A Sustainability Assessment of the Greenseeker N Management Tool: A Lysimetric Experiment on Barley
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. GHGs Emission Measurements and Flux Estimation
2.3. Soil and Crop Analysis
2.4. Economic Evaluation Method
2.5. Statistical Analysis
3. Results and Discussion
3.1. Meteorological Conditions during the Study Period
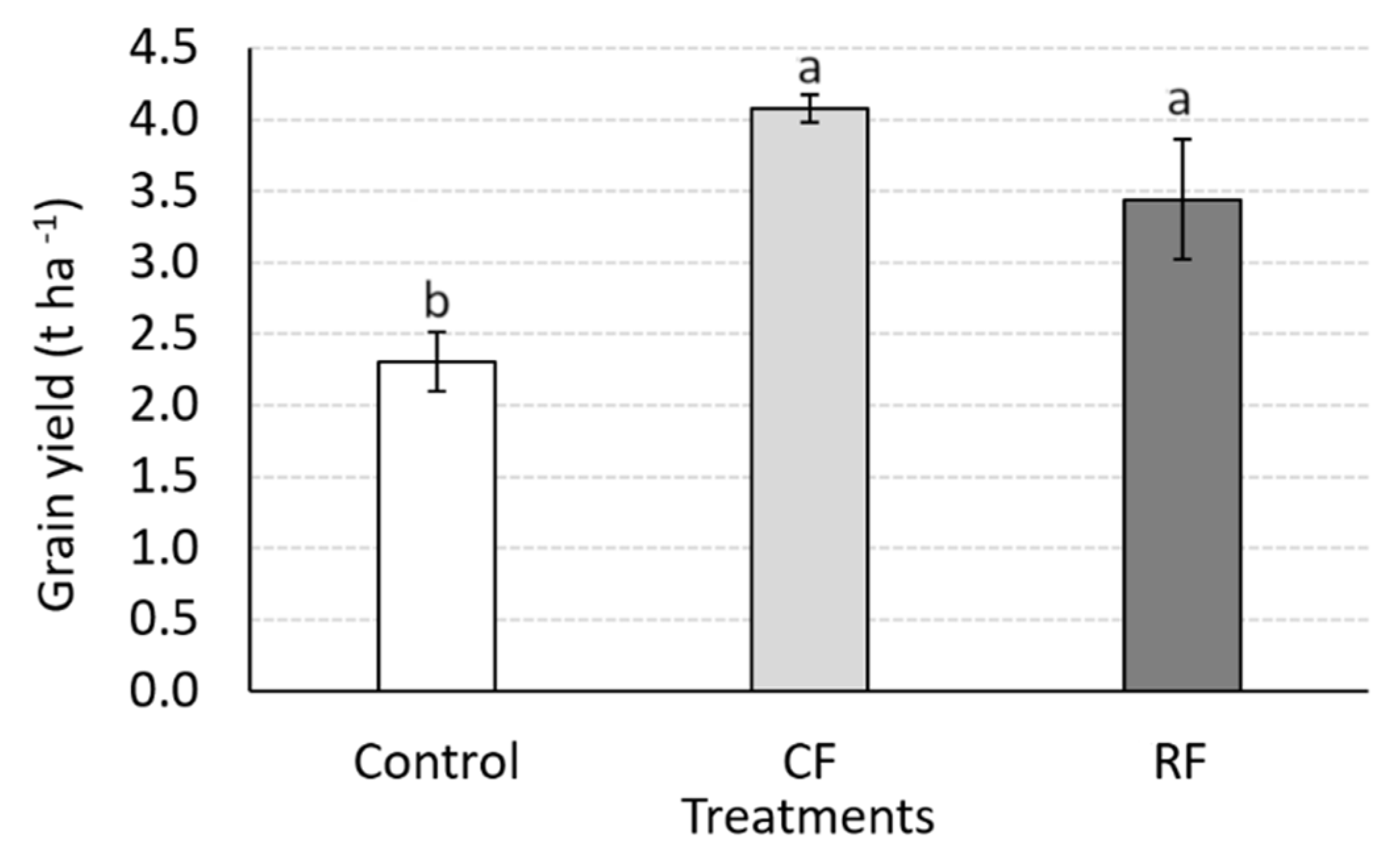
3.2. Yield Responses to N Rates and Economic Benefits
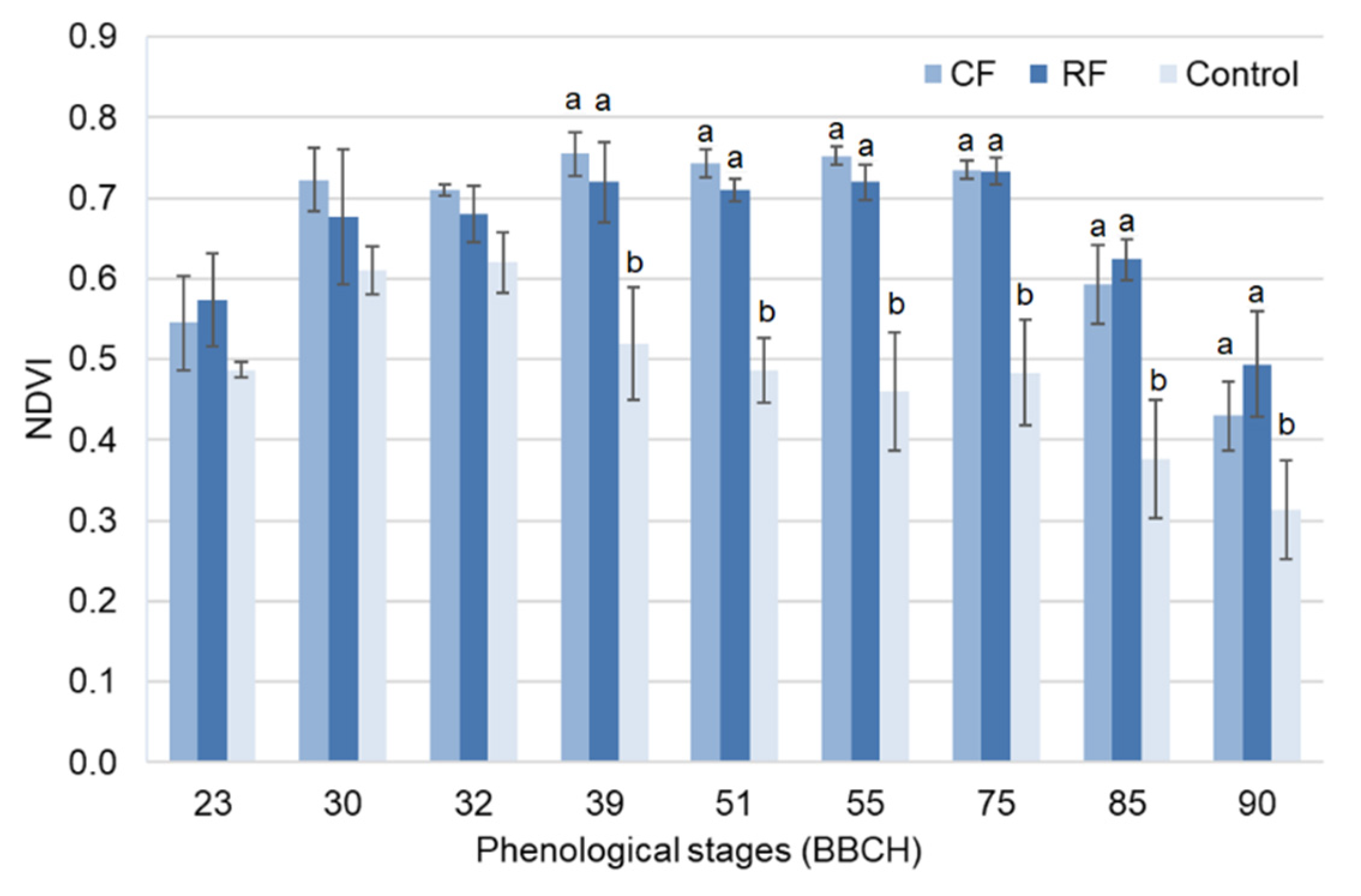
3.3. NDVI Trend during Crop Development
3.4. Possible Use of NDVI Measurements to Predict Plant, Grain Weight, and N Content
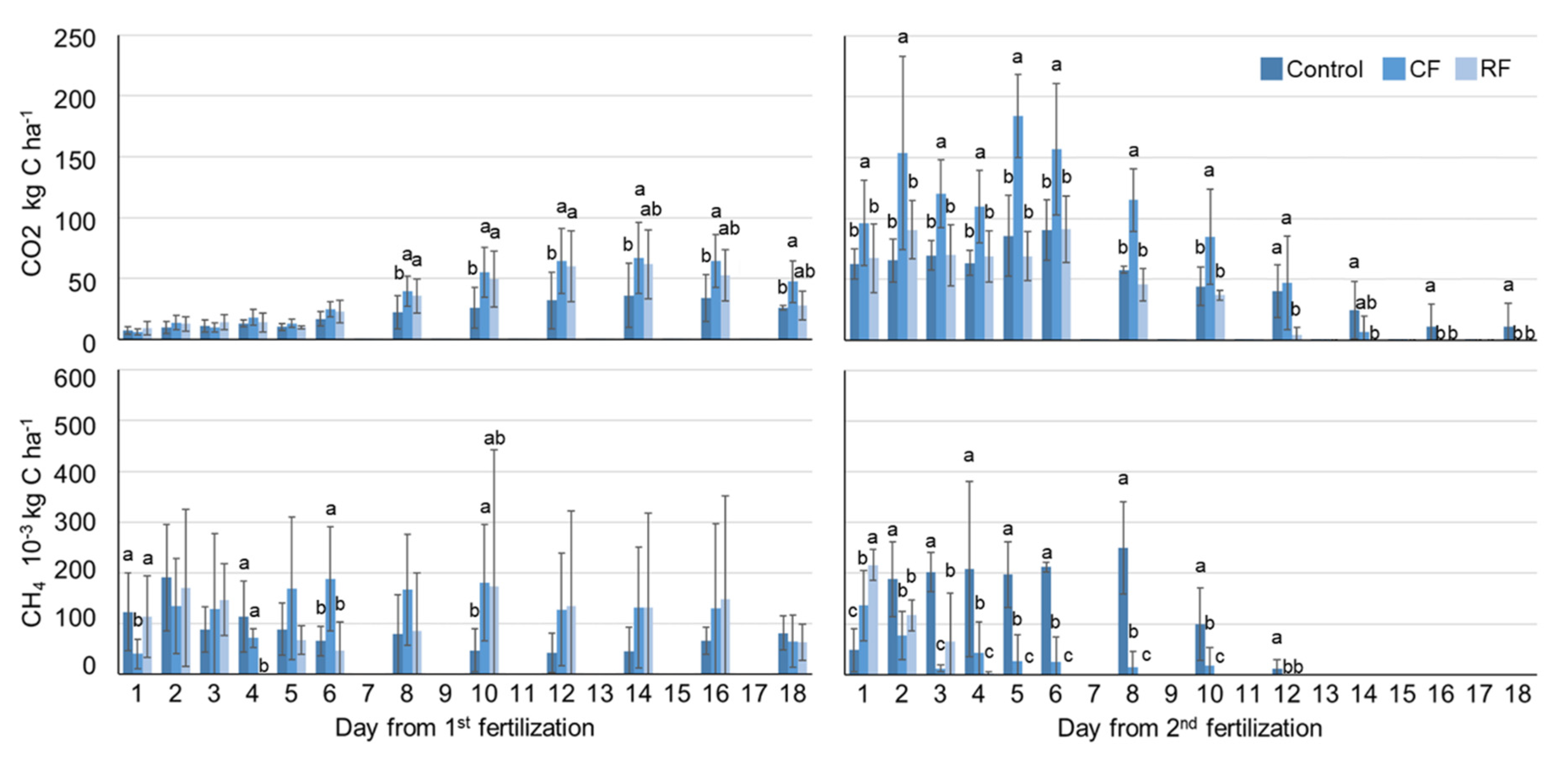
3.5. Effect of Fertilization Method on GHGs Emissions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lindblom, J.; Lundström, C.; Ljung, M.; Jonsson, A. Promoting sustainable intensification in precision agriculture: Review of decision support systems development and strategies. Precis. Agric. 2017, 18, 309–331. [Google Scholar] [CrossRef]
- Brisco, B.; Brown, R.J.; Hirose, T.; McNairn, H.; Staenz, K. Precision agriculture and the role of remote sensing: A review. Can. J. Remote Sens. 2014, 24, 315–327. [Google Scholar] [CrossRef]
- Robertson, M.J.; Llewellyn, R.S.; Mandel, R.; Lawes, R.; Bramley, R.G.V.; Swift, L.; Metz, N.; O’Callaghan, C. Adoption of variable rate fertilizer application in the Australian grains industry: Status, issues and prospects. Precis. Agric. 2012, 13, 181–199. [Google Scholar] [CrossRef]
- Naser, M.A.; Khosla, R.; Longchamps, L.; Dahal, S. Using NDVI to differentiate wheat genotypes productivity under dryland and irrigated conditions. Remote Sens. 2020, 12, 824. [Google Scholar] [CrossRef]
- Ranđelović, P.; Đorđević, V.; Milić, S.; Balešević-Tubić, S.; Petrović, K.; Miladinović, J.; Đukić, V. Prediction of soybean plant density using a machine learning model and vegetation indices extracted from RGB images taken with a UAV. Agronomy 2020, 10, 1108. [Google Scholar] [CrossRef]
- Guo, A.; Huang, W.; Ye, H.; Dong, Y.; Ma, H.; Ren, Y.; Ruan, C. Identification of wheat yellow rust using spectral and texture features of hyperspectral images. Remote Sens. 2020, 12, 1419. [Google Scholar] [CrossRef]
- Sandino, J.; Pegg, G.; Gonzalez, F.; Smith, G. Aerial mapping of forests affected by pathogens using UAVs, hyperspectral sensors, and artificial intelligence. Sensors 2018, 18, 944. [Google Scholar] [CrossRef]
- Siqueira, R.; Longchamps, L.; Dahal, S.; Khosla, R. Use of fluorescence sensing to detect nitrogen and potassium variability in maize. Remote Sens. 2020, 12, 1752. [Google Scholar] [CrossRef]
- Alvar-Beltrán, J.; Fabbri, C.; Verdi, L.; Truschi, S.; Dalla Marta, A.; Orlandini, S. Testing proximal optical sensors on Quinoa growth and development. Remote Sensing 2020, 12, 1958. [Google Scholar] [CrossRef]
- Aranguren, M.; Castellón, A.; Aizpurua, A. Crop sensor based non-destructive estimation of nitrogen nutritional status, yield, and grain protein content in wheat. Agriculture 2020, 10, 148. [Google Scholar] [CrossRef]
- Zhao, Y.; Potgieter, A.B.; Zhang, M.; Wu, B.; Hammer, G.L. Predicting wheat yield at the field scale by combining high-resolution Sentinel-2 satellite imagery and crop modelling. Remote Sensing 2020, 12, 1024. [Google Scholar] [CrossRef]
- Tagarakis, A.C.; Ketterings, Q.M. In-season estimation of corn yield potential using proximal sensing. Agron. J. 2017, 109, 1323–1330. [Google Scholar] [CrossRef]
- Holland, K.H.; Schepers, J.S. Derivation of a variable rate nitrogen application model for in-season fertilization of corn. Agron. J. 2010, 102, 1415–1424. [Google Scholar] [CrossRef]
- Padilla, F.M.; Peña-Fleitas, M.T.; Gallardo, M.; Thompson, R.B. Threshold values of canopy reflectance indices and chlorophyll meter readings for optimal nitrogen nutrition of tomato. Ann. Appl. Biol. 2015, 166, 271–285. [Google Scholar] [CrossRef]
- Padilla, F.M.; Peña-Fleitas, M.T.; Gallardo, M.; Thompson, R.B. Proximal optical sensing of cucumber crop N status using chlorophyll fluorescence indices. Eur. J. Agron. 2016, 73, 83–97. [Google Scholar] [CrossRef]
- Lu, J.; Miao, Y.; Shi, W.; Li, J.; Hu, X.; Chen, Z.; Kusnierek, K. Developing a proximal active canopy sensor-based precision nitrogen management strategy for high-yielding rice. Remote Sens. 2020, 12, 1440. [Google Scholar] [CrossRef]
- Ali, A.M. Using hand-held chlorophyll meters and canopy reflectance sensors for fertilizer nitrogen management in cereals in small farms in developing countries. Sensors 2020, 20, 1127. [Google Scholar]
- Padilla, F.M.; Gallardo, M.; Peña-Fleitas, M.T.; de Souza, R.; Thompson, R.B. Proximal optical sensors for nitrogen management of vegetable crops: A review. Sensors 2018, 18, 2083. [Google Scholar] [CrossRef]
- Marino, S.; Alvino, A. Proximal sensing and vegetation indices for site-specific evaluation on an irrigated crop tomato. Eur. J. Remote Sens. 2014, 47, 271–283. [Google Scholar] [CrossRef]
- De Souza, R.; Peña-Fleitas, M.T.; Thompson, R.B.; Gallardo, M.; Padilla, F.M. Assessing performance of vegetation indices to estimate nitrogen nutrition index in pepper. Remote Sens. 2020, 12, 763. [Google Scholar] [CrossRef]
- Pallottino, F.; Biocca, M.; Nardi, P.; Figorilli, S.; Menesatti, P.; Costa, C. Science mapping approach to analyze the research evolution on precision agriculture: World, EU and Italian situation. Precis. Agric. 2018, 19, 1011–1026. [Google Scholar] [CrossRef]
- Lukina, E.V.; Freeman, K.W.; Wynn, K.J.; Thomason, W.E.; Mullen, R.W.; Stone, M.L.; Solie, J.B.; Klatt, A.R.; Johnson, G.V.; Elliott, R.L.; et al. Nitrogen fertilization optimization algorithm based on in-season estimates of yield and plant nitrogen uptake. J. Plant Nutr. 2001, 24, 885–898. [Google Scholar] [CrossRef]
- Scharf, P.C.; Shannon, D.K.; Palm, H.L.; Sudduth, K.A.; Drummond, S.T.; Kitchen, N.R.; Mueller, L.J.; Hubbard, V.C.; Oliveira, L.F. Sensor-based nitrogen applications out-performed producer-chosen rates for corn in on-farm demonstrations. Agron. J. 2011, 103, 1683–1691. [Google Scholar] [CrossRef]
- Robertson, M.; Carberry, P.; Brennan, L. Economic benefits of variable rate technology: Case studies from Australian grain farms. Crop Pasture Sci. 2009, 60, 799–807. [Google Scholar] [CrossRef]
- McFadden, B.R.; Brorsen, B.W.; Raun, W.R. Nitrogen fertilizer recommendations based on plant sensing and Bayesian updating. Precis. Agric. 2018, 19, 79–92. [Google Scholar] [CrossRef]
- Molin, J.P.; Motomiya, A.V.D.A.; Frasson, F.R.; Faulin, G.D.C.; Tosta, W. Test procedure for variable rate fertilizer in coffee. Acta Scientiarum. Agron. 2010, 32, 569–575. [Google Scholar] [CrossRef]
- Bragagnolo, J.; Amado, T.J.C.; Nicoloso, R.S.; Santi, A.L.; Fiorin, J.E.; Tabaldi, F. Optical crop sensor for variable-rate nitrogen fertilization in corn: II-indices of fertilizer efficiency and corn yield. Revista Brasileira de Ciência do Solo 2013, 37, 1299–1309. [Google Scholar] [CrossRef]
- Colaço, A.F.; Molin, J.P. Variable rate fertilization in citrus: A long-term study. Precis. Agric. 2017, 18, 169–191. [Google Scholar] [CrossRef]
- Basso, B.; Dumont, B.; Cammarano, D.; Pezzuolo, A.; Marinello, F.; Sartori, L. Environmental and economic benefits of variable rate nitrogen fertilization in a nitrate vulnerable zone. Sci. Total Environ. 2016, 545–546, 227–235. [Google Scholar] [CrossRef]
- Phillips, S.B.; Keahey, D.A.; Warren, G.W.; Mullins, G.L. Nitrogen management. Agron. J. 2004, 96, 591–600. [Google Scholar]
- Jana Havrankova, J.; Richard John Godwin, R.J.; Vladimir Rataj, V.; Gavin Adam Wood, G.A. Benefits from Application of Ground Based Remote Sensing Systems in Winter Wheat Nitrogen Management in Europe. In 2008 Providence, Rhode Island, USA, 29 June–2 July 2008; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2008; p. 1. [Google Scholar]
- Diacono, M.; Rubino, P.; Montemurro, F. Precision nitrogen management of wheat. A review. Agron. Sustain. Dev. 2013, 33, 219–241. [Google Scholar] [CrossRef]
- Stefanini, M.; Larson, J.A.; Lambert, D.M.; Yin, X.; Boyer, C.N.; Scharf, P.; Buschermohle, M.J. Effects of optical sensing based variable rate nitrogen management on yields, nitrogen use and profitability for cotton. Precis. Agric. 2019, 20, 591–610. [Google Scholar] [CrossRef]
- Li, A.; Duval, B.D.; Anex, R.; Scharf, P.; Ashtekar, J.M.; Owens, P.R.; Ellis, C. A case study of environmental benefits of sensor-based nitrogen application in corn. J. Environ. Qual. 2016, 45, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Lenerts, A.; Popluga, D. Facilitate calculations of GHG emissions in Latvian agriculture at farm level. In Proceedings of the 15th International Multidisciplinary Scientific Geoconference SGEM 2015 “Ecology, Economics, Education and Legislation” Conference Proceedings, Albena, Bulgaria, 18–24 June 2015; Volume III, pp. 287–294. [Google Scholar]
- Kritikos, M. Precision agriculture in Europe. Legal, Social and Ethical Considerations STUDY EPRS|European Parliamentary Research Service Science and Technology Options Assessment; STOA: Brussels, Belgium, 2017. [Google Scholar]
- Domínguez, P.; Fellmann, T.; Weiss, F.; Witzke, P.; Barreiro-Hurlé, J.; Himics, M.; Jansson, T.; Salputra, G.; Leip Ignacio Pérez Domínguez, A. EU Impact Analysis on GHG-Emission Proposal: Focus on Agriculture EcAMPA 2; JRC Science for Policy Report; European Commission, Publications Office of the European Union: Luxembourg, 2016. [Google Scholar]
- Millar, N.; Robertson, G.P.; Diamant, A.; Gehl, R.J.; Grace, P.R.; Hoben, J.P. Quantifying N2O Emissions Reductions in US Agricultural Crops through N Fertilizer Rate Reduction; Verified Carbon Standard: Washington, DC, USA, 2013. [Google Scholar]
- Oertel, C.; Matschullat, J.; Zurba, K.; Zimmermann, F.; Erasmi, S. Greenhouse gas emissions from soils—A review. Chemie der Erde 2016, 76, 327–352. [Google Scholar] [CrossRef]
- Lu, M.; Zhou, X.; Luo, Y.; Yang, Y.; Fang, C.; Chen, J.; Li, B. Minor stimulation of soil carbon storage by nitrogen addition: A meta-analysis. Agric. Ecosyst. Environ. 2011, 140, 234–244. [Google Scholar] [CrossRef]
- Al-Kaisi, M.M.; Kruse, M.L.; Sawyer, J.E. Effect of Nitrogen Fertilizer Application on Growing Season Soil Carbon Dioxide Emission in a Corn-Soybean Rotation. J. Environ. Qual. 2008, 37, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, B.; Ziadi, N.; Rochette, P.; Chantigny, M.H.; Angers, D.A.; Bertrand, N.; Smith, W.N. Soil-surface carbon dioxide emission following nitrogen fertilization in corn. Can. J. Soil Sci. 2016, 96, 219–232. [Google Scholar] [CrossRef]
- Chu, H.; Hosen, Y.; Yagi, K. NO, N2O, CH4 and CO2 fluxes in winter barley field of Japanese Andisol as affected by N fertilizer management. Soil Biol. Biochem. 2007, 39, 330–339. [Google Scholar] [CrossRef]
- Bin-Feng, S.; Zhao, H.; Yi-Zhong, L.Ü.; Fei, L.U.; Wang, X.-K. The effects of nitrogen fertilizer application on methane and nitrous oxide emission/uptake in Chinese croplands. J. Integr. Agric. 2016, 15, 440–450. [Google Scholar]
- Balafoutis, A.; Beck, B.; Fountas, S.; Vangeyte, J.; Wal, T.; Soto, I.; Gómez-Barbero, M.; Barnes, A.; Eory, V.; Balafoutis, A.; et al. Precision Agriculture Technologies Positively Contributing to GHG Emissions Mitigation, Farm Productivity and Economics. Sustainability 2017, 9, 1339. [Google Scholar] [CrossRef]
- Willer, H.; Yussefi-Menzler, M.; Sorensen, N. The World of Organic Agriculture—Statistics and Emerging Trends 2008; Earthscan: Bohn, Germany, 2010. [Google Scholar]
- SIR No Title. Available online: http://www.sir.toscana.it/ricerca-dati (accessed on 2 March 2019).
- Poorter, H.; Bühler, J.; van Dusschoten, D.; Climent, J.; Postma, J.A. Pot size matters: A meta-analysis of the effects of rooting volume on plant growth. Funct. Plant Biol. 2012, 39, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; McConkey, B.; Wang, H.; Janzen, H. Root distribution by depth for temperate agricultural crops. Field Crops Res. 2016, 189, 68–74. [Google Scholar] [CrossRef]
- Napoli, M.; Cecchi, S.; Zanchi, C.A.; Orlandini, S. Leaching of glyphosate and aminomethylphosphonic acid through silty clay soil columns under outdoor conditions. J. Environ. Qual. 2015, 44, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.; Atwell, S.; Dunn, D. Sensor-based nitrogen fertilization for midseason rice production on Southeast Missouri. Crop Forage Turf. Man. 2017, 3, 1–7. [Google Scholar] [CrossRef][Green Version]
- Verdi, L.; Kuikman, P.J.; Orlandini, S.; Mancini, M.; Napoli, M.; Dalla Marta, A. Does the use of digestate to replace mineral fertilizers have less emissions of N2O and NH3? Agric. For. Meteorol. 2019, 269–270, 112–118. [Google Scholar] [CrossRef]
- Parkin, T.B.; Venterea, R.T. Chamber-based trace gas flux measurements. In Sampling Protocols; Follet, R.F., Ed.; USDA-ARS: Beltsville, MD, USA, 2010; pp. 3-1–3-39. [Google Scholar]
- Napoli, M.; Cecchi, S.; Orlandini, S.; Mugnai, G.; Zanchi, C.A. Simulation of field-measured soil loss in Mediterranean hilly areas (Chianti, Italy) with RUSLE. Catena 2016, 145, 246–256. [Google Scholar] [CrossRef]
- Gee, G.W.; Bauder, J.W. Particle-size analysis. In Methods of Soil Analysis, Part 1. Physical and Mineralogical Methods, Agronomy Monograph No. 9; Klute, A., Ed.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Allison, L.E.; Moodie, C.D. Carbonate. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Black, C.A., Ed.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 1379–1398. [Google Scholar]
- Santi, C.; Certini, G.; D’Acqui, L.P. Direct determination of organic carbon by dry combustion in soils with carbonates. Commun. Soil Sci. Plant Anal. 2006, 37, 155–162. [Google Scholar] [CrossRef]
- Matejovic, I. Determination of carbon and nitrogen in samples of various soils by the dry combustion. Commun. Soil Sci. Plant Anal. 1997, 28, 1499–1511. [Google Scholar] [CrossRef]
- AOAC. AOAC Official Methods of Analysis, 13th ed.; Horwitz, W., Ed.; Association of Otlicial Analytical Chemists: Washington, DC, USA, 1980. [Google Scholar]
- FICAMCOM Listino dei Prezzi all’Ingrosso Rilevati sul Mercato di Firenze—Listino n. 8 dal 18 al 24 Febbraio. 2019. Available online: https://www.fi.camcom.gov.it/regolazione-del-mercato/tutela-di-consumatori-e-imprese/prezzi/listini-settimanali-dei-prezzi-allingrosso (accessed on 12 March 2019).
- MOCAMCOM Listino dei Prezzi all’Ingrosso Rilevati sul Mercato di Modena—Anno 2018—n° 53 del 31 Dicembre 2018. Available online: https://www.mo.camcom.it/tutela-del-mercato/borsamerci/listini (accessed on 12 March 2019).
- IPCC. The Physical Science Basis. Working Group I Contribution to the 5th Assessment Report of the IPCC. Available online: http://www.climatechange2013.org (accessed on 20 January 2020).
- Brentrup, F.; Pallière, C. Energy efficiency and greenhouse gas emissions in European nitrogen fertilizer production and use. In Proceedings of International Fertiliser Society; The International Fertiliser Society: Cambridge, UK, 2008; pp. 1–25. [Google Scholar]
- Bragagnolo, J.; Jorge Carneiro Amado, T.; da Silveira Nicoloso, R.; Jasper, J.; Kunz, J.; de Gregori Teixeira, T. Optical crop sensor for variable-rate nitrogen fertilization in corn: i-plant nutrition and dry matter production. Rev. Bras. Ciênc. Solo 2013, 37, 1288–1298. [Google Scholar] [CrossRef]
- Mayfield, A.H.; Trengove, S.P. Grain yield and protein responses in wheat using the N-Sensor for variable rate N application. Crop Pasture Sci. 2009, 60, 818–823. [Google Scholar] [CrossRef]
- Keeler, B.L.; Gourevitch, J.D.; Polasky, S.; Isbell, F.; Tessum, C.W.; Hill, J.D.; Marshall, J.D. The social cost of nitrogen. Sci. Adv. 2016, 2, e1600219. [Google Scholar] [CrossRef] [PubMed]
- Van Grinsven, H.J.; Holland, M.; Jacobsen, B.H.; Klimont, Z.; Sutton, M.A.; Jaap Willems, W. Costs and benefits of nitrogen for Europe and implications for mitigation. Environ. Sci. Technol. 2013, 47, 3571–3579. [Google Scholar] [CrossRef] [PubMed]
- de Motomiya, A.V.; Molin, J.P.; Chiavegato, E.J. Utilização de sensor óptico ativo para detectar deficiência foliar de nitrogênio em algodoeiro 1. Rev. Bras. Eng. Agrícola e Ambient. 2009, 13, 137–145. [Google Scholar] [CrossRef][Green Version]
- Hassan, M.A.; Yang, M.; Rasheed, A.; Yang, G.; Reynolds, M.; Xia, X.; Xiao, Y.; He, Z. A rapid monitoring of NDVI across the wheat growth cycle for grain yield prediction using a multi-spectral UAV platform. Plant Sci. 2019, 282, 95–103. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, B.; Singh, M.; Thind, S.K. Hyperspectral indices, correlation and regression models for estimating growth parameters of wheat genotypes. J. Indian Soc. Remote Sens. 2015, 43, 551–558. [Google Scholar] [CrossRef]
- Spitkó, T.; Nagy, Z.; Zsubori, Z.T.; Szőke, C.; Berzy, T.; Pintér, J.; Marton, C.L. Connection between normalized difference vegetation index and yield in maize. Plant Soil Environ. 2016, 62, 293–298. [Google Scholar] [CrossRef]
- Huete, A.R. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Prabhakara, K.; Dean Hively, W.; McCarty, G.W. Evaluating the relationship between biomass, percent groundcover and remote sensing indices across six winter cover crop fields in Maryland, United States. Int. J. Appl. Earth Obs. Geoinf. 2015, 39, 88–102. [Google Scholar] [CrossRef]
- Dalla Marta, A.; Grifoni, D.; Mancini, M.; Orlando, F.; Guasconi, F.; Orlandini, S. Durum wheat in-field monitoring and early yield prediction: Assessment of potential use of high resolution satellite imagery in a hilly area of Tuscany, Central Italy. J. Agric. Sci. 2015, 153, 68–77. [Google Scholar] [CrossRef]
- Chandel, N.S.; Tiwari, P.S.; Singh, K.P.; Jat, D.; Gaikwad, B.B.; Tripathi, H.; Golhani, K. Yield prediction in wheat (Triticum aestivum L.) using spectral reflectance indices. Curr. Sci. 2019, 116, 272–278. [Google Scholar] [CrossRef]
- Boeckx, P.; Vermoesen, A.; Van Cleemput, O. Emission of gaseous hydrocarbons and NH3 out of soils. In Biosphere-Atmosphere Exchange of Pollutants and Trace Substances; Springer: Berlin/Heidelberg, Germany, 1997; pp. 405–412. [Google Scholar]
- Song, C.; Wang, L.; Tian, H.; Liu, D.; Lu, C.; Xiaofeng Xu, X.; Zhang, L.; Yang, G.; Wan, Z. Effect of continued nitrogen enrichment on greenhouse gas emissions from a wetland ecosystem in the Sanjiang Plain, Northeast China: A 5 year nitrogen addition experiment. J. Geophys. Res. Biogeosci. 2013, 118, 741–751. [Google Scholar] [CrossRef]
- Chapuis-Lardy, L.; Wrage, N.; Metay, A.; Chotte, J.-L.; Bernoux, M. Soils, a sink for N2O? A review. Glob. Chang. Biol. 2007, 13, 1–17. [Google Scholar] [CrossRef]
- Fog, K. The effect of added nitrogen on the rate of decomposition of organic matter. Biol. Rev. 1988, 63, 433–462. [Google Scholar] [CrossRef]
- Sainju, U.M.; Jabro, J.D.; Stevens, W.B. Soil carbon dioxide emission and carbon content as affected by irrigation, tillage, cropping system, and nitrogen fertilization. J. Environ. Qual. 2008, 37, 98–106. [Google Scholar] [CrossRef]
- Tanveer, S.K.; Wen, X.; Lu, X.L.; Zhang, J.; Liao, Y. Tillage, mulch and N fertilizer affect emissions of CO2 under the rain fed condition. PLoS ONE 2013, 8, e72140. [Google Scholar] [CrossRef]
- Ma, L.; Huang, W.; Guo, C.; Wang, R.; Xiao, C. Soil microbial properties and plant growth responses to carbon and water addition in a temperate steppe: The importance of nutrient availability. PLoS ONE 2012, 7, e35165. [Google Scholar] [CrossRef]
- Liang, L.L.; Eberwein, J.R.; Allsman, L.A.; Grantz, D.A.; Jenerette, G.D. Regulation of CO2 and N2O fluxes by coupled carbon and nitrogen availability. Environ. Res. Lett. 2015, 10, 034008. [Google Scholar] [CrossRef]
- Allison, S.D.; Vitousek, P.M. Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol. Biochem. 2005, 37, 937–944. [Google Scholar] [CrossRef]
- Cai, Z.; Xing, G.; Yan, X.; Xu, H.; Tsuruta, H.; Yagi, K.; Minami, K. Methane and nitrous oxide emissions from rice paddy fields as affected by nitrogen fertilisers and water management. Plant Soil 1997, 196, 7–14. [Google Scholar] [CrossRef]
- Liu, L.; Greaver, T.L. A review of nitrogen enrichment effects on three biogenic GHGs: The CO2 sink may be largely offset by stimulated N2O and CH4 emission. Ecol. Lett. 2009, 12, 1103–1117. [Google Scholar] [CrossRef]
- Shang, Q.; Yang, X.; Gao, C.; Wu, P.; Liu, J.; Xu, Y.; Shen, Q.; Zou, J.; Guo, S. Net annual global warming potential and greenhouse gas intensity in Chinese double rice-cropping systems: A 3-year field measurement in long-term fertilizer experiments. Glob. Chang. Biol. 2011, 17, 2196–2210. [Google Scholar] [CrossRef]
- Venterea, R.T.; Burger, M.; Spokas, K.A. Nitrogen oxide and methane emissions under varying tillage and fertilizer management. J. Environ. Qual. 2005, 34, 1467–1477. [Google Scholar] [CrossRef] [PubMed]
- Mosier, A.R.; Halvorson, A.D.; Reule, C.A.; Liu, X.J. Net global warming potential and greenhouse gas intensity in irrigated cropping systems in northeastern colorado. J. Environ. Qual. 2006, 35, 1584–1598. [Google Scholar] [CrossRef] [PubMed]
- Neumann, R.B.; Moorberg, C.J.; Lundquist, J.D.; Turner, J.C.; Waldrop, M.P.; McFarland, J.W.; Euskirchen, E.S.; Edgar, C.W.; Turetsky, M.R. Warming effects of spring rainfall increase methane emissions from thawing permafrost. Geophys. Res. Lett. 2019, 46, 1393–1401. [Google Scholar] [CrossRef]


| Treatments | N ‰ | Organic Matter % | Particle-Size Distribution (USDA) | Bulk Density Mg m−3 | pH | Total CaCO3 % | ||
|---|---|---|---|---|---|---|---|---|
| Sand % | Silt % | Clay % | ||||||
| Control | 0.15 | 1.36 | 13.1 ± 1.1 | 35.2 ± 0.2 | 51.7 ± 0.9 | 1.26 ± 0.14 | 7.8 ± 0.1 | 13.8 ± 0.2 |
| RF | 0.17 | 1.53 | ||||||
| CF | 0.15 | 1.37 | ||||||
| Treatments | N Rate kg ha−1 | Environmental Costs | Fertilizer Cost € kg−1 | Sale Grain € ha−1 | Economic Profit € ha−1 | Environmental Profit € ha−1 | |
|---|---|---|---|---|---|---|---|
| Fertilizer Production € ha−1 | GHGs Release € ha−1 | ||||||
| Control | 0 | 0 c | 217.11 b | 0 c | 582.74 b | 582.74 b | 365.63 a |
| RF | 75 | 230 b | 233.52 ab | 81 b | 870.27 a | 789.50 a | 326.47 a |
| CF | 150 | 459 a | 393.67 a | 162 a | 1073.79 a | 912.25 a | 59.58 b |
| ANOVA | *** | * | *** | *** | ** | ** | |
| Phenological Stage (BBCH Scale) | Mass of Dry Matter of Total Biomass kg ha−1 | Mass of Dry Matter of Grain kg ha−1 | Whole Plant Nitrogen Content % |
|---|---|---|---|
| 23 | 0.23 | 0.23 | 0.08 |
| 30 | 0.74 ** | 0.74 ** | 0.41 |
| 32 | 0.79 ** | 0.86 ** | 0.45 * |
| 39 | 0.84 ** | 0.88 ** | 0.54 * |
| 51 | 0.8 ** | 0.86 ** | 0.64 ** |
| 55 | 0.81 ** | 0.85 ** | 0.52 * |
| 75 | 0.71 ** | 0.78 ** | 0.48 * |
| 85 | 0.44 * | 0.53 * | 0.26 |
| 90 | 0.16 | 0.21 | 0.07 |
| Fertilization | Treatments | Days CO2 Emission | Average CO2 Emission kg C ha−1 day−1 | Days CH4 Emission | Average CH4 Emission kg C ha−1 day−1 |
|---|---|---|---|---|---|
| First fertilization | Control | 18 | 23.17 ± 9.85 b | 18 | 0.084 ± 0.014 a |
| RF | 18 | 37.36 ± 13.71 a | 18 | 0.116 ± 0.084 a | |
| CF | 18 | 42.22 ± 14.75 a | 18 | 0.146 ± 0.065 a | |
| Second fertilization | Control | 15 ± 2.05 | 56.22 ± 6.21 b | 11 ± 0.94 | 1.763 ± 0.450 a |
| RF | 11 ± 0.47 | 60.57 ± 14.85 b | 3 ± 0.47 | 1.251 ± 0.453 a | |
| CF | 13 ± 1.24 | 114.98 ± 14.40 a | 5 ± 3.20 | 0.703 ± 0.198 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabbri, C.; Napoli, M.; Verdi, L.; Mancini, M.; Orlandini, S.; Dalla Marta, A. A Sustainability Assessment of the Greenseeker N Management Tool: A Lysimetric Experiment on Barley. Sustainability 2020, 12, 7303. https://doi.org/10.3390/su12187303
Fabbri C, Napoli M, Verdi L, Mancini M, Orlandini S, Dalla Marta A. A Sustainability Assessment of the Greenseeker N Management Tool: A Lysimetric Experiment on Barley. Sustainability. 2020; 12(18):7303. https://doi.org/10.3390/su12187303
Chicago/Turabian StyleFabbri, Carolina, Marco Napoli, Leonardo Verdi, Marco Mancini, Simone Orlandini, and Anna Dalla Marta. 2020. "A Sustainability Assessment of the Greenseeker N Management Tool: A Lysimetric Experiment on Barley" Sustainability 12, no. 18: 7303. https://doi.org/10.3390/su12187303
APA StyleFabbri, C., Napoli, M., Verdi, L., Mancini, M., Orlandini, S., & Dalla Marta, A. (2020). A Sustainability Assessment of the Greenseeker N Management Tool: A Lysimetric Experiment on Barley. Sustainability, 12(18), 7303. https://doi.org/10.3390/su12187303








