The Use of the Sous-Vide Method in the Preparation of Poultry at Home and in Catering—Protection of Nutrition Value Whether High Energy Consumption
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
- –
- SV64 (64 °C, 60 min)—parameter according to sous-vide equipment manufacturer’s guideline.
- –
- –
2.2. Technological Parameters
2.3. Proximate Composition and Fatty Acid Profile
2.4. Microbiological Analyses
2.5. Statistical Analysis
3. Results
3.1. Energy Consumption
3.2. The Yield of the Various Heat Treatment Methods of the Chicken Breast
3.3. Changes in the pH Value of the Chicken Breast Processed with Various Heat Treatment Methods
3.4. The Proximate Composition of the Chicken Breast after Heat Treatment
3.5. Profile of Fatty Acid Content in the Chicken Breast After the Heat Treatment Method
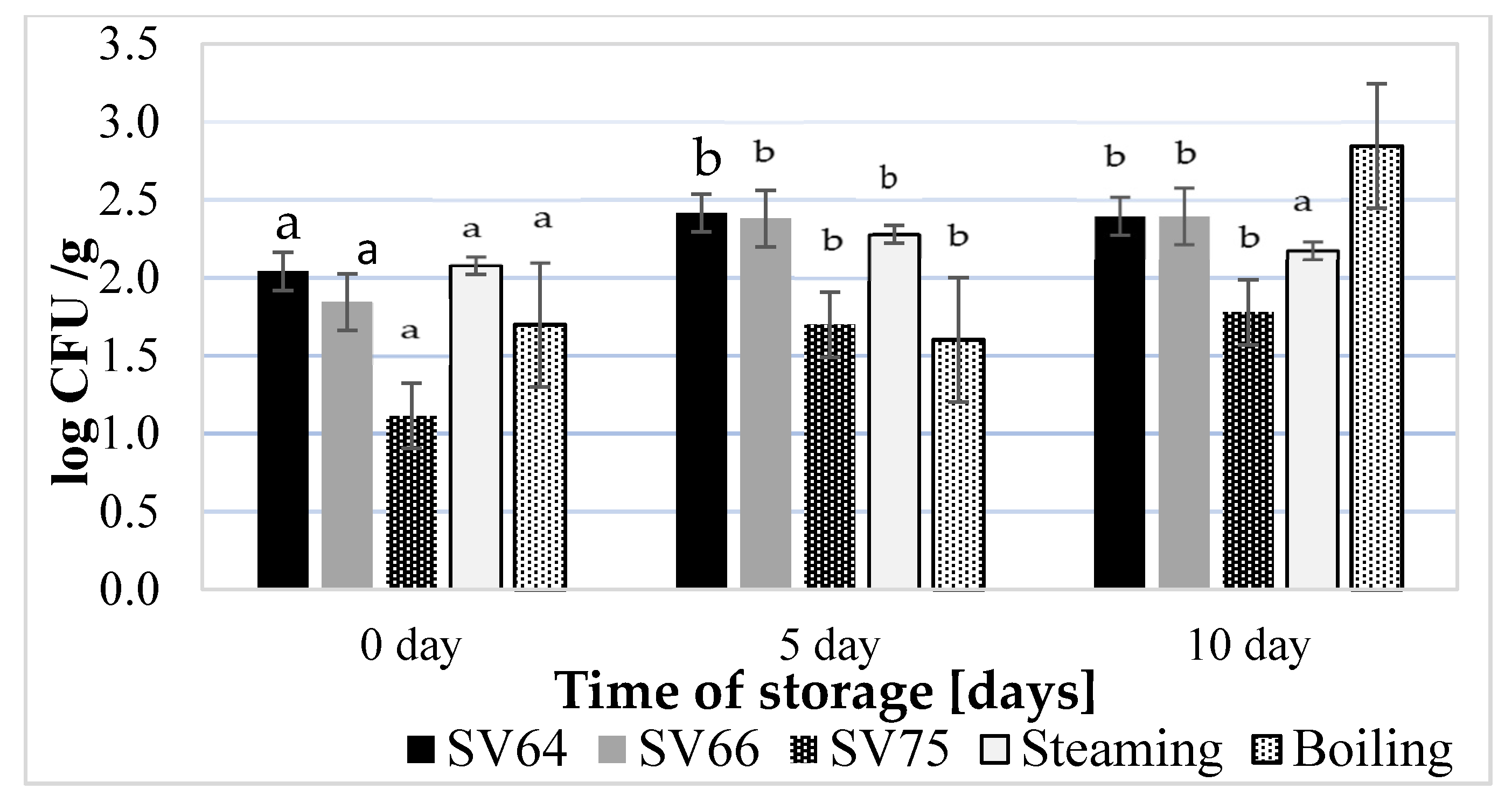
3.6. Effect of Heat Treatment on the Microbiological Quality of the Chicken Breast
4. Discussion
4.1. Technological Quality of the Chicken Breast Cooking
4.2. Nutritional Value and Fatty Acid Profile of the Chicken Breast
4.3. Microbiological Quality of Chicken Breasts
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- FAO. Food Wastage Footprint: Impacts on Natural Resources; FAO: Rome, Italy, 2013. [Google Scholar]
- Frequently Asked Questions (FAQs): Reducing Food Waste in the EU. European Commission Q & A: Brussels, 15 June 2020. MEMO/19. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/fw_lib_reduce-food-waste-eu_faqs.pdf (accessed on 31 August 2020).
- Parfitt, J.; Barthel, M.; Macnaughton, S. Food waste within food supply chains: Quantification and potential for change to 2050. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 3065–3081. [Google Scholar] [CrossRef]
- Papargyropoulou, E.; Steinberger, J.K.; Wright, N.; Lozano, R.; Padfield, R.; Ujang, Z. Patterns and Causes of Food Waste in the Hospitality and Food Service Sector: Food Waste Prevention Insights from Malaysia. Sustainability 2019, 11, 6016. [Google Scholar] [CrossRef]
- Bilska, B.; Tomaszewska, M.; Kołozyn-Krajewska, D. Analysis of the behaviors of polish consumers in relation to food waste. Sustainability 2020, 12, 304. [Google Scholar] [CrossRef]
- Zielińska, D.; Bilska, B.; Marciniak-Łukasiak, K.; Łepecka, A.; Trząskowska, M.; Neffe-Skocińska, K.; Tomaszewska, M.; Szydłowska, A.; Kołozyn-Krajewska, D. Consumer understanding of the date of minimum durability of food in association with quality evaluation of food products after expiration. Int. J. Environ. Res. Public Health 2020, 17, 1632. [Google Scholar] [CrossRef]
- Sałek, P.; Przybylski, W.; Jaworska, D.; Adamczak, L.; Zielińska, D.; Głuchowski, A. The effects on the quality of poultry meat of supplementing feed with zinc-methionine complex. Acta Sci. Pol. Technol. Aliment. 2020, 19, 73–82. [Google Scholar] [CrossRef]
- Lafarga, T.; Viñas, I.; Bobo, G.; Simó, J.; Aguiló-Aguayo, I. Effect of steaming and sous vide processing on the total phenolic content, vitamin C and antioxidant potential of the genus Brassica. Innov. Food Sci. Emerg. Technol. 2018, 47, 412–420. [Google Scholar] [CrossRef]
- Beretta, C.; Hellweg, S. Potential environmental benefits from food waste prevention in the food service sector. Resour. Conserv. Recycl. 2019, 147, 169–178. [Google Scholar] [CrossRef]
- Rinaldi, M.; Dall’Asta, C.; Paciulli, M.; Cirlini, M.; Manzi, C.; Chiavaro, E. A novel time/temperature approach to sous vide cooking of beef muscle. Food Bioprocess. Technol. 2014, 7, 2969–2977. [Google Scholar] [CrossRef]
- Ramane, K.; Galoburda, R.; Kreicbergs, V.; Vanaga, I. Amino acid profile of Sous vide cooked poultry breast meat products. In Proceedings of the 11th International Congress on Engineering and Food (ICEF11), Athens, Greece, 22–26 May 2011; Volume 3, pp. 2199–2200. [Google Scholar]
- Rotola-Pukkila, M.K.; Pihlajaviita, S.T.; Kaimainen, M.T.; Hopia, A.I. Concentration of umami compounds in pork meat and cooking juice with different cooking times and temperatures. J. Food Sci. 2015, 80, C2711–C2716. [Google Scholar] [CrossRef] [PubMed]
- Oz, F.; Zikirov, E. The effects of sous-vide cooking method on the formation of heterocyclic aromatic amines in beef chops. LWT Food Sci. Technol. 2015, 64, 120–125. [Google Scholar] [CrossRef]
- Oz, F.; Seyyar, E. Formation of Heterocyclic Aromatic Amines and Migration Level of Bisphenol-A in Sous-Vide-Cooked Trout Fillets at Different Cooking Temperatures and Cooking Levels. J. Agric. Food Chem. 2016, 64, 3070–3082. [Google Scholar] [CrossRef] [PubMed]
- Rasińska, E.; Rutkowska, J.; Czarniecka-Skubina, E.; Tambor, K. Effect of cooking methods on changes in fatty acids contents, lipid oxidation and volatile compounds of rabbit meat. LWT Food Sci. Technol. 2019, 110, 64–70. [Google Scholar] [CrossRef]
- Falowo, A.B.; Muchenje, V.; Hugo, A. Effect of sous-vide technique on fatty acid and mineral compositions of beef and liver from Bonsmara and non-descript cattle. Ann. Anim. Sci. 2017, 17, 565–580. [Google Scholar] [CrossRef]
- Silva, F.L.; de Lima, J.P.; Melo, L.S.; da Silva, Y.S.; Gouveia, S.T.; Lopes, G.S.; Matos, W.O. Comparison between boiling and vacuum cooking (sous-vide) in the bioaccessibility of minerals in bovine liver samples. Food Res. Int. 2017, 100, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Lassen, A.; Kall, M.; Hansen, K.; Ovesen, L. A comparison of the retention of vitamins B1, B2 and B6, and cooking yield in pork loin with conventional and enhanced meal-service systems. Eur. Food Res. Technol. 2002, 215, 194–199. [Google Scholar] [CrossRef]
- Barnett, M.P.; Chiang, V.S.; Milan, A.M.; Pundir, S.; Walmsley, T.A.; Grant, S.; Markworth, J.F.; Quek, S.-Y.; George, P.M.; Cameron-Smith, D. Plasma elemental responses to red meat ingestion in healthy young males and the effect of cooking method. Eur. J. Nutr. 2018, 1–8. [Google Scholar] [CrossRef]
- Díaz, P.; Nieto, G.; Garrido, M.D.; Bañón, S. Microbial, physical–chemical and sensory spoilage during the refrigerated storage of cooked pork loin processed by the sous vide method. Meat Sci. 2008, 80, 287–292. [Google Scholar] [CrossRef]
- Roldán, M.; Antequera, T.; Martín, A.; Mayoral, A.I.; Ruiz, J. Effect of different temperature–time combinations on physicochemical, microbiological, textural and structural features of sous-vide cooked lamb loins. Meat Sci. 2013, 9, 572–578. [Google Scholar] [CrossRef]
- Hong, G.E.; Kim, J.H.; Ahn, S.J.; Lee, C.H. Changes in Meat Quality Characteristics of the Sous-vide Cooked Chicken Breast during Refrigerated Storage. Korean J. Food Sci. Anim. Resour. 2015, 35, 757–764. [Google Scholar] [CrossRef]
- Soletska, A.; Krasota, A. Prospects of applying vacuum technology in the manufacture of culinary poultry meat products. Food Environ. Saf. J. 2016, 15, 3–9. [Google Scholar]
- EC. EU Agricultural Outlook for Markets and Income, 2019–2030. European Commission, DG Agriculture and Rural Development: Brussels; p. 46. Available online: https://ec.europa.eu/info/sites/info/files/food-farming-fisheries/farming/documents/agricultural-outlook-2019-report_en.pdf (accessed on 31 August 2020).
- Babic, J.; Milićević, D.; Vranic, D.; Lukic, M.; Petrovic, Z. The effect of season of transportation on the welfare of broilers and selected parameters of broiler meat quality. Tehnologija Mesa 2014, 55, 46–53. [Google Scholar] [CrossRef]
- Marangoni, F.; Corsello, G.; Cricelli, C.; Ferrara, N.; Ghiselli, A.; Lucchin, L.; Poli, A. Role of poultry meat in a balanced diet aimed at maintaining health and wellbeing: An Italian consensus document. Food Nutr. Res. 2015, 59, 27606. [Google Scholar] [CrossRef] [PubMed]
- Donma, M.M.; Donma, O. Beneficial effects of poultry meat consumption on cardiovascular health and the prevention of childhood obesity. Med. ONE 2017, 2, e170018. [Google Scholar] [CrossRef]
- Hammerschlag, K.; Venkat, K. Meat-eater’s Guide to Climate Change and Health: Lifecycle Assessments—Methodology and Results; Environmental Working Group: Washington, DC, USA, 2011. [Google Scholar]
- Röös, E.; Sundberg, C.; Tidåker, P.; Strid, I.; Hansson, P.A. Can carbon footprint serve as an indicator of the environmental impact of meat production? Ecol. Indic. 2013, 24, 573–581. [Google Scholar] [CrossRef]
- Arrieta, E.M.; González, A.D. Energy and carbon footprints of food: Investigating the effect of cooking. Sust. Prod. Consum. 2019, 19, 44–52. [Google Scholar] [CrossRef]
- Pathare, P.B.; Roskilly, A.P. Quality and energy evaluation in meat cooking. Food Eng. Rev. 2016, 8, 435–447. [Google Scholar] [CrossRef]
- Baldwin, D.E. Sous vide cooking: A review. Int. J. Gastron. Food Sci. 2012, 1, 15–30. [Google Scholar] [CrossRef]
- USDA. Safe Minimum Internal Temperature Chart. 2012. Available online: https://www.fsis.usda.gov/wps/wcm/connect/625d9435-4f14-46fe-b207-5d6688cb4db5/Safe_Miminum_Internal_Temperature_Chart.pdf?MOD=AJPERES (accessed on 10 December 2017).
- Baldwin, D.E. A Practical Guide to Sous Vide Cooking. 2008. Available online: https://www.hotex.hu/data/upload/file/SousVidePracticalGuide%C2%B0.pdf (accessed on 27 January 2018).
- PN-ISO 1442:2000. Polish Standard. Meat and Meat Products. Determination of Moisture Content (Reference Method); Polish Committee for Standardization: Warszawa, Poland, 2000. [Google Scholar]
- PN-ISO 1444:2000. Meat and Meat Products. Determination of Free Fat Content; Polish Committee for Standardization: Warsaw, Poland, 2000. [Google Scholar]
- Rasińska, E.; Czarniecka-Skubina, E.; Rutkowska, J. Fatty acid and lipid contents differentiation in cuts of rabbit meat. CyTA J. Food 2018, 16, 807–813. [Google Scholar] [CrossRef]
- PN-EN ISO 4833-1:2013-12. Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms; Polish Committee for Standardization: Warsaw, Poland, 2013. [Google Scholar]
- PN-EN ISO 21527-1:2009. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds; Polish Committee for Standardization: Warsaw, Poland, 2009. [Google Scholar]
- PN-EN ISO 6888-2:2001 + A1:2004. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus aureus and Other Species); Polish Committee for Standardization: Warsaw, Poland, 2004. [Google Scholar]
- PN-EN ISO 16649-2:2004. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia coli; Polish Committee for Standardization: Warsaw, Poland, 2004. [Google Scholar]
- PN-EN ISO 21528-2:2017-08. Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae; Polish Committee for Standardization: Warsaw, Poland, 2017. [Google Scholar]
- PN-EN ISO 11290-2:2000 + A1:2005 + Ap1:2006 + Ap2:2007. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Detection and Enumeration of Listeria Monocytogenes; Polish Committee for Standardization: Warsaw, Poland, 2005. [Google Scholar]
- PN-EN ISO 6579-1:2017-04. Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella; Polish Committee for Standardization: Warsaw, Poland, 2017. [Google Scholar]
- Centre for food Safety. Microbiological Guidelines for Food. For. Ready-to-Eat Food in General and Specific Food Items; Centre for Food Safety: Hong Kong, China, 2014; pp. 7–8. Available online: https://www.cfs.gov.hk/english/food_leg/files/food_leg_Microbiological_Guidelines_for_Food_e.pdf (accessed on 18 May 2020).
- Commission Regulation (EC) No 1441/2007 of 5 December 2007 amending Regulation (EC) No 2073/2005 on microbiological criteria for foodstuffs (L 322 z 7.12.2007). Off. J. EU 2007, 12–29. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:322:0012:0029:EN:PDF (accessed on 1 September 2020).
- Marzano, M.A.; Balzaretti, C.M. Cook-serve method in mass catering establishments: Is it still appropriate to ensure a high level of microbiological quality and safety? Food Control 2011, 22, 1844–1850. [Google Scholar] [CrossRef]
- Wattanachant, S.; Benjakul, S.; Ledward, D.A. Effect of heat treatment on changes in texture. structure and properties of Thai indigenous chicken muscle. Food Chem. 2005, 93, 337–348. [Google Scholar] [CrossRef]
- Chumngoen, W.; Chen, C.F.; Chen, H.Y.; Tan, F.J. Influences of end-point heating temperature on the quality attributes of chicken meat. Br. Poult. Sci. 2016, 57, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Nithyalakshmi, V.; Preetha, R. Effect of cooking conditions on physico-chemical and textural properties of Emu (Dromaius novaehollandiae) meat. Int. Food Res. J. 2015, 22, 1924–1930. [Google Scholar]
- Bıyıklı, M.; Akoğlu, A.; Kurhan, Ş.; Akoğlu, İ.T. Effect of different Sous Vide cooking temperature-time combinations on the physicochemical, microbiological, and sensory properties of turkey cutlet. Int. J. Gastron. Food Sci. 2020, 20, 100204. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, S. Effects of cooking on thermal-induced changes of Qingyuan partridge chicken breast. Food Sci. Biotechnol. 2012, 21, 1525–1531. [Google Scholar] [CrossRef]
- Li, C.; Wang, D.; Xu, W.; Gao, F.; Zhou, G. Effect of final cooked temperature on tenderness, protein solubility and microstructure of duck breast muscle. LWT Food Sci. Technol. 2013, 51, 266–274. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, B.; Oh, E.; Kim, Y.S.; Choi, Y.M. Combined effects of sous-vide cooking conditions on meat and sensory quality characteristics of chicken breast meat. Poult. Sci. 2020, 99, 3286–3291. [Google Scholar] [CrossRef]
- Karpińska-Tymoszczyk, M.; Draszanowska, A.; Danowska-Oziewicz, M.; Kurp, L. The effect of low-temperature thermal processing on the quality of chicken breast fillets. Food Sci. Technol. Int. 2020. [Google Scholar] [CrossRef]
- Silva, F.A.; Ferreira, V.C.; Madruga, M.S.; Estévez, M. Effect of the cooking method (grilling. roasting. frying and sous-vide) on the oxidation of thiols. tryptophan. alkaline amino acids and protein cross-linking in jerky chicken. J. Food Sci. Technol. 2016, 53, 3137–3146. [Google Scholar] [CrossRef]
- Ormian, M.; Augustyńska-Prejsnar, A.; Sokołowicz, Z. Wpływ obróbki termicznej na wybrane cechy jakości mięśni piersiowych kurcząt z chowu wybiegowego. Postępy Techniki Przetwórstwa Spożywczego 2015, 2, 43–46. [Google Scholar]
- Mora, B.; Curti, E.; Vittadini, E.; Barbanti, D. Effect of different air/steam convection cooking methods on turkey breast meat: Physical characterization, water status and sensory properties. Meat Sci. 2011, 88, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Savage, E.M. Validation of a combi oven cooking method for preparation of chicken breast meat for quality assessment. J. Food Sci. 2008, 73, S424–S430. [Google Scholar] [CrossRef]
- Fusi, A.; Guidetti, R.; Azapagic, A. Evaluation of environmental impacts in the catering sector: The case of pasta. J. Clean. Prod. 2016, 132, 146–160. [Google Scholar] [CrossRef]
- Takacs, B.; Borrion, A. The Use of Life Cycle-Based Approaches in the Food Service Sector to Improve Sustainability: A Systematic Review. Sustainability 2020, 12, 3504. [Google Scholar] [CrossRef]
- Warthesen, J.J.; Vickers, Z.M.; Whitney-West, S.; Wolf, I.D. Cookery methods for vegetables: Influence on sensory quality, nutrient retention, and energy consumption. Home Econ. Res. J. 1984, 13, 61–79. [Google Scholar] [CrossRef]
- Rhee, K.S.; Drew, F. Energy Consumption and Acceptability: Comparison of Cooking Methods and Appliances for Beef Patties 1. Home Econ. Res. J. 1977, 5, 269–282. [Google Scholar] [CrossRef]
- Das, T.; Subramanian, R.; Chakkaravarthi, A.; Singh, V.; Ali, S.Z.; Bordoloi, P.K. Energy conservation in domestic rice cooking. J. Food Eng. 2006, 75, 156–166. [Google Scholar] [CrossRef]
- Baker, R.C.; Darfler, J.M.; Rehkugler, G.E. Electrical energy used and time consumed when cooking foods by various home methods: Chickens. Poult. Sci. 1981, 60, 2062–2070. [Google Scholar] [CrossRef]
- Joseph, J.K.; Awosanya, B.; Adeniran, A.T.; Otagba, U.M. The effects of end-point internal cooking temperatures on the meat quality attributes of selected Nigerian poultry meats. Food Qual. Prefer. 1997, 8, 57–61. [Google Scholar] [CrossRef]
- Weber, J.; Bochi, V.C.; Ribeiro, C.P.; Victório, A.D.M.; Emanuelli, T. Effect of different cooking methods on the oxidation, proximate and fatty acid composition of silver catfish (Rhamdia quelen) fillets. Food Chem. 2008, 106, 140–146. [Google Scholar] [CrossRef]
- Ersoy, B.; Özeren, A. The effect of cooking methods on mineral and vitamin contents of African catfish. Food Chem. 2009, 115, 419–422. [Google Scholar] [CrossRef]
- Bastías, J.M.; Balladares, P.; Acuña, S.; Quevedo, R.; Muñoz, O. Determining the effect of different cooking methods on the nutritional composition of salmon (Salmo salar) and chilean jack mackerel (Trachurus murphyi) fillets. PLoS ONE 2017, 12, e0180993. [Google Scholar] [CrossRef] [PubMed]
- Ramane, K.; Strautniece, E.; Galoburda, R. Chemical and sensory parameters of heat-treated vacuum-packaged broiler and hen fillet products. Proc. Latv. Univ. Agric. 2012, 27, 54–58. [Google Scholar] [CrossRef]
- Danowska-Oziewicz, M.; Karpińska-Tymoszczyk, M.; Borowski, J.; Bialobrzewski, I.; Zapotoczny, P. The effect of cooking in a steam-convention oven and storage in vacuum on the quality of turkey meat. Food Sci. Technol. Int. 2009, 15, 345–356. [Google Scholar] [CrossRef]
- Wang, S.H.; Chang, M.H.; Chen, T.C. Shelf-life and microbiological profiler of chicken wing products following sous vide treatment. Int. J. Poult. Sci. 2004, 3, 326–332. [Google Scholar] [CrossRef][Green Version]
- Jørgensen, F.; Sadler-Reeves, L.; Shore, J.; Aird, H.; Elviss, N.; Fox, A.; Kaye, M.; Willis, C.; Amar, C.; De Pinna, E.; et al. An assessment of the microbiological quality of lightly cooked food (including sous-vide) at the point of consumption in England. Epidemiol. Infect. 2017, 145, 1500–1509. [Google Scholar] [CrossRef]
- McIntyre, L.; Jorgenson, V.; Ritson, M. Sous vide style cooking practices linked to Salmonella Enteritidis illnesses. Environ. Health Rev. 2017, 60, 42–49. [Google Scholar] [CrossRef]
- Stringer, S.C.; Metris, A. Predicting bacterial behaviour in sous vide food. Int. J. Gastron. Food Sci. 2018, 13, 117–128. [Google Scholar] [CrossRef]
- Stringer, S.C.; Fernandes, M.A.; Metris, A. Safety of Sous - Vide Foods: Feasibility of Extending ComBase to Describe the Growth/Survival/Death Response of Bacterial Foodborne Pathogens Between 40 °C and 60 °C; Institute of Food Research: Norwich, UK, 2012; pp. 4–5. [Google Scholar]
| Parameter | Raw | Heat Treatment Method ± SE | SEM | ||||
|---|---|---|---|---|---|---|---|
| Sous-Vide Method | Steaming (SP100) | Boiling (BP100) | |||||
| 64°C × 60 min (SV64) | 66°C × 80 min (SV66) | 75°C × 35 min (SV75) | |||||
| Total process duration (min) | - | 126 | 149 | 117 | 55 | 42 | 0.31 |
| Energy consumption (kWh)* (only for cooking process) | - | 0.548 (0.023) | 0.585 (0.025) | 0.743 (0.017) | 0.116 (0.058) | 0.057 (0.025) | 0.02 |
| Yield (%) | - | 89.40 e | 82.40 d | 83.10 c | 72.44 b | 69.49 a | 0.71 |
| pH | 6.14 a | 6.16 a | 6.30 b | 6.32 b | 6.33 b,c | 6.40 c | 0.04 |
| Parameter | Raw | Heat Treatment Method % [( ± SE] | SEM | ||||
|---|---|---|---|---|---|---|---|
| Sous-Vide Method | Steaming (SP100) | Boiling (BP100) | |||||
| 64°C × 60 min (SV64) | 66°C × 80 min (SV66) | 75°C × 35 min (SV75) | |||||
| Water content | 76.90 c | 74.50 c | 71.30 b | 70.50 a,b | 71.20 b | 68.50 a | 0.81 |
| Protein content | 22.60 a | 22.90 a | 27.40 b | 24.50 a | 27.40 b | 29.20 b | 0.89 |
| Fat content | 0.49 a | 1.94 c | 1.23 b | 1.94 c | 1.48 b | 1.42 b | 0.09 |
| Parameter | Raw | Heat Treatment Method [ (g·100 g−1 KT) ±SE] | ||||
|---|---|---|---|---|---|---|
| Sous-Vide Method | Steaming (SP100) | Boiling (BP100) | ||||
| 64°C × 60 min (SV64) | 66°C × 80 min (SV66) | 75°C × 35 min (SV75) | ||||
| SFA | 33.50 a ± 0.10 | 33.09 a ± 0.01 | 34.02 a ± 0.12 | 34.24 a ± 0.03 | 37.08 b ± 0.01 | 35.67 b ± 0.10 |
| C12:0 | 3.72 a ± 0.12 | 3.41 a ± 0.03 | 3.79 a ± 0.09 | 3.74 a ± 0.05 | 3.65 a ± 0.01 | 4.04 b ± 0.04 |
| C14:0 | 3.06 b ± 0.03 | 2.86 a ± 0.01 | 3.14 b ± 0.00 | 3.09 b ± 0.00 | 3.28 c ± 0.00 | 3.18 c ± 0.00 |
| C16:0 | 20.30 a ± 0.06 | 20.20 a ± 0.03 | 20.90 a,b ± 0.01 | 21.00 b ± 0.02 | 22.50 b ± 0.03 | 21.60 b ± 0.04 |
| C17:0 | 0.13 a ± 0.01 | 0.13 a ± 0.00 | 0.16 a ± 0.00 | 0.14 a ± 0.00 | 0.14 a ± 0.00 | 0.12 a ± 0.00 |
| C18:0 | 6.08 a ± 0.02 | 6.26 a,b ± 0.01 | 5.82 a ± 0.02 | 6.04 a ± 0.00 | 7.30 c ± 0.01 | 6.57 b,c ± 0.01 |
| C20:0 | 0.10 a ± 0.01 | 0.08 a ± 0.01 | 0.08 a ± 0.01 | 0.07 a ± 0.01 | 0.09 a ± 0.00 | 0.08 a ± 0.00 |
| C22:0 | 0.03 a ± 0.03 | 0.07 a ± 0.01 | 0.00 a ± 0.00 | 0.00 a ± 0.00 | 0.05 a ± 0.00 | 0.05 a ± 0.01 |
| C23:0 | 0.16 a ± 0.01 | 0.08 a ± 0.01 | 0.11 a ± 0.01 | 0.10 a ± 0.02 | 0.08 a ± 0.02 | 0.07 a ± 0.00 |
| MUFA | 39.84 a ± 0.08 | 38.81 a ± 0.18 | 43.91 b ± 0.07 | 40.85 a ± 0.02 | 39.66 a ± 0.01 | 40.00 a ± 0.09 |
| C14:1 (cis-9) | 0.29 a ± 0.04 | 0.37 b ± 0.01 | 0.34 b ± 0.01 | 0.38 b ± 0.01 | 0.35 b ± 0.00 | 0.35 b ± 0.00 |
| C16:1 (cis-9) n-7 | 3.49 a ± 0.00 | 3.97 b ± 0.13 | 3.88 b ± 0.00 | 3.97 b ± 0.00 | 3.54 a ± 0.00 | 3.85 b ± 0.12 |
| C17:1 (cis-10) | 0.11 a ± 0.00 | 0.11 a ± 0.00 | 0.07 a ± 0.00 | 0.10 a ± 0.00 | 0.09 a ± 0.00 | 0.09 a ± 0.00 |
| C18:1 n-9 (OA) | 33.10 a ± 0.10 | 31.60 b ± 0.05 | 36.40 c ± 0.05 | 33.50 a ± 0.01 | 32.90 a ± 0.00 | 32.90 a ± 0.05 |
| C18:1 n-3 (cis 11) | 2.24 a ± 0.02 | 2.24 a ± 0.00 | 2.63 a ± 0.00 | 2.40 a ± 0.00 | 2.27 a ± 0.01 | 2.32 a ± 0.00 |
| C20:1 n-9 | 0.56 c ± 0.01 | 0.49 a,b ± 0.01 | 0.57 c ± 0.01 | 0.46 a ± 0.02 | 0.47 a ± 0.00 | 0.50 b ± 0.02 |
| PUFA | 24.774 b ± 0.10 | 26.00 b ± 0.15 | 19.07 a ± 0.01 | 21.63 a ± 0.02 | 20.86 a ± 0.04 | 21.51 a ± 0.04 |
| C18:2 n-6 (LA) | 21.00 e ± 0.11 | 21.40 e ± 0.04 | 16.10 a ± 0.02 | 18.70 d ± 0.05 | 17.60 b ± 0.01 | 18.20 c ± 0.04 |
| C18:3 n-6 (GLA) | 0.19 b ± 0.04 | 0.22 b ± 0.00 | 0.10 a ± 0.01 | 0.12 a ± 0.03 | 0.16 b ± 0.01 | 0.11 a ± 0.02 |
| C18:3 n-3 (ALA) | 2.11 e ± 0.01 | 2.19 e ± 0.00 | 1.42 a ± 0.01 | 1.61 c ± 0.02 | 1.55 b ± 0.01 | 1.70 d ± 0.01 |
| C20:2 n-6 | 0.25 d ± 0.00 | 0.29 e ± 0.00 | 0.22 b ± 0.00 | 0.21 a ± 0.00 | 0.25 d ± 0.00 | 0.24 c ± 0.00 |
| C20:3 n-6 | 0.27 b ± 0.01 | 0.37 d ± 0.00 | 0.23 a ± 0.00 | 0.20 a ± 0.00 | 0.30 c ± 0.00 | 0.31 c ± 0.01 |
| C20:4 n-6 (AA) | 0.84 c ± 0.02 | 1.30 e ± 0.01 | 0.74 a ± 0.02 | 0.81 b ± 0.01 | 0.89 d ± 0.02 | 0.88 d ± 0.00 |
| C20:5 n-3 (EPA) | 0.00 a ± 0.00 | 0.08 d ± 0.00 | 0.10 e ± 0.00 | 0.00 a ± 0.00 | 0.06 b ± 0.00 | 0.07 c ± 0.00 |
| C22:6 n-3 (DHA) | 0.06 a ± 0.00 | 0.12 c ± 0.01 | 0.15 c ± 0.00 | 0.06 a ± 0.00 | 0.08 b ± 0.00 | 0.08 b ± 0.00 |
| Non identified FAs | 1.97 a ± 0.08 | 2.10 b ± 0.24 | 2.99 d ± 0.04 | 3.25 e ± 0.06 | 2.40 b ± 0.02 | 2.79 c ± 0.05 |
| n-3 g/100g | 2.17 b ± 0.01 | 2.39 b ± 0.01 | 1.67 a ± 0.02 | 1.67 a ± 0.02 | 1.69 a ± 0.01 | 1.84 a ± 0.01 |
| n-6 g/100g | 22.57 c ± 0.16 | 23.61 d ± 0.04 | 17.40 a ± 0.01 | 19.96 b ± 0.06 | 19.18 b ± 0.04 | 19.67 b ± 0.05 |
| n-6: n-3 | 9.42 | 9.83 | 10.24 | 11.76 | 11.29 | 10.94 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Głuchowski, A.; Czarniecka-Skubina, E.; Buła, M. The Use of the Sous-Vide Method in the Preparation of Poultry at Home and in Catering—Protection of Nutrition Value Whether High Energy Consumption. Sustainability 2020, 12, 7606. https://doi.org/10.3390/su12187606
Głuchowski A, Czarniecka-Skubina E, Buła M. The Use of the Sous-Vide Method in the Preparation of Poultry at Home and in Catering—Protection of Nutrition Value Whether High Energy Consumption. Sustainability. 2020; 12(18):7606. https://doi.org/10.3390/su12187606
Chicago/Turabian StyleGłuchowski, Artur, Ewa Czarniecka-Skubina, and Maria Buła. 2020. "The Use of the Sous-Vide Method in the Preparation of Poultry at Home and in Catering—Protection of Nutrition Value Whether High Energy Consumption" Sustainability 12, no. 18: 7606. https://doi.org/10.3390/su12187606
APA StyleGłuchowski, A., Czarniecka-Skubina, E., & Buła, M. (2020). The Use of the Sous-Vide Method in the Preparation of Poultry at Home and in Catering—Protection of Nutrition Value Whether High Energy Consumption. Sustainability, 12(18), 7606. https://doi.org/10.3390/su12187606







