Ecosystems, Threats, and Management, Missoula, MT, USA, 23–27 May 1999; Cole, D.N., McCool, S.F., Borrie, W.T., O’Loughlin, J., Eds.; Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 1999; pp. 23–48. [Google Scholar]
- Kołodziejczyk, K. Standards for Infrastructure on Tourist Trails Based on Selected European Examples, 1st ed.; Institute of Geography and Regional Development: Wrocław, Poland, 2015; 459p. [Google Scholar]
- Kuss, F.R.; Graefe, A.R. Effects of recreation trampling on natural area vegetation. J. Leis. Res. 1985, 17, 165–183. [Google Scholar] [CrossRef]
- Wichmann, M.C.; Alexander, M.J.; Soons, M.B.; Galsworthy, S.; Dunne, L.; Gould, R.; Fairfax, Ch.; Niggemann, M.; Hails, R.S.; Bullock, J.M. Human-mediated dispersal of seeds over long distances. Proc. R. Soc. B Biol. Sci. 2008, 276, 523–532. [Google Scholar] [CrossRef]
- Pickering, C.; Mount, A. Do tourists disperse weed seed? A global review of unintentional human-mediated terrestrial seed dispersal on clothing, vehicles and horses. J. Sustain. Tour. 2010, 18, 239–256. [Google Scholar] [CrossRef]
- Anderson, L.G.; Rocliffe, S.; Haddaway, N.R.; Dunn, A.M. The role of tourism and recreation in the spread of non-native species: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0140833. [Google Scholar] [CrossRef]
- Sun, D.; Liddle, M.J. Plant morphological characteristics and resistance tosimulated trampling. Environ. Manag. 1993, 17, 511–521. [Google Scholar] [CrossRef]
- Kissling, M.; Hegetschweiler, K.T.; Rusterholz, H.P.; Baur, B. Short-term and long-term effects of human trampling on above-ground vegetation, soildensity, soilorganic matter and soil microbial processes in suburban beech forests. Appl. Soil Ecol. 2009, 42, 303–314. [Google Scholar] [CrossRef]
- Pickering, C.M.; Growcock, A.J. Impacts of experimental trampling on tall alpine herb fields and subalpine grasslands in the Australian Alps. J. Environ. Manag. 2009, 91, 532–540. [Google Scholar] [CrossRef]
- Dumitraşcu, M.; Marin, A.; Preda, E.; Ţîbîrnac, M.; Vădineanu, A. Tramplingeffectsonplantspeciesmorphology. Rom. J. Biol.- Plant Biol. 2010, 55, 89–96. [Google Scholar]
- Bernhardt-Römermann, M.; Gray, A.; Vanbergen, A.J.; Bergès, L.; Bohner, A.; Brooker, R.W.; DeBruyn, L.; DeCinti, B.; Dirnböck, T.; Grandin, U.; et al. Functional traits and local environment predict vegetation responses to disturbance: A pan-European multi-site experiment. J. Ecol. 2011, 99, 777–787. [Google Scholar] [CrossRef]
- Korkanç, S.Y. Impacts of recreational human trampling on selected soil and vegetation properties of Aladag Natural Park, Turkey. Catena 2014, 113, 219–225. [Google Scholar] [CrossRef]
- Mason, S.; Newsome, D.; Moore, S.; Admiraal, S. Recreational trampling negatively impacts vegetation structure of an Australian biodiversity hot spot. Biodivers Conserv. 2015, 24, 2685–2707. [Google Scholar] [CrossRef]
- Runnström, M.C.; Ólafsdóttir, R.; Blanke, J.; Berlin, B. Image analysis to monitor experimental trampling and vegetation recovery in Icelandic plant communities. Environments 2019, 6, 99. [Google Scholar] [CrossRef]
- Grabherr, G. The impact of trampling by tourists on a high altitudinal grasslandin the Tyrolean Alps, Austria. Vegetatio 1982, 48, 209–219. [Google Scholar]
- Gmyrek-Gołąb, K.; Krauz, K.; Łabaj, M.; Mroczka, A.; Tadel, A.; Witkowski, Z. Tourist dispersion around a trail in ‘WawozHomole’[HomoleGeorge] naturereserve. Nat. Conserv. 2005, 61, 61–64. [Google Scholar]
- Wenjun, L.; Xiaodong, G.; Chunyan, L. Hiking trail sand tourism impact assessment in protected area: Jiuzhaigou Biosphere Reserve, China. Environ. Monit. Assess. 2005, 108, 279–293. [Google Scholar]
- Kolasińska, A.; Adamski, P.; Ciapała, S.; Švajda, J.; Witkowski, Z. Trail management, off-trail walking and visitor impact in the Pieniny Mts National Park (PolishCarpathians). Eco-Mont 2015, 7, 26–36. [Google Scholar]
- Dale, D.; Weaver, T. Trampling effects on vegetation of the trail corridors of North Rocky Mountain Forests. J. Appl. Ecol. 1974, 11, 767–772. [Google Scholar] [CrossRef]
- Bright, J.A. Hiker impact on herbaceous vegetation along trails in an evergreen woodland of Central Texas. Biol. Conserv. 1986, 36, 53–69. [Google Scholar] [CrossRef]
- Hall, C.N.; Kuss, F.R. Vegetation alternation along trials in Shenandoah National Park, Virginia. Biol. Conserv. 1989, 48, 211–227. [Google Scholar] [CrossRef]
- Roovers, P.; Verheyen, K.; Hermy, M.; Gulinck, H. Experimental trampling and vegetation recovery in some forest and heathland communities. Appl. Veg. Sci. 2004, 7, 111–118. [Google Scholar] [CrossRef]
- Skłodowski, J.W.; Bartosz, S.; Dul, Ł.; Grzybek, D.; Jankowski, S.; Kajetanem, M.; Kalisz, P.; Korenkiewicz, U.; Mazur, G.; Myszek, J.; et al. An attempt to assess the effect of tourist trail width on adjacen tforest environment. Sylwan 2009, 153, 699–709. [Google Scholar]
- Zdanowicz, E.; Skłodkowski, S. Evaluation of changes in environment around recreational routes on the example of Bielański Forest Reserve in Warsaw. Studia I Mater. CEPL W Rogowie 2013, 37, 348–355. [Google Scholar]
- Atik, M.; Sayan, S.; Karaguzel, O. Impact of recreational trampling on the natural vegetation in Termessos National Park, Antalya-Turkey. Tarim Bilimleri Dergisi 2009, 15, 249–258. [Google Scholar]
- Gremmen, N.J.M.; Smith, V.R.; vanTongeren, O.F.R. Impact of trampling on the vegetation of subantarctic Marion Island. Arc. Antarc. Alp. Res. 2003, 35, 442–446. [Google Scholar] [CrossRef]
- Jägerbrand, A.K.; Alatalo, J.M. Effects of human trampling on abundance and diversity of vascular plants, bryophytes and lichens in alpine heath vegetation, Northern Sweden. Springerplus 2015, 4, 1–12. [Google Scholar] [CrossRef]
- Kutiel, P.; Zhevelev, H.; Harrison, R. The effect of recreational impacts on soil and vegetation of stabilised coastal dunes in the Sharon Park, Israel. Ocean Coast. Manag. 1999, 42, 1041–1060. [Google Scholar] [CrossRef]
- Interpretation Manual of European Union Habitats-Eur 27. Available online: http://ec.europa.eu/environment/nature/legislation/habitatsdirective/docs/2007_07_im.pdf (assessed on 7 December 2019).
- Matuszko, D.; Piotrowicz, K. Characteristics of urban climate and the climate of Krakow. In The City in the Study of Geographers, 1st ed.; Trzepacz, P., Więcław-Michniewska, J., Brzosko-Sermak, A., Kołos, A., Eds.; Institute of Geography and Spatial Management, Jagiellonian University: Kraków, Poland, 2015; pp. 221–241. [Google Scholar]
- Perzanowska, J. Podgórki Tynieckie. In Treasures of Nature and Culture of Krakow and the Surrounding Area. Ecological Educational Paths; Grzegorczyk, M., Perzanowska, J., Eds.; Institute of Nature Conservation PAS and, WAM: Kraków, Poland, 2005; pp. 307–341. [Google Scholar]
- Csapodý, V. Keimlingsbestimmungs-Buch der Dikotyledonen; Akademiai Kiado: Budapeszt, Hungary, 1968; 286p. [Google Scholar]
- Muller, F.M. Seedlings of the North-Western European Lowland. A Flora of Seedlings, 1st ed.; Junk, B.V., Ed.; Springer Netherlands: Haarlem, The Netherlands, 1978; 653p. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie, Grundzüge der Vegetationskunde, 3rd ed.; Springer: Berlin, Germany, 1964; 631p. [Google Scholar]
- Matuszkiewicz, W.A. Guide for Identification of Polish Plant Communities; Polish Scientific Publishers PWN: Warsaw, Poland, 2017; 536p. [Google Scholar]
- Raunkiaer, C. The Life Forms of Plants and Statistical Plant Geography; Oxford University Press: London, UK, 1934; 632p. [Google Scholar]
- Fitter, A.H.; Peat, H.J. The Ecological Flora Database. J. Ecol. 1994, 82, 415–425. Available online: http://www.ecoflora.co.uk (assessed on 7 December 2019). [CrossRef]
- Ellenberg, H.; Weber, H.E.; Düll, R.; Wirth, V.; Werner, W.; Paulißen, D. Zeigerwerte von Pflanzen in Mitteleuropa. ScriptaGeobot. 1992, 18, 3–258. [Google Scholar]
- Pladias. Database of the Czech Flora and Vegetation. 2014–2019. Available online: http://www.pladias.org (assessed on 7 December 2019).
- Sádlo, J.; Chytrý, M.; Pergl, J.; Pyšek, P. Plant dispersal strategies: A new classification based on the multiple dispersal modes of individuals pecies. Preslia 2018, 90, 1–22. [Google Scholar] [CrossRef]
- Alien Species in Poland. 2009. Available online: http://www.iop.krakow.pl/ias/species (assessed on 7 December 2019).
- Pliszko, A. Additional data to the occurrence of Erigeron acris subsp. Serotinus (Weihe) Greuter (Asteraceae) in Europe. Steciana 2014, 18, 29–31. [Google Scholar]
- Calculation for the Chi-Square Test: An Interactive Calculation Tool for Chi-Square Tests of Goodness of Fit and Independence. 2001. Available online: http://quantpsy.org (assessed on 7 December 2019).
- Root-Bernstein, M.; Svenning, J.C. Human paths have positive impacts on plant richness and diversity: Ameta-analysis. Ecol. Evol. 2018, 8, 11111–11121. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, M.; Pickering, C.M.; McDougall, K.L.; Wright, G.T. Sustained impacts of a hiking trail on changing Windswept Feldmark vegetation in the Australian Alps. Aust. J. Bot. 2014, 62, 263–275. [Google Scholar] [CrossRef]
- Tikka, P.M.; Högmander, H.; Koski, P.S. Road and railway verges serve as dispersal corridors for grasslandplants. Landsc. Ecol. 2001, 16, 659–666. [Google Scholar] [CrossRef]
- Sun, D. Trampling resistance, recovery and growth rate of eight plant species. Agric. Ecosyst. Environ. 1992, 38, 165–273. [Google Scholar] [CrossRef]
- Snetsinger, S.D.; White, K. Recreation and Trail Impacts on Wildlife Species of Interest in Mount Spokane State Park; Pacific Biodiversity Institute, Winthrop: Washington, DC, USA, 2009; 60p. [Google Scholar]
- Harper, J.L. The Population Biology of Plants, 1st ed.; Academic Press: London, UK, 1977; 892p. [Google Scholar]
- Grubb, P.J. The maintenance of species-richness in plant communities: The importance of the regeneration niche. Biol. Rev. 1977, 52, 107–145. [Google Scholar] [CrossRef]
- Bullock, J.M. Gaps and seedling colonization. In Seeds: The Ecology of Regeneration in Plant Communities, 2nd ed.; Fenner, M., Ed.; CABI Publishing: NewYork, NY, USA, 2000; pp. 375–395. [Google Scholar]
- Kalamees, R.; Zobel, M. The role of the seed bank in gap regeneration in a calcareous grassland community. Ecology 2002, 83, 1017–1025. [Google Scholar] [CrossRef]
- Bullock, J.M.; Hill, B.C.; Silvertown, J.; Sutton, M. An experimental study of the effects of sheep grazing on vegetation change in a species-poor grasslands and the role of seedling recruitment intogaps. J. Appl. Ecol. 1994, 31, 493–507. [Google Scholar] [CrossRef]
- Bąba, W.; Kompała-Bąba, A. Do small-scale gaps in calcareous grassland swards facilitates eedling establishment? Acta Soc. Bot. Pol. 2005, 74, 125–131. [Google Scholar] [CrossRef]
- Heise, W. The Impact of Landscape Structure and Use History on the Flora and Vegetation of Calcareous Grasslands in Krakow. Ph.D. Thesis, Institute of Botany, Jagiellonian University, Kraków, Poland, 2014. [Google Scholar]
- Mydłowski, M. (Ed.) Directions of Development and Management of Green Areas in Krakow for 2017–2030. Anex II: Nature Conservation; Department of Environmental Management: Krakow, Poland, 2016; 256p. [Google Scholar]
- Tokarska-Guzik, B.; Dajdok, Z.; Zając, M.; Zając, A.; Urbisz, A.; Danielewicz, W.; Hołdyński, C. Alien Plants in Poland with Particular Reference to Invasive Species; General Directorate for Environmental Protection: Warsaw, Poland, 2012; 197p. [Google Scholar]
- Dobay, G.; Dobay, B.; Falusi, E.; Hajnáczki, S.; Penksza, K.; Bajor, Z.; Lampert, R.; Bakó, G.; Wichmann, B.; Szerdahelyi, T. Effects of sport tourism on temperate grasslandcommunities (Duna-IpolyNationalPark, Hungary). Appl. Ecol. Environ. Res. 2017, 15, 457–472. [Google Scholar] [CrossRef]
- Pescott, O.L.; Stewart, G.B. Assessing the impact of human trampling on vegetation: A systematic review and meta-analysis of experimental evidence. Peer J. 2014, 2, e360. [Google Scholar] [CrossRef] [PubMed]
- Malo, J.E.; Jiménez, B.; Suárez, F. Herbivore dunging and endozoochorous seed deposition in a Mediterranean dehesa. J. Rangel. Manag. 2000, 53, 322–328. [Google Scholar] [CrossRef]
- Cosyns, E.; Claerbout, S.; Lamoot, I.; Hoffmann, M. Endozoochorous seed dispersal by cattle and horse in as patially heterogeneous landscape. Plant Ecol. 2005, 178, 149–162. [Google Scholar] [CrossRef]
- Kuiters, A.T.; Huiskes, H.P.J. Potential of endozoochorous seed dispersal by sheep in calcareous grasslands: Correlations with seed traits. Appl. Veg. Sci. 2010, 13, 163–172. [Google Scholar] [CrossRef]
- Protection Plans for Natura 2000 Areas. Available online: http://krakow.rdos.gov.pl/plany-zadan-ochronnych (assessed on 2 January 2020).

| Study Site | Tourist/Recreation Infrastructure | Width of Pathway (cm) | Coordinates and Elevation of Pathway | |||
|---|---|---|---|---|---|---|
| within the Study Area | in the Vicinity of the Study Area | Narrow | Wide | Narrow | Wide | |
| Bogucianka | Vantage point, information board | Football stadium | 46 | 150 | 50°00.555’ | 50°00.675’ |
| N/19°48.909’; | N/19°48.914’; | |||||
| 244 m a.s.l. 1 | 251 m a.s.l. | |||||
| Fort Bodzów | Vantage points, benches, bins, shelters, motor sports paths | Rope park | 50 | 240 | 50°02.031’ | 50°01.978’ |
| N/19°52.576’; | N/19°51.891’; | |||||
| 250 m a.s.l. | 238 m a.s.l. | |||||
| Górka Pychowicka | Vantage points, benches, bins, shelters, fire circles, information board | Motor sports paths, bike paths | 50 | 180 | 50°01.823’ | 50°01.850’ |
| N/19°52.996’; | N/19°52.996’; | |||||
| 225 m a.s.l. | 225 m a.s.l. | |||||
| Tyniec | Vantage point, motor sports paths, | - | 36 | 335 | 50°00.301’ | 50°00.313’ |
| N/19°49.095’; | N/19°49.125’; | |||||
| 256 m a.s.l. | 254 m a.s.l. | |||||
| Uroczysko Kowadza | Vantage point, benches | - | 35 | 131 | 50°00.884’ | 50°00.880’ |
| N/19°46.638’; | N/19°49.648’; | |||||
| 268 m a.s.l. | 266 m a.s.l. | |||||
| UroczyskoWielkanoc | Vantage point, benches, bins, information board | - | 30 | 115 | 50°00.959’ | 50°00.938’ |
| N/19°48.850’; | N/19°48.840’; | |||||
| 264 m a.s.l. | 260 m a.s.l. | |||||
| Zakrzówek | Vantage points, climbing walls, information board | Lagoon created inthe lime quarry, bike paths | 33 | 120 | 50°02.365’ | 50°02.415’ |
| N/19°54.987’; | N/19°54.752’; | |||||
| 203 m a.s.l. | 213 m a.s.l. | |||||
| Study Sites | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Górka Pychowicka | Fort Bodzów | Bogucianka | Uroczysko Wielkanoc | Zakrzówek | Uroczysko Kowadza | Tyniec | |||
| Light intensity | Narrow | CL | 2000 | 1150 | 2000 | 2000 | 1720 | 2000 | 740 |
| (±0.0) | (±400.6) | (±0.0) | (±0.0) | (±489.4) | (±0.0) | (±508.1) | |||
| FU | 2000 | 1020 | 2000 | 2000 | 1690 | 2000 | 690 | ||
| (±0.0) | (±498.4) | (±0.0) | (±0.0) | (±499.8) | (±0.0) | (±499.8) | |||
| Wide | CL | 1820 | 1850 | 2000 | 1950 | 1460 | 2000 | 1090 | |
| (±423.73) | (±337.4) | (±0.0) | (±158.1) | (±614.9) | (±0.0) | (±502.1) | |||
| FU | 1680 | 1680 | 2000 | 1800 | 1270 | 1900 | 830 | ||
| (±474.6) | (±518.1) | (±0.0) | (±421.6) | (±551.8) | (±316.2) | (±447.3) | |||
| Soil moisture | Narrow | CL | 2.5 | 1 | 1.3 | 6.7 | 4 | 3.7 | 4.5 |
| (±0.6) | (±0.0) | (±0.3) | (±2.1) | (±1.0) | (±2.3) | (±2.2) | |||
| FU | 1.7 | 1 | 1.2 | 4.3 | 3.6 | 3.5 | 4.6 | ||
| (±0.8) | (±0.0) | (±0.3) | (±2.3) | (±1.1) | (±1.5) | (±2.2) | |||
| Wide | CL | 1 | 1 | 1.2 | 5.8 | 4.9 | 3.7 | 5.5 | |
| (±0.0) | (±0.0) | (±0.2) | (±1.9) | (±1.6) | (±1.8) | (±1.8) | |||
| FU | 1 | 1 | 1.1 | 5.4 | 3.9 | 3 | 4.4 | ||
| (±0.0) | (±0.0) | (±0.2) | (±1.8) | (±1.1) | (±0.7) | (±1.7) | |||
| Soil pH | Narrow | CL | 7.4 | 7.5 | 7.5 | 7.3 | 7.3 | 7.4 | 7.2 |
| (±0.2) | (±0.0) | (±0.0) | (±0.4) | (±0.3) | (±0.2) | (±0.3) | |||
| FU | 7.5 | 7.5 | 7.5 | 7.4 | 7.4 | 7.2 | 7.1 | ||
| (±0.2) | (±0.0) | (±0.0) | (±0.2) | (±0.2) | (±0.3) | (±0.2) | |||
| Wide | CL | 7.8 | 7.5 | 7.5 | 7.3 | 7.4 | 7.2 | 7.3 | |
| (±0.3) | (±0.2) | (±0.0) | (±0.3) | (±0.2) | (±0.3) | (±0.3) | |||
| FU | 7.7 | 7.5 | 7.5 | 7.5 | 7.4 | 7.4 | 7.4 | ||
| (±0.3) | (±0.0) | (±0.0) | (±0.2) | (±0.3) | (±0.2) | (±0.2) | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostrakiewicz-Gierałt, K.; Pliszko, A.; Gmyrek-Gołąb, K. The Effect of Visitors on the Properties of Vegetation of Calcareous Grasslands in the Context of Width and Distances from Tourist Trails. Sustainability 2020, 12, 454. https://doi.org/10.3390/su12020454
Kostrakiewicz-Gierałt K, Pliszko A, Gmyrek-Gołąb K. The Effect of Visitors on the Properties of Vegetation of Calcareous Grasslands in the Context of Width and Distances from Tourist Trails. Sustainability. 2020; 12(2):454. https://doi.org/10.3390/su12020454
Chicago/Turabian StyleKostrakiewicz-Gierałt, Kinga, Artur Pliszko, and Katarzyna Gmyrek-Gołąb. 2020. "The Effect of Visitors on the Properties of Vegetation of Calcareous Grasslands in the Context of Width and Distances from Tourist Trails" Sustainability 12, no. 2: 454. https://doi.org/10.3390/su12020454
APA StyleKostrakiewicz-Gierałt, K., Pliszko, A., & Gmyrek-Gołąb, K. (2020). The Effect of Visitors on the Properties of Vegetation of Calcareous Grasslands in the Context of Width and Distances from Tourist Trails. Sustainability, 12(2), 454. https://doi.org/10.3390/su12020454






