A Simple Home-Based Lifestyle Intervention Program to Improve Cardiac Autonomic Regulation in Patients with Increased Cardiometabolic Risk
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Assessment (Necessary Also to Exclude Any Contraindication to Exercise)
- History, evaluation of previous medical tests, standard medical examination, anthropometric and hemodynamic data
- Blood tests (fasting plasma glucose; total, HDL (high density lipoproteins) and LDL (low density lipoproteins) cholesterol; triglycerides; blood count; ALT (alanine transaminase) and AST (aspartate transaminase) levels measured at T0 in all subjects and at T1 in 73% of subjects, due to economic constraints.
- BIA bioelectrical impedance analysis (BodyStat Quadscan 4000, BodystatR_ Quadscan 4000, Body Stat Ltd., Isle of Man, British Isles) [33] was employed in order to estimate percentage of fat mass (FM) and of free fat mass (FFM) using the proprietary equation provided by the manufacturer.
2.2. Cardiac Autonomic Regulation
2.3. Lifestyle Assessment
- Physical activity (weekly physical activity volume) was assessed by a modified version of the commonly employed short version of the International Physical Activity Questionnaire [37,38], which focuses on intensity (nominally estimated in metabolic equivalents—MET—according to the type of activity) and duration (in minutes) of physical activity. We considered the following levels: brisk walking (≈3.3 METs), other activities of moderate intensity (≈4.0 METs) and activities of vigorous intensity (≈8.0 METs).
- Nutrition was assessed using the AHA Diet Score [6], taking into consideration fruit/vegetables, fish, sweetened beverages, whole grain and sodium consumption (the assessment of the latter was adapted to Italian eating habits using a single question inquiring as to the habits of eating sodium-rich foods or adding sodium to food [39,40].
- Perceptions of stress, fatigue, control and somatic symptoms (4SQ) were assessed using a self-administered questionnaire [12,36] providing nominal self-rated Likert scales from 0 (“no perception”) to 10 (“highest perception”) for each measure. The 4SQ considers 18 somatic symptoms; thus, the total score ranged from 0 to 180.
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Cardiac Autonomic Regulation
4.2. Lifestyle Modification Program (LMP)
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- American College of Lifestyle Medicine. The American College of Lifestyle Medicine Standards. 2015. Available online: www lifestylemedicineorg/standards (accessed on 16 September 2020).
- Willett, W.C. Balancing life-style and genomics research for disease prevention. Science 2002, 296, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.F.; Villani, G.Q. Lifestyle modification in secondary prevention. Eur. J. Prev. Cardiol. 2017, 24 (Suppl. 3), 101–107. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [PubMed]
- Janssen, V.; De Gucht, V.; Dusseldorp, E.; Maes, S. Lifestyle modification programmes for patients with coronary heart disease: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Prev. Cardiol. 2012, 20, 620–640. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and setting national goals for cardiovascular health promotion and disease reduction. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef]
- Spring, B.; Ockene, J.K.; Gidding, S.S.; Mozaffarian, D.; Moore, S.; Rosal, M.C.; Brown, M.D.; Vafiadis, D.K.; Cohen, D.L.; Burke, L.E.; et al. Better population health through behavior change in adults: A call to action. Circulation 2013, 128, 2169–2176. [Google Scholar] [CrossRef]
- Stead, M.; Craigie, A.M.; Macleod, M.; McKell, J.; Caswell, S.; Steele, R.J.C.; Anderson, A.S. Why are some people more successful at lifestyle change than others? Factors associated with successful weight loss in the BeWEL randomised controlled trial of adults at risk of colorectal cancer. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 87. [Google Scholar] [CrossRef]
- Murray, J.; Craigs, C.; Hill, K.; Honey, S.; House, A. A systematic review of patient reported factors associated with uptake and completion of cardiovascular lifestyle behaviour change. BMC Cardiovasc. Disord. 2012, 12, 120. [Google Scholar] [CrossRef]
- Milani, R.V.; Lavie, C.J. Health Care 2020: Reengineering Health Care Delivery to Combat Chronic Disease. Am. J. Med. 2015, 128, 337–343. [Google Scholar] [CrossRef]
- Murphy, A.W.; Cupples, M.E.; Smith, S.M.; Byrne, M.; Newell, J. Effect of tailored practice and patient care plans on secondary prevention of heart disease in general practice: Cluster randomised controlled trial. BMJ 2009, 339, b4220. [Google Scholar] [CrossRef]
- Lucini, D.; Riva, S.; Pizzinelli, P.; Pagani, M. Stress management at the worksite: Reversal of symptoms profile and cardiovascular dysregulation. Hypertension 2007, 49, 291–297. [Google Scholar] [CrossRef]
- Rao, G.; Burke, L.E.; Spring, B.; Ewing, L.J.; Turk, M.; Lichtenstein, A.H.; Cornier, M.-A.; Spence, J.D.; Coons, M. New and emerging weight management strategies for busy ambulatory settings: A scientific statement from the American Heart Association endorsed by the Society of Behavioral Medicine. Circulation 2011, 124, 1182–1203. [Google Scholar] [CrossRef] [PubMed]
- Graham, I.M.; Stewart, M.; Hertog, M.l.G. Cardiovascular Round Table Task Force. Factors impeding the implementation of cardiovascular prevention guidelines: Findings from a survey conducted by the European Society of Cardiology. Eur. J. Cardiovasc. Prev. Rehabilit. 2006, 13, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, M.W.; Schneider, E.C.; Rosenthal, M.B.; Volpp, K.G.; Werner, R.M. Association between participation in a multipayer medical home intervention and changes in quality, utilization, and costs of care. JAMA 2014, 311, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Engel, B.T. Psychosomatic medicine, behavioral medicine, just plain medicine. Psychosom. Med. 1986, 48, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Ahn, A.C.; Tewari, M.; Poon, C.S.; Phillips, R.S. The clinical applications of a systems approach. PLoS Med. 2006, 3, e209. [Google Scholar] [CrossRef]
- Joyner, M.J.; Green, D.J. Exercise protects the cardiovascular system: Effects beyond traditional risk factors. J. Physiol. 2009, 587, 5551–5558. [Google Scholar] [CrossRef] [PubMed]
- La Rovere, M.T.; Bigger, J.T., Jr.; Marcus, F.I.; Mortara, A.; Schwartz, P.J. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes after Myocardial Infarction) Investigators. Lancet 1998, 351, 478–484. [Google Scholar] [CrossRef]
- Lucini, D.; Mela, G.S.; Malliani, A.; Pagani, M. Impairment in cardiac autonomic regulation preceding arterial hypertension in humans. Circulation 2002, 106, 2673–2679. [Google Scholar] [CrossRef]
- Ondicova, K.; Mravec, B. Role of nervous system in cancer aetiopathogenesis. Lancet Oncol. 2010, 11, 596–601. [Google Scholar] [CrossRef]
- Lucini, D.; Zuccotti, G.V.; Scaramuzza, A.; Malacarne, M.; Gervasi, F.; Pagani, M. Exercise might improve cardiovascular autonomic regulation in adolescents with type 1 diabetes. Acta Diabetol. 2013, 50, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Grassı, G.; Seravalle, G.; Colombo, M.; Bolla, G.; Cattaneo, B.M.; Cavagnini, F.; Mancia, G. Body weight reduction, sympathetic nerve traffic, and arterial baroreflex in obese normotensive humans. Circulation 1998, 97, 2037–2042. [Google Scholar] [CrossRef] [PubMed]
- Soares-Miranda, L.; Sattelmair, J.; Chaves, P.; Duncan, G.E.; Siscovick, D.S.; Stein, P.K.; Mozaffarian, D. Physical activity and heart rate variability in older adults: The Cardiovascular Health Study. Circulation 2014, 129, 2100–2110. [Google Scholar] [CrossRef] [PubMed]
- Lucini, D.; Milani, R.V.; Costantino, G.; Lavie, C.J.; Porta, A.; Pagani, M. Effects of cardiac rehabilitation and exercise training on autonomic regulation in patients with coronary artery disease. Am. Hear. J. 2002, 143, 977–983. [Google Scholar] [CrossRef]
- Lucini, D.; Malacarne, M.; Oggionni, G.; Gatzmeier, W.; Santoro, A.; Pagani, M. Endocrine Adjuvant Therapy might Impair Cardiac Autonomic Regulation in Breast Cancer Survivors. Cardiol. Cardiovasc. Med. 2019, 3, 034–049. [Google Scholar] [CrossRef][Green Version]
- Sala, R.; Malacarne, M.; Solaro, N.; Pagani, M.; Lucini, D. A composite autonomic index as unitary metric for heart rate variability: A proof of concept. Eur. J. Clin. Investig. 2017, 47, 241–249. [Google Scholar] [CrossRef]
- Rollnick, S.; Butler, C.C.; Kinnersley, P.; Gregory, J.; Mash, B. Motivational interviewing. BMJ 2010, 340, c1900. [Google Scholar] [CrossRef] [PubMed]
- Stefani, L.; Klika, R.; Mascherini, G.; Mazzoni, F.; Lunghi, A.; Petri, C.; Petreni, P.; Di Costanzo, F.; Maffulli, N.; Galanti, G. Effects of a home-based exercise rehabilitation program for cancer survivors. J. Sports Med. Phys. Fit. 2019, 59, 846–852. [Google Scholar] [CrossRef]
- American College of Sports Medicine; Riebe, D.; Ehrman, J.K.; Liguori, G.; Magal, M. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Lippincot Williams & Wilkins: Philadelphia, PA, USA, 2018. [Google Scholar]
- Mozaffarian, D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef]
- Lucini, D.; Vigo, C.; Malacarne, M.; Gatzemeier, W.; Pagani, M. Lifestyle changes as internal medicine. Eur. J. Intern. Med. 2017, 43, e40–e42. [Google Scholar] [CrossRef]
- Benton, M.J.; Swan, P.D.; Schlairet, M.C.; Sanderson, S. Comparison of body composition measurement with whole body multifrequency bioelectrical impedance and air displacement plethysmography in healthy middle-aged women. Health Care Women Int. 2011, 32, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Lucini, D.; Solaro, N.; Pagani, M. Autonomic differentiation map: A novel statistical tool for interpretation of heart rate variability. Front. Physiol. 2018, 9, 401. [Google Scholar] [CrossRef] [PubMed]
- Oggionni, G.; Spataro, A.; Pelliccia, A.; Malacarne, M.; Pagani, M.; Lucini, D. Left Ventricular Hypertrophy in World Class Elite Athletes Is Associated with Signs of Improved Cardiac Autonomic Regulation. Available online: https://journals.sagepub.com/doi/10.1177/2047487319830534 (accessed on 12 February 2019).
- Lucini, D.; Solaro, N.; Lesma, A.; Gillet, V.B.; Pagani, M.; Dickerson, J.; Ivannikov, M. Health promotion in the workplace: Assessing stress and lifestyle with an intranet tool. J. Med. Internet Res. 2011, 13, e88. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Minetto, M.A.; Motta, G.; Gorji, N.E.; Lucini, D.; Biolo, G.; Pigozzi, F.; Portincasa, P.; Maffiuletti, N.A. Reproducibility and validity of the Italian version of the International Physical Activity Questionnaire in obese and diabetic patients. J. Endocrinol. Investig. 2017, 41, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Lucini, D.; Zanuso, S.; Solaro, N.; Vigo, C.; Malacarne, M.; Pagani, M. Reducing the risk of metabolic syndrome at the worksite: Preliminary experience with an ecological approach. Acta Diabetol. 2015, 53, 63–71. [Google Scholar] [CrossRef]
- Lucini, D.; Zanuso, S.; Blair, S.; Pagani, M. A simple healthy lifestyle index as a proxy of wellness: A proof of concept. Acta Diabetol. 2014, 52, 81–89. [Google Scholar] [CrossRef]
- Lean, M.E.; Leslie, W.S.; Barnes, A.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. Lancet 2018, 391, 541–551. [Google Scholar] [CrossRef]
- Solaro, N.; Malacarne, M.; Pagani, M.; Lucini, D. Cardiac baroreflex, HRV, and statistics: An interdisciplinary approach in hypertension. Front. Physiol. 2019, 10, 478. [Google Scholar] [CrossRef]
- Chen, L.M.; Farwell, W.R.; Jha, A.K. Primary care visit duration and quality. Arch. Intern. Med. 2009, 169, 1866–1872. [Google Scholar] [CrossRef]
- Lianov, L.; Johnson, M. Physician competencies for prescribing lifestyle medicine. JAMA 2010, 304, 202. [Google Scholar] [CrossRef] [PubMed]
- Hivert, M.; Arena, R.; Forman, D.E.; Kris-Etherton, P.M.; McBride, P.E.; Pate, R.R.; Spring, B.; Trilk, J.; Van Horn, L.; Kraus, W.E. Medical training to achieve competency in lifestyle counseling: An essential foundation for prevention and treatment of cardiovascular diseases and other chronic medical conditions: A scientific statement from the American Heart Association. Circulation 2016, 134, e308–e327. [Google Scholar] [CrossRef] [PubMed]
- Trilk, J.L.; Nelson, L.; Briggs, A.; Muscato, D. Including Lifestyle Medicine in Medical Education: Rationale for American College of Preventive Medicine/American Medical Association Resolution 959. Am. J. Prev. Med. 2019, 56, e169–e175. [Google Scholar] [CrossRef] [PubMed]
- Gates, A.B.; Kerry, R.; Moffatt, F.; Ritchie, I.K.; Meakins, A.; Thornton, J.S.; Rosenbaum, S.; Taylor, A. Movement for movement: Exercise as everybody’s business? Br. J. Sports Med. 2017, 51, 767–768. [Google Scholar] [CrossRef]
| 1st Visit Actions | Allotted Time | Goals |
|---|---|---|
| Welcome | 5 min | ▪ Understand patient’s concerns and circumstances ▪ Introduce the program and define patient’s and physician’s roles and responsibilities ▪ Establish an empathic, maieutic relationship |
| Clinical history and clinical assessment | 10 min | ▪ Definition of patient’s health status ▪ Take note of pharmacological plan and discuss potential side effects ▪ Discussion of lifestyle assessment (ad hoc questionnaire was filled in before encounter with physician) ▪ Collection of anthropometric data ▪ If required, prescription of further clinical tests |
| Explanation of diagnosis and of specific benefits derived from lifestyle change | 5 min | ▪ Make the patient aware about her clinical condition ▪ Clear up facts from patient’s interpretation of the personal implications ▪ Support the patient in learning how lifestyle change may ameliorate her health and reduce cardiometabolic/oncologic risk, using her history ▪ Allow the patient to elicit desire, motivation to change ▪ Give the opportunity to the patient to raise any questions in order to have tailored answers |
| Setting of specific individual goals | 5 min | ▪ Allow the patient to express her own reasons for change ▪ Assure the patient that she will have all the required support ▪ Prioritize behavior/issues for change (patient–physician alliance) ▪ Define long-term goal/s and steps (short-term goals) to reach it/them |
| Education about nutrition and definition of tailored nutritional plan | 10 min | ▪ Provide information about food choice ▪ Support the patient in discovering how to improve nutrition based on her/his history, clinical status and lifestyle assessment ▪ Create a personalized nutrition program that the patient considers realistic, tailoring general clinical guidelines to personalized actions ▪ Elicit in the patient the desire to adhere to the tailored program, taking into account medical guidelines, personal clinical needs and patient’s preferences |
| Education about physical activity and tailored exercise prescription | 10 min | ▪ Provide information about exercise ▪ Be sure that the patient understands the importance of reducing sedentary behaviors and of increasing structured exercise ▪ Help the patient to discover and define practical strategies to reduce sedentariness during her normal daily activities ▪ Ensure that the patient realizes the importance of exercise and may be capable of appreciating any single improvement ▪ Elicit in the patient the desire to adhere to the tailored exercise prescription, taking into account medical guidelines, personal clinical needs and patient’s preferences |
| Follow-Up Visit Actions | Allotted Time | Goals |
| Welcome | 1 min | ▪ Reinforce the empathic, maieutic relationship |
| Clinical history (from previous encounter to present) and clinical assessment | 9 min | ▪ Assess changes in clinical status and lifestyle from previous visit to present ▪ Collection of present anthropometric data ▪ Discussion of tests results prescribed during or after first visit |
| Analysis of results and of encountered barriers | 5 min | ▪ Provide feedback on progression towards set goals ▪ Empower the patient and reflect on her central role in determining her health status and the success of the lifestyle change program ▪ Reinforce the collaborative relationship with the physician and the possibility to get all the required support ▪ Make the patient aware of the (even small) result/s obtained and how it/them has/have improved her quality of life |
| Problem solving | 10 min | ▪ Help the patient to find solution to overcome the encountered problems ▪ Help the patient to discover the required resources and to select those which best account for her needs ▪ Raise patient awareness of consequence of her/his behavior on her/his quality of life and wellbeing ▪ Make the patient aware that to encounter barriers and to find the resources to overcome them is a critical, always present, part of a lifestyle change program |
| Setting of further specific individual goals | 5 min | ▪ Assist the patient to define further steps towards the long-term goal ▪ Make the patient aware that she is regaining control over her/his life and wellbeing |
| Variables | Before | After | t-Test |
|---|---|---|---|
| Age (years) | 52.9 ± 8.1 | ||
| Gender (f/m) | 23/3 | ||
| Smoke (no/yes) | 19/7 | 19/7 | |
| Height (cm) | 162.2 ± 8.5 | ||
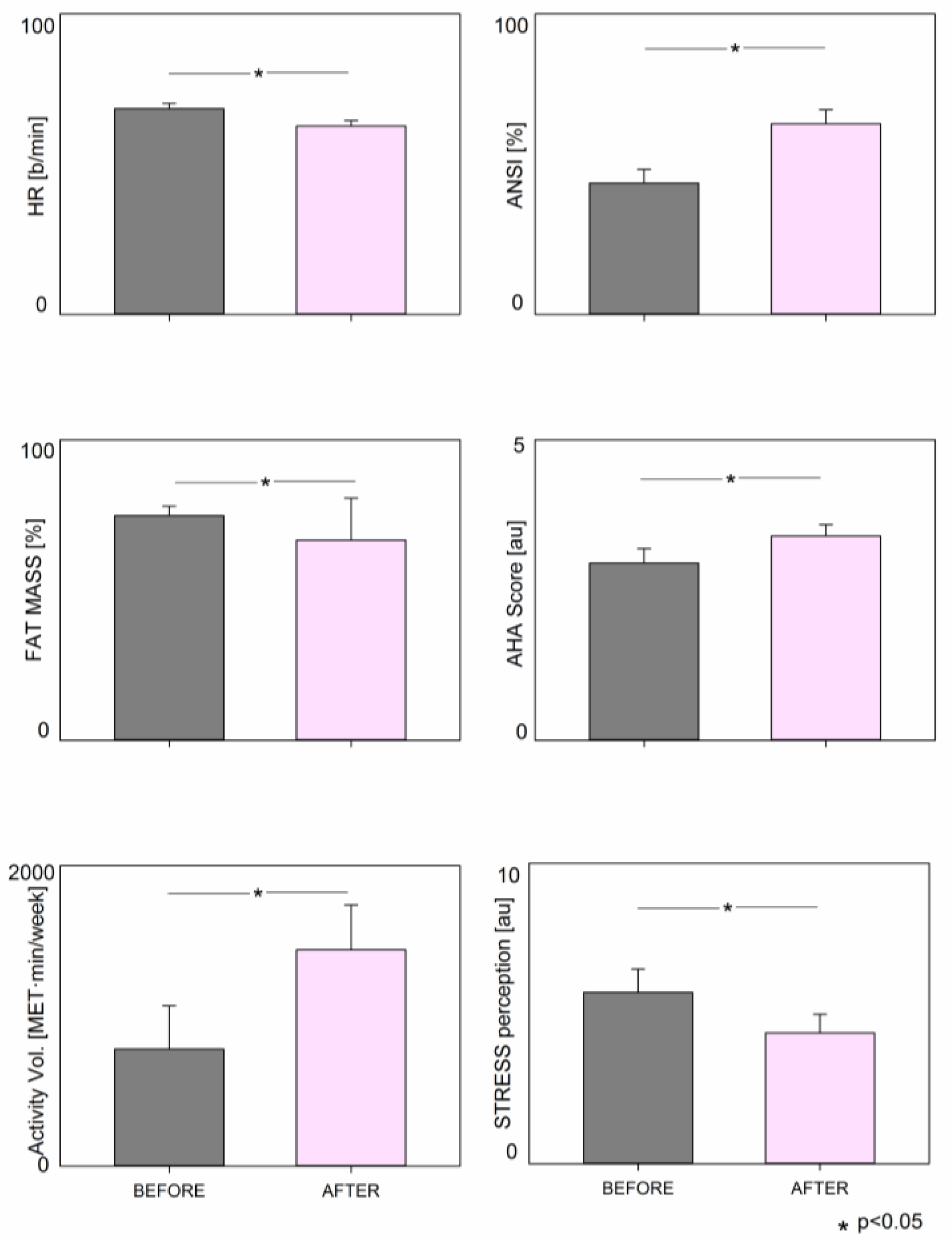
| Weight (kg) | 74.9 ± 18.6 | 65.9 ± 15.8 | <0.001 |
| BMI (kg/m2) | 28.1 ± 5.7 | 24.8 ± 4.8 | <0.001 |
| Waist circumference (cm) | 96.1 ± 14.8 | 89.9 ± 15.1 | <0.001 |
| Fat Mass (%) | 37.4 ± 7.5 | 33.3 ± 7.0 | <0.001 |
| SAP (mmHg) | 114.7 ± 17.4 | 111.5 ± 14.5 | 0.198 |
| DAP (mmHg) | 75.1 ± 9.5 | 69.8 ± 10.2 | 0.001 |
| HR (b/min) | 68.5 ± 8.9 | 62.7 ± 9.1 | 0.001 |
| DP ((b/min) × mmHg) | 7861.7 ± 1593.0 | 7016.4 ± 1429.0 | 0.001 |
| AHA Diet Score (a.u.) | 2.95 ± 1.09 | 3.40 ± 0.88 | 0.045 |
| ActivityVolume (MET·min/week) | 778.5 ± 1290.7 | 1439.8 ± 1335.0 | <0.001 |
| SEDENTARINESS (h/week) | 38.2 ± 21.8 | 32.4 ± 22.7 | 0.250 |
| STRESS perception (au) | 5.70 ± 3.45 | 4.35 ± 2.77 | 0.034 |
| FATIGUE perception (au) | 6.45 ± 2.37 | 4.90 ± 3.25 | 0.103 |
| 4SQ Score (au) | 58.0 ± 29.5 | 44.8 ± 26.4 | 0.005 |
| CONTROL perception (au) | 5.55 ± 2.60 | 7.35 ± 2.08 | 0.075 |
| Fasting glucose (mg/dL) | 94.1 ± 9.4 | 92.5 ± 13.5 | 0.642 |
| Total cholesterol (mg/dL) | 234. ± 29.1 | 203.9 ± 29.1 | 0.036 |
| HDL cholesterol (mg/dL) | 58.2 ± 12.1 | 66.1 ± 16.8 | 0.035 |
| LDL cholesterol (mg/dL) | 133.4 ± 29.0 | 104.7 ± 33.7 | 0.256 |
| Triglycerides (mg/dL) | 104.6 ± 33.8 | 90.1 ± 26.8 | 0.169 |
| Variable | Before | After | p | |
|---|---|---|---|---|
| RR | (msec) | 891.6 ± 125.2 | 977.4 ± 156.1 | 0.001 |
| RR VAR | (msec2) | 1227.8 ± 1053.1 | 1727.8 ± 1215.6 | 0.008 |
| RR LFa | (msec2) | 323.3 ± 343.7 | 559.8 ± 880.9 | 0.118 |
| RR HFa | (msec2) | 312.9 ± 382.9 | 445.9 ± 379.1 | 0.007 |
| RR LFnu | (nu) | 47.3 ± 22.8 | 45.4 ± 24.9 | 0.748 |
| RR HFnu | (nu) | 45.7 ± 21.9 | 48.6 ± 24.3 | 0.621 |
| RR LF/HF | (.) | 1.84 ± 2.26 | 2.25 ± 3.39 | 0.959 |
| RRRo | (au) | 0.30 ± 0.09 | 0.29 ± 0.09 | 0.592 |
| RR p_0v | (%) | 23.0 ± 13.7 | 21.4 ± 13.4 | 0.623 |
| RR p_1v | (%) | 48.7 ± 6.9 | 48.9 ± 10.1 | 0.921 |
| RR p_2l | (%) | 10.9 ± 7.1 | 11.1 ± 6.2 | 0.948 |
| RR p_2u | (%) | 16.9 ± 8.9 | 18.4 ± 9.8 | 0.218 |
| A.XXAR | (msec/mmHg) | 2.72 ± 23.0 | 2.58 ± 2.46 | 0.852 |
| SAP mean | (mmHg) | 117.6 ± 18.2 | 114.5 ± 21.0 | 0.439 |
| SAP LFa | (mmHg2) | 3.5 ± 4.2 | 4.2 ± 7.1 | 0.675 |
| α Index | (msec/mmHg) | 14.6 ± 8.7 | 16.2 ± 8.9 | 0.482 |
| ANSI | (%) | 43.7 ± 23.0 | 63.5 ± 22.9 | 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucini, D.; Malacarne, M.; Gatzemeier, W.; Pagani, M. A Simple Home-Based Lifestyle Intervention Program to Improve Cardiac Autonomic Regulation in Patients with Increased Cardiometabolic Risk. Sustainability 2020, 12, 7671. https://doi.org/10.3390/su12187671
Lucini D, Malacarne M, Gatzemeier W, Pagani M. A Simple Home-Based Lifestyle Intervention Program to Improve Cardiac Autonomic Regulation in Patients with Increased Cardiometabolic Risk. Sustainability. 2020; 12(18):7671. https://doi.org/10.3390/su12187671
Chicago/Turabian StyleLucini, Daniela, Mara Malacarne, Wolfgang Gatzemeier, and Massimo Pagani. 2020. "A Simple Home-Based Lifestyle Intervention Program to Improve Cardiac Autonomic Regulation in Patients with Increased Cardiometabolic Risk" Sustainability 12, no. 18: 7671. https://doi.org/10.3390/su12187671
APA StyleLucini, D., Malacarne, M., Gatzemeier, W., & Pagani, M. (2020). A Simple Home-Based Lifestyle Intervention Program to Improve Cardiac Autonomic Regulation in Patients with Increased Cardiometabolic Risk. Sustainability, 12(18), 7671. https://doi.org/10.3390/su12187671




