Abstract
The pretreatment of lignocellulosic biomass (LC biomass) prior to the anaerobic digestion (AD) process is a mandatory step to improve feedstock biodegradability and biogas production. An important potential is provided by lignocellulosic materials since lignocellulose represents a major source for biogas production, thus contributing to the environmental sustainability. The main limitation of LC biomass for use is its resistant structure. Lately, biological pretreatment (BP) gained popularity because they are eco-friendly methods that do not require chemical or energy input. A large number of bacteria and fungi possess great ability to convert high molecular weight compounds from the substrate into lower mass compounds due to the synthesis of microbial extracellular enzymes. Microbial strains isolated from various sources are used singly or in combination to break down the recalcitrant polymeric structures and thus increase biogasgeneration. Enzymatic treatment of LC biomass depends mainly on enzymes like hemicellulases and cellulases generated by microorganisms. The articles main purpose is to provide an overview regarding the enzymatic/biological pretreatment as one of the most potent techniques for enhancing biogas production.
1. Introduction
Bioenergy represents energy from biomass and plays an important role in promoting renewable alternatives. LC biomass is one of the most generous renewable bioresources in nature containing lignin, cellulose, and hemicelluloses. Lignocellulosic materials are the best sources used for biofuel production, such as biogas, and include residues from agriculture and forests, energy crops, and municipal and food waste [1]. According to the latest statistical report for biogas, in Europe, almost 72% of the feedstocks used in the anaerobic digestion (AD) process for biogas production come from the agricultural sector, such as energy crops, manure, and other agricultural residues [2]. The main issue of using lignocellulosic (LC) biomass for the biogas production is biomass recalcitrance, which represents biomass resistance to chemical and biological breakdown [3].
The main drawback of the conventional AD process is the lower hydrolysis rate because of the complex structure that composes LC biomass used as feedstock for biogas production. Thus, the LC biomass pretreatment before AD is considered a significant step to improve its biodegradability, and also the biogas production [4]. This pretreatment method is starting to gain more and more interest because this techniques presents a short reaction time an increased need for nutrition for enzymatic reactions, and also because most enzymes don’t react in the presence of inhibitors and other microbial metabolites.The advantages of biological pretreatment (BP) compared with nonbiological procedures are:
- -
- the possible production of useful results that can of great use further
- -
- decreased development of inhibitory substances because the conditions for operating are more moderated,
- -
- the reduced application chemicals,
- -
- a reduced energy input,
- -
- lower costs for waste deposit.
The use of an enzyme secreting bacterial consortium for biomass is beneficial, as commercial and pure enzymes are too expensive for the AD process.
Pretreatment is important to decompose the mix of lignin and hemicellulose and expand the active contact surface between cellulose and enzymes. Lately, distinct pretreatment methods were recommended for the enhancement of LC biomass digestibility in order to improve AD efficiency. The pretreatment methods include physical, chemical, and biological, but these can be used singly or in combinations [5].
Physical and chemical pretreatments used for disintegration of lignocellulosic resources are energy demanding and use chemicals, which could negatively affect the environment [6]. In this context, biological pretreatment (BP) is an attractive alternative conducted under much milder environmental conditions, with low energy input and no chemical requirement, being an environmentally friendly method. Therefore, the main goal of BP is to maximize the lignin removal and to break the cellulose crystalline structure in order to make it more accessible to the attack of enzymes or microorganisms. BP for biogas production improvement has concentrated on the use of enzymes, fungal strains, and microbial populations for aerobic and anaerobic conditions [7,8].
The paper makes a comparative analysis, as complete as possible, of the research results of the last years regarding the different methods of BP of the substrate for biogas production, without considering the other physical and chemical methods. The main techniques that use microorganisms (bacteria and fungi) and enzymes are specified, and the results obtained in increasing the biogas yield are mentioned. This detailed study aimed to include as many types of biological treatment as possible and their comparative analysis, so as to facilitate the approach of future research to optimize the AD process.
2. Structure of Lignocellulosic Biomass
In order to produce biogas, we need substrate, the most important material used as food for methanogen microorganisms. Substrates are the organic feeding material for all AD applications [9]. The substrate used for biogas production can be of various types like crop residues, animal manure and slurries, organic waste from households, organic fraction of municipal solid waste, and organic waste from dairy production, food industries, and agro-industries. Furthermore, algal biomass, and micro and macroalgae, are being used for studies given their biogas yielding potential [9]. A substrate type that has not been mentioned until recently is represented by energy crops that in recent years have become more and more used for obtaining an alternative source of energy.
Co-digestion is another popular method, where two or more substrates are used in the AD process, with the purpose to balance the C/N ratio of the feedstock. An example for this process can be given by giant cane in combination with pig slurry, co-digested in a continuously stirred tank lab scale reactor [10]. Substrate properties influence the efficiency and stability of the anaerobic process used for obtaining biogas. Moreover, its composition affects the quality and the quantity of the gas obtained.
Since the desire and the necessity to reduce global warming, scientists have studied different types of alternative sources of energies than can be applied, thus mentioning that LC biomass can be of great interest due to the fact that it is the most abundant organic source (forest residues, industrial activities, agricultural activities, or energy crops) [11].
The LC biomass is composed from three major fractions—cellulose, hemicellulose, and lignin. The structure formed by these three major components is resistant to lignocellulosic material bioconversion and that is why a pretreatment process is used to disintegrate the composition in order to obtain a higher biogas production rate.
2.1. Lignocellulosic Biomass Composition
The two main carbohydrate polymers that can be found in the composition of LC biomass are, cellulose and hemicellulose, and lignin, but also other elements found in smaller percentages like other carbohydrates, ash, pectin, and proteins. Given the fact that lignocellulosic material is the principal component of plant cell walls, it is considered an abundant organic source in the world. The average amount of cellulose, hemicellulose, and lignin in most LC biomass is 30 to 60% cellulose, 20 to 40% hemicellulose, and 15 to 25% lignin, but these percentages vary for different materials [12].
There are also studies that give different percentage intervals for lignocellulose biomass composition, such as hemicellulose 10–40%, lignin 5–25%, and cellulose 40–80% [13].
2.2. Cellulose
As part of the lignocellulosic material, cellulose is a polymer, type polysaccharide of glucose disaccharide units, with cellobiose tightly connected by b-1, 4-glycoside bonds [14]. Cellulose is also the principal compound of plant cell walls, making it an abundant source of organic compound on earth [15]. Hydrogen bons link the molecules of cellulose with different orientations, leading to diverse levels of crystallinity, which has an important part in the biodegradation of cellulose, with a higher crystallinity leading to problems in the process [7].
2.3. Hemicellulose
Hemicellulose is branched heterogeneous polymer of diverse polysaccharides like pentoses, hexoses, and sugar acids [14]. Given its branched nature, it allows the formation of almost indestructible bonds with cellulose and lignin, increasing lignocellulosic material rigidity [16].
2.4. Lignin
The second abundant compound of LC biomass on earth is lignin. Lignin is made out of phenylpropane units. Lignin links cellulose and hemicellulose in order to form a solid three-dimensional structure of the plant cell wall [7].
2.5. Lignocellulose Structure
Looking at the structure of lignocellulosic matter, we can see that a skeleton is formed out of cellulose and by hemicellulose and lignin, similar to a matrix, and binding materials [17]. The high resistance of lignocellulose to biological degradation is linked to a high degree of refractory lignin presence [18]. An amorphous matrix material is formed by hemicellulose since it is non-covalently bonded to the external area of the surface of cellulose fibrils [19]. Even though it is the compound that has the weakest bond compound and it has a high chemical sensitivity, it plays a crucial role in strengthening the structure of lignocellulose. Lignin is a macromolecule that is crosslinked and relatively hydrophobic and aromatic, but it has a high resistance to biological degradation [20]. Cellulose is hydrophilic, because of the existence of groups R-OH, and internal hydrogen bonds, but not highly soluble in water because its large size [21]. To express better the composition of the lignocellulosic matter by high lightening the structure we added data that it is presented in Table 1. The chemical composition is mentioned for each structure cellulose, hemicellulose and lignin. Thus, the table below presents the composition of different lignocellulosic substrates.

Table 1.
Chemical composition of different lignocellulosic substrates. (Data from—see reference column).
AD is a well known method for converting organic materials into bioenergy, and its effectiveness and sustainability have been demonstrated by different research papers and industrial investigations [41]. The AD process uses microorganisms to degrade organic material and convert it to biogas. Degradation of lignocellulosic matter is dependent on enzymes produced by microorganisms. AD is separated in four main sequential steps: hydrolysis, acidogenesis, acetogenesis, and methanogenesis [42].
Even though AD is a proven method of converting organic matter into biomethane, the result was not the same in the case of LC biomass rigidity [16], due to the fact that LC biomass properties and composition inhibits the property of microorganisms and enzymes involved in AD to decompose the organicmaterial. This is because of lignin, which is like a rigid cover for cellulose and hemicellulose [43]. Multiple research papers have outlined the fact that greater lignin content in the organic fraction results in reduced biogas and methane yield [44]. Therefore, in order to have a productive AD process on lignocellulosic material, high in lignin content, different pretreatment steps are needed to expedite the hydrolysis of LC biomass, enhancing the biogas production.
To better illustrate the way LC biomass is structured, we can observe Figure 1. The molecules for lignin, cellulose, and hemicellulose are presented, as well as the three lignin monomers, coniferyl (G) alcohols, coumaryl (H) and sinapyl (S), and, with a detailed image of the crystalline cellulose.
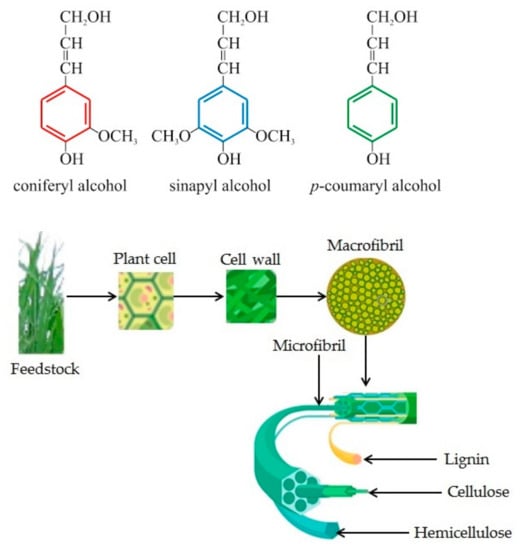
Figure 1.
Lignocellulosic biomass substrate structure, representing plant structure and lignin, cellulose, hemicellulose molecule and carbohydrate polymers and aromatic polymers of lignocellulosic biomass (adapted from [45,46]).
Using enzymes in the LC biomass pretreatment is a common way to increase biogas yield, but its effect depends on the enzymes being used, and also the structure of the treated biomass, given enzyme specificity [47]. As an example, there is an enzyme called laccase, derived from phenoloxidase, which works by catalyzing phenol, anilines, and aromatic thiols oxidation in the feedstock, leading to microbial growth and fermentation ability being enhanced in the AD [48].
Efficiency of different enzymes for the AD process depend on several factors, including substrate composition, incubationperiod, temperature, pH levels, and reactor design [49].
Barley shell, pearl millet husk, rice straw, wheat bran, and wheat straw where subjected to a xylanases enzyme that was synthesized from Aspergillus niger. The results reported a saccharification of approximately 34.5% for rice straw after 8 h of incubation [50].
BP uses a variety of microorganisms and enzymes to selectively degrade lignin and hemicelluloses, resulting in enhanced biomass saccharification and biomass yield [51]. The best microorganisms for BP are the species of white-rot fungi from the Basidiomycetes class. Phanerochaete chrysosporium gives maximum efficiency due to its high growth rate and lignin degradation capabilities [52]. Microorganisms from the natural environment (Mcons), like soil, cow dung, goat dung, etc., are used for pretreating lignocellulosic material, having the ability to degrade both cellulose and hemicellulose.
Zhang et al. [53] created thermophilic Mcons from different sources, such as thermophilic landfill and decaying straw. This mixture was mixed with distillery wastewater in order to pretreat cassava waste at 55 °C for 12 h, resulting in a 96% higher methane yield.
Most pretreatment methods for lignocellulosic AD processes require high amounts of energy and chemical input in order to properly work, but they also create detrimental impacts [54]. Compared to these methods, BP is highly economical regarding energy and chemical requirements, and also does not produce inhibitory byproducts. BP is realized by the external addition of microorganisms and/or industrial and lignolytic enzymes and cellulase, to break down the lignocellulosic components [55]. Due to the fact that microbial enzymes are efficient in degrading lignocellulosic compounds, BP is known as one of the best ways of degrading this type of biomass, even though the enzymatic reaction rate is very slow [56].
3. Biological Pretreatment
In many circumstances, the use of microorganisms (bacteria and fungi) is far more cost effective than the use of hydrolytic enzymes. Microorganisms are known for their great ability to convert high molecular weight compounds in the substrate into lower mass compounds that can enter the fermentation process. This process is due to the synthesis of microbial extracellular enzymes capable of breaking down the recalcitrant polymeric structures in the substrate. A large number of bacteria and fungi produce cellulosolytic, lignolytic, amylolytic, pectinolytic, proteolytic, lipolytic, and other enzymes, which provide their necessary nutrients.
BP based on the action of microorganisms involves both the synthesis of enzymes and the multiplication of decomposing microorganisms. Therefore, in this type of pretreatment, it is necessary to take into account the conditions of survival and growth of useful microorganisms, such as nutrients, inhibitors, temperature, oxygen concentration, pH, and others. Modification of each of these parameters can lead to major changes in the structure of the populations of microorganisms used in the decomposition of the substrate. In fact, these changes can be made depending on the desire and requirements of the biogas process. However, unlike the addition of enzymes, whose action is much faster, the use of microorganisms involves longer periods of time, stricter operating conditions, and the possibility of growth of unwanted microorganisms. The value of the generation time for the given conditions must be taken into account for each species. It is also known that the doubling time for bacteria is much shorter than for fungi, so the use of microorganisms should be done after prior studies.
3.1. Bacterial Pretreatment
In BP, bacteria can be used as an inoculum containing a single species, bacterial consortia, or in combination with fungi. Bacteria can come from different sources such as microbial cultures from previous fermentations, sludge, substrates with lignocellulosic structure, rumen fluid, and various agri-food by-products. They adapt relatively easily to new conditions and substrates and, after a longer or shorter lag phase, begin to multiply. Several bacteria species are known for their characteristics in alteration, solubilization, and degradation of lignin. The most representative species include Streptomycetes, Actinomycetes, Nocardia, and Eubacteria [57].
3.1.1. Microbial Consortia
Various studies have shown that a consortia of multi-species produce more active lignocellulosic enzyme complexes than single strain cultures, because of their higher stability to environmental conditions (temperature, pH, inhibitors, and others) and higher redundancy [58,59].
Ali et al. [60] isolated a microbial consortium from sawdust containing a mixture of lignocellulosic materials, using a serial dilution plating technique. The microbial consortium contained microorganisms with lignocellulolytic activity, among which the bacterial genera Citrobacter, Klebsiella, Exiguobacterium, Lactococcus, Micrococcus, and the yeasts Sugiyamaella and Vanrija were identified. The results showed that the treatment with these microorganisms (5 bacteria and 2 yeasts) for 10 days could significantly enhance the hydrolysis and biomethanation of sawdust, demonstrating the positive effect of the isolated microbial consortium [60].
In another study, in 2017, Ali et al. [58] isolated a bacterial consortium containing several strains of Bacillus, Pseudomonas, Streptomyces, and Staphylococcus, which were analyzed and quantified by the amplification of 16S rRNA, FISH (Fluorescence In Situ Hybridization), and real-time PCR techniques. The data obtained demonstrated that the hydrolysis of treated sawdust had a higher rate by the use of consortium [61]. In 2020, Ali et al. obtained and tested two new bacterial consortia, called CS-5 and BC-4, in order to increase the methane yield in AD concomitant hydrolysis of catalpa sawdust and chlorophenols (CPs). The authors obtained ninety-seven bacterial strains isolated from sawdust, and Eichhornia crassipes composting. The bacterial strains have been identified using the 16S rRNA technique [58]. The main strains of microorganisms used for the CS-5 aerobic consortium were Micrococcus luteus SR-1, Citrobacter freundii SR-3, Exiguobacterium acetylicum SR-5, Acidisoma tundrae alien SR-14, and Dyella sp. strain SR-16. For the anaerobic consortium BC-4, the strains were represented by Thermoanaerobacterium aciditolerans strain SR-4, Ruminococcus flavefaciens strain SR-7, Caproiciproducens galcititolivorans strain SR-8, and Methanobrevibacter thaueri strain SR-13. The use of the bacterial consortia resulted in a higher degree of hydrolysis of the lignocellulosic material in the substrate, and thus, the yield of methane increased by more than 60% [58].
For enhancing AD some researchers used bacterial consortium like the one contained Clostridium straminisolvens (CSK1), Clostridium sp. FG4b, Pseudoxanthomonas sp. strain M1-3, Brevibacilus sp. M1-5, and Bordetella sp. M1-6 [62]. The key bacterium for cellulose degradation is considered to be CSK1, an anaerobic cellulolytic bacterium [63]. In 2016, Yuan et al. [64] studied the possibility of enhancing AD of cotton stalk by BP, using a consortium of bacteria called MC1 comprised of thermophilic bacteria isolated from compost materials. The authors demonstrated that the pretreatment with the MC1 bacterial consortium was effective in improving biodegradability and enhancing methane production from cotton stalks. MC1 increased the sCOD (soluble Chemical Oxygen Demand) concentration of the hydrolysates, and decreased cellulose and hemicellulose concentration. The use of the MC1 consortium in biological treatment resulted in an increase of the production of biogas and methane from the cotton stalk compared to the untreated substrate [64].
In another study, the rotted silage maize straw was treated with the MC1 consortium and the results demonstrated that the carboxymethyl cellulase activities and the proportions of key strains in MC1 were the same in the treated and untreated substrate. This demonstrates that MC1 was resistant to microbial contamination in rotted maize straw [65].
In a bacterial pretreatment study, Sankaran et al. [57] showed that Cupriavidus basilensis B-8, removed the content of lignin with 41.5% and the content of carbon with 37.7% after 7 days of treatment, due to the depolymerization of lignin [57]. In another study, the corn straw was treated with a microbial consortium containing yeasts (Saccharomyces cerevisiae sp., Coccidioides immitis sp., and Hansenula anomala sp.), cellulolytic bacteria (Bacillus licheniformis sp., Bacillus subtilis sp., Pseudomonas sp.), the fungus Pleurotus florida sp., and the lactic acid bacteria Lactobacillus deiliehii sp. It was found that B. licheniformis sp., B. subtilis sp., and Pseudomonas sp. showed cellulolytic and hemicellulolytic activities, whereas the fungus P. florida sp. produced ligninases. Yeasts metabolize the available sugars produced by hydrolysis, for multiplication and biomass accumulation. L. deiliehii sp. maintains the pH at optimal values for other microorganisms. The treated corn straw yielded a higher production of biogas (with 33.07%) [66].
Suksong et al. [67] studied in 2019 the use of thermotolerant cellulolytic bacterial consortia containing Clostridiaceae isolated from digestate sludge and Lachnospiraceae isolated from cow manure. The AD process and the production of biogas from oil palm empty fruit bunches was increased by a maximum of 3–11-fold.
The effect of using lignocellulose-degrading microbial consortia isolated from horse manure and decomposed wood was studied by Tantayotai et al. The results of the research to obtain biogas from rice straw showed that microorganisms produce endo-β-glucanase, exo-β-glucanase, and β-glucosidase, and these microbial consortia enhanced the biogas production by 4.21 and 6.20 times, respectively, compared to the control [68].
Vervaeren et al. [69] observed the effect of several biological silage additives on biogas production using corn as a substrate. Several additives containing lactic acid bacteria, yeasts, fungi, and enzymes were tested. Bonsilage Mais (Lactosan, Ringe, Denmark) and Silasil Energy (Lactosan) have both homo- and hetero-fermentative bacterial populations. Sil-All 4 4 (Alltech, Nicholasville, KY, USA) contains homo- and hetero-fermentative lactic acid bacteria and four enzymes (amylase, cellulase, hemicellulase, and pentosanase). Microferm (EM-Agriton) contains mixed populations of lactic acid bacteria, yeasts, and fungi. The results obtained demonstrated that the addition of bioactive compounds, such as yeasts and enzymes for ensiling, improves storage and AD processes.
In 2019, Zhao et al. [70] demonstrated that BP enhances the hydrolytic ability of microbial populations and the production of biogas during AD. The corn stover was pretreated with a microbial consortium (BYND-9), which led to an increase of 62.85% compared to the control. Methanosarcina (5.21%) was the predominant archaea in the untreated stover, and Methanosaeta (10.82%) was the most active in the BP. This is relevant, because of methanogenic archaea, Methanosaeta characterized by the property of capturing acetic acid, can significantly increase the methane production [70].
3.1.2. Fluid Rumen Bacteria
The complex rumen microbial communities contain seven predominant genera, such as Prevotella, Butyrivibrio, Ruminococcus, and the unclassified Lachnospiraceae, Ruminococcaceae, Bacteroidales, and Clostridiales. The next-generation sequencing technology is often used to demonstrate the properties of these microorganisms [71].
These bacteria grow rapidly on the lignocellulose inside the rumen, and secrete digestive enzymes at the same time, such as cellulase, hemicellulase, and β-glycosidase. In this way, rumen microorganisms degrade cellulose, hemicellulose, and pectin into monosaccharide, which could be gradually degraded into volatile fatty acids (VFAs) [72].
Takizawa et al. [73] studied the effects of pretreatment with rumen fluid on biogas production from paper sludge. Experiments showed that the amount of methane was 3.4 times higher than that from untreated substrate. Rumen microorganisms produce enzymes that degrade lignocellulosic material in the paper sludge, especially acid detergent lignin, and the values of the dissolved COD (Chemical Oxygen Demand) and VFAs were higher.
Other research has shown that pretreatment of corn stover with rumen fluid improved the hydrolysis of lignocellulosic material and the concentration of soluble COD was several times higher, and the structure of corn stover changed. It demonstrated the increase of specific surface area and the minimization of crystallinity degree. The authors concluded that the pretreatment efficiency is due to the participation of the enzymes secreted by rumen microorganisms [72].
Jin et al. [74] investigated the effect of rumen cultures on anaerobic co-digestion of corn straw with pig manure. Cellulase and xylanase activities of fermenting fluid confirmed the effect of rumen microorganisms. It was found that there was a remarkable improvement on anaerobic acidification and co-digestion of corn straw with pig manure using rumen microorganisms.
In 2017, Baba et al. [75] used cattle rumen fluid, considered as slaughterhouse waste. They demonstrated that the pretreatment of rapeseed (Brassica napus L.) with rumen fluid, produced 1.5 times more methane compared to untreated substrate. The bacterial population was analyzed with the MiSeq next-generation sequencer. It was found that the predominant bacterial populations belong, in the first stages, to phylum Bacteroidetes, containing Prevotella spp., capable of hydrolyzing starch, and, after 6 h, the phylum Firmicutes, containing Ruminococcus spp., becomes predominant. The authors detected in the treated substrate, 7 cellulolytic, 25 cello-oligosaccharolytic, and 11 xylanolytic bacteria.
Lee et al. [76] analyzed the cellulase and hemicellulase-producing bacteria using cow rumen fluid to enhance the biogas production and Clostridia (e.g., Ruminococcus and Clostridium) were identified with the metagenomic analysis as the major populations in this system. The number of Prevotella and Fibrobacter decreased after the treatment with rumen fluid. In addition, Calicellulosiruptor had the main cellulolytic activity in this process. This research showed that the composition of cellulolytic bacteria in the pretreatment process differs significantly from those in the rumen.
Other experiments were conducted to study the effect of microorganisms of cow rumen in improving the biodegradation of substrate represented by wheat straw, as can be seen in Figure 2. The experiments were conducted for three months and it was shown that members of the Clostridiales order and Basidiomycota phylum were found to be the dominant lignocellulolytic bacteria and fungi, respectively [71].
Figure 2.
Lignocellulosic metabolic pathways of the rumen microorganisms during rumen fermentation (adapted from [71]).
Other studies on the microbial and biotechnological benefits of rumen liquid used to degrade LC biomass in biogas plants have been performed by Nagler et al. [77], who showed that rumen liquid addition is a promising strategy for enhanced and accelerated exploitation of lignocellulose-rich biomass for biomethanization.
Takizawa et al. [78] studied the effect of conditions of rumen liquid preservation at various temperatures, on the pretreatment of waste paper, and demonstrated that the temperature of 4 °C was optimal for protozoa and lignocellulolytic enzymes.
3.1.3. Single Strain Bacterial Cultures
A two-stage system of AD using a recombinant Bacillus megaterium strain with keratinolytic properties has been studied for the BP of chicken feather waste. The authors state that one day treatment with this strain resulted in 155% of methane production of untreated feathers compared to an untreated control [79].
Yadav et al. [80] studied the biogas production potential of straw treated only with fungal species, compared to a complex treatment with fungi and the chitinolytic bacterium Bacillus subtilis. They showed that there was a 46 and 51% increase in methane production in the two treatment cases studied (fungal and complex treatment).
3.1.4. Pretreatment of Microalgae and Water Plants with Bacteria
In addition to lignocellulosic biomass, microalgae can also be used in the process of AD. It is known that the recalcitrant substrate represented by the microalgal biomass containing a large amount of cellulose complicates the process of AD. Kavitha et al. [81] isolated, by a method of screening on carboxymethyl cellulose agar (CMC) medium, four morphologically different cellulase-secreting bacteria, belonging to the genus Bacillus. The authors stated that the bacterially pretreated microalgae showed superior biodegradability than did the control.
For the pretreatment of microalgae, there are generally used hydrolytic bacteria, capable of breaking the cell wall structures, such as polysaccharides, proteins, other biopolymers, and calcified compounds [82,83]. For example, Bacillus licheniformis bacteria have been used to break the thick cell wall of Chlorella sp., in a study for biogas improvement. The results of this research demonstrated that the methane yield was enhanced by 22.7% [57,84].
He et al. [84] studied the production of methane from microalgae Chlorella sp. by anaerobic bio-pretreatment using the hydrolytic and acidogenic facultative anaerobic bacterium Bacillus licheniformis. The results revealed that the soluble chemical oxygen demand was 16.4–43.4% higher, the concentration of volatile fatty acids (VFAs) increased by 17.3–44.2%, and the methane production was enhanced (9.2–22.7%).
Microorganisms are also used for the pretreatment of water hyacinth (Eichhornia crassipes), a freshwater lignocellulosic floating weed that is found in the aquatic ecosystem, characterized by incessant reproductive potential. Barua et al. isolated Bordetella muralis VKVVG5, Citrobacter werkmanii VKVVG4, and Paenibacillus sp. VKVVG1 from various sources and tested these bacteria for hydrolysis of water hyacinth. The most active bacterial species was Citrobacter werkmanii VKVVG4, which led to a 3 times increase in the cumulative biogas generation of the microbial pretreated water hyacinth [85].
In another study, Barua et al. [86] found that the ideal food to microorganism (F/M) ratio for biogas production from water hyacinth was 1.5. After the pretreatment of the substrate with the bacterium Citrobacter werkmanii VKVVG4 with a concentration of 109 CFU/mL for 4 days, the biogas production increased to the value of 156 ± 11 mL CH4/g VS in a 1 L anaerobic reactor, for the optimal F/M ratio.
3.1.5. Selection of Bacteria by Thermophilic Pretreatment
The selection of microbial communities that increase biogas production can be made indirectly, even from pre-existing microorganisms in the substrate, by applying a heat pretreatment. Tapadia-Maheshwari et al. [87] performed a study in a reactor containing particulate rice straw, using cattle dung slurry as inoculum, and demonstrated that the methane yield increased to 274 mL g−1 volatile solids. The authors chose the optimal conditions for AD at 37 °C, pH-7, with addition of urea and zinc as trace elements, at 21 days HRT (Hydraulic Retention Time). These optimal conditions have determined the selection of bacteria from the genera Clostridium, Bacteroides, and Ruminococcus as the dominant hydrolytic bacteria, and Methanosarcina as the methanogen. It was shown that the thermophilic microaerobic pretreatment (TMP) of corn straw can have the effect of increasing the hydrolytic activity of microorganisms in the AD process. The proportion of Bacilli class (belongs to phylum Firmicutes) in TMP was 124.89% higher than that of thermophilic treatment under an anaerobic condition, which determines an improved AD performance of biogas production [88].
In the last period, numerous microorganisms are used within pretreatment for anaerobic fermentation; these include naturally occurring organisms, laboratory selected mutants, or even genetically modified organisms (GMOs). Genetic engineering plays a major part in all aspects of biotechnology and also in biofuel production. Current research is known to use u the power of genetic engineering to improve the biogas production. This is obtained by manipulating genes in specific pathways and/or incorporating specific DNA fragments into target species, although the focus of research is on genetic engineering in enzyme production [89].
3.2. Fungal Pretreatment
Lately, BP applied to lignocellulosic substrate for improvement of biogas generation was concentrated on the use of fungal treatment, bacterial consortium, and enzymatic treatments [7,90].
Fungal pretreatment improves degradation of lignin and hemicellulose, which is important for the AD process [4]. Different fungi classes were tested for pretreatment of LC biomass decomposition in order to increase the biogas yield. These classes include the ascomycetes (e.g., Trichoderma reesei) and basidiomycetes species, that are grouped into white-, brown-, and soft-rot fungi. In addition, some anaerobic genera (e.g., Orpinomyces sp.) were discovered, having the capacity to decompose cellulose in the tracts of ruminants [90,91].
It was found that the basidiomycetes white-rot fungi are the most efficient between all fungal genera for delignification process. White-rot fungi differ according to lignin decomposition ways, identified as selective and non-selective lignin decomposition. In the selective one, lignin and hemicellulose are mainly broken, at the same time using a low quantity of cellulose. For non-selective delignification, lignin, hemicellulose, and cellulose are broken unevenly [4,7,90,92].
Sanchez [93] reported that fungi present two categories of extracellular enzymatic structures—the hydrolytic structure, which generates hydrolases that have the ability to decompose polysaccharide, and an extracellular ligninolytic structure, which breaks lignin and unlocks phenyl groups. The ligninolytic system of white-rot fungi comprises three main oxidizing enzymes: lignin peroxidase, manganese peroxidase, and laccase (or phenol oxidase). It must be mentioned that the specified enzymes are not generated from all white-rot fungi [91,94,95].
White-rot fungi are able to degrade lignin, synthesizing a group of extracellular ligninolytic enzymes, having no inhibitors for the AD process and biogas production. Their unique enzymatic structure allows them to break down the phenolic groups and to convert lignin into carbon dioxide. Lignin biodegradation by white-rot fungi is an oxidative reaction catalyzed mainly by laccases [93,95].
The most used species of white-rot fungi for biomass treatment before AD process contain Phanerochaete chrysosporium, Pleurotus ostreatus, Trametes versicolor, Flammulina velutipes, Ceriporiopsis subvermispora, Streptomyces viridosporus, and Trichoderma viride [96,97].
Compared with the white-rot fungi, the brown-rot fungi class uses enzymes to degrade preferentially pectin and cellulose, followed by hemicellulose, with minimal removal of lignin [94,96].
LC biomass degradation by soft-rot fungi is not well known. However, some soft-rot fungi are able to decompose lignin, due to the fact that they degrade the secondary cell wall and reduce the bulk of Klason lignin in angiosperm wood [93].
The majority of fungi produce enzymes such as cellobiose dehydrogenase, aryl alcohol oxidases, glucose oxidase, glyoxal oxidase, copper oxidase, and hydrolytic enzymes, leading to cellulose, hemicellulose, and lignin decomposition [13,98]. Fungi are frequently isolated from the soil, different plants, or agricultural residues [3].
Throughout fungal pretreatment, the humidity level represents one of the main parameters. Water is important in nutrient transport, but too much water could inhibit the fungi growing by reducing the available oxygen [99].
Several studies showed that white-rot fungal pretreatment of lignocellulosic substrate used in AD led to biogas production improvement. Furthermore, it was observed that there was a higher methane content in the biogas mixture.
Mustafa et al. [100] used Pleurotus ostreatus and Trichoderma reesei for straw pretreatment in order to increase its decomposition and methane yield. They reported that humidity level and incubation period greatly influenced the performance of fungal pretreatment. Fungal pretreatment by P. ostreatus led to a significant degradation of lignin and hemicellulose. Lignin removal was about 33.4% at 75% humidity level and 20 days incubation period, and a 120% enhancement in methane production versus unprocessed rice straw was observed. For T. reesei treatment in the same conditions, lignin removal was 23.6%, and a 78.3% enhancement in methane production.
Zhao et al. [99] investigated the fungal pretreatment result of garden waste on methane generation. Garden wastes were subjected to treatment with Ceriporiopsis subvermispora, a white-rot fungus that decomposes lignin. It was proved that fungal pretreatment enhanced the methane production of garden waste by 85–154%, versus untreated garden waste. Lignin decomposition increased from 0% for the initial substrate to about 21%, and the methane production was increased by 154%.
The sugarcane bagasse was pretreated with three wood–decay fungi—the brown-rot fungus Laetiporus sulphureus, the white-rot fungus Pleurotus ostreatus, and selective white-rot fungus Ceriporiopsis subvermispora for periods varying from 7 to 60 days [101]. After 60 days of biotreatment, P. ostreatus degraded all the sugarcane bagasse components to a similar extent. Glucan, xylan, and lignin losses reached 8.4, 15.7, and 11.1%, respectively. Selective white-rot C. subvermispora provided the most efficient pretreatment of sugarcane bagasse even if glucan losses were minimal, but lignin and xylan losses reached 48% and 47% at the end of pretreatment.
Bioaugmentation with Piromyces rhizinflata YM600 in an anaerobic two-stage system for biogas production using as substrate corn silage and cattail, was studied for a period of 60 days by Nkemka et al. [102]. They concluded that bioaugmentation with P. rhizinflata YM600 did not improve the overall methane production of corn silage and cattail but improved the volatile fatty acid degradation rate. This is beneficial in avoiding volatile fatty acid accumulation and inhibition in AD processes.
Wood fiber, grass, corn stalks, and wheat straw were pretreated for 21 days using Phanerochaete flavido-alba fungus in order to enhance their AD [103]. Pretreatment of lignocellulosic substrates with P. flavido-alba led to a decrease in all lignocellulose segments at different levels in each substrate. In grass, lignin remained unmodified but observed a decrease by 20% in the other tested substrates. Biogas generation was increased just in wood fiber, recording 124 NL biogas kg−1 dry wood fiber with 64% methane, after 21 days of AD.
Tisma et al. [104] investigated corn silage pretreatment with white-rot fungus Trametes versicolor for biogas production [104]. The pretreated whole-plant corn silage was used as substrate in anaerobic co-digestion with cow manure for biogas production. The methane content of the obtained biogas (0.236 ) was higher using corn silage treated with T. versicolor as substrate instead of ensiled corn silage and corn grits (0.167 ).
Fungal strain Curvularia lunata was used for BP optimization of wheat and pearl millet for biogas generation [105]. The ideal conditions were achieved to be 32 °C, 65% humidity level, and 23 days of treatment period for Curvularia lunata. The authors certified that Curvularia lunata has the capacity to secrete laccase. After the biological optimized treatment with Curvularia lunata of wheat straw, the biogas production grew from 449 to 533 mL/gVS, whereas methane yield grew from 274 to 336 mL/gVS. For pearl millet straw, the biogas production grew from 360 to 463 mL/gVS, whereas methane yield grew from 220 to 305 mL/gVS after optimized treatment.
White-rot fungi Trametes versicolor used for pretreatment of cereal crops and cow manure enhance AD efficiency; the methane production being improved by 10 to 18% and cellulose decomposition up to 80% [106].
Yildirim et al. [107] studied the effect of anaerobic rumen fungi mixture (Orpinomyces sp., Piromyces sp., Anaeromyces sp., and Neocallimastix frontalis) on biogas and biomethane production. Cow manure was used as substrate in an anaerobic reactor. The results showed that the substrate pretreatment with anaerobic rumen fungi in a concentration of 15% increased the biogas production after 40 days with about 5500 mL/d, compared with the control reactor (1500 mL/d).
The influence of the rice straw fungal pretreatment with Pleurotus ostreatus, Phanerochaete chrysosporium, and Ganoderma lucidum on the methane production resulted from AD was evaluated by Kainthola et al. [108]. Phanerochaete chrysosporium fungal strain was the most efficient in the rice straw pretreatment. Maximum lignocellulosic biodegradation was 36% more than an untreated sample and the maximum methane production was 339.31 mL/g VS added after 35 days of incubation.
The enzyme secreted by Trichoderma viride and Aspergillus sp. were tested for maize straw pretreatment with the purpose of AD process enhancement. The methane yield resulted from mixed enzyme pretreatment was 31.74% higher than the control [109].
Fang et al. [110] pretreated raw and oyster champost with fungal strains Trametes versicolor and Pleurotus sajor-caju for improvement of fermentative volatile fatty acid generation. Pleurotus sajor-caju was the most effective to raw champost on selectively degrading lignin and improved volatile fatty acid production.
White-rot fungi can produce ligninolytic enzymes to decompose lignin even if the drawback is that the rate of decomposition is slow [90]. Lignocellulosic fungal pretreatment used for the AD process may take weeks or months, depending on the rate of the fungal growth and on the substrate composition [95]. Thus, using fungal pretreatment with other pretreatment techniques could reduce the time required for the AD process [111].
Ali and Sun [112] investigated the results of chemical and physical methods followed by fungal treatment by Aspergillus terreus and Trichoderma viride for park wastes and cattle dung in order to enhance the biogas generation. The authors concluded that the biogas production was improved (by 22.7%) by chemical and biological feedstock pretreatment (125.9 L/Kg VS) versus untreated feedstock (102.6 L/Kg VS). Moreover, the methane production was improved by 30% after the use of fungal and alkaline pretreatment (2.5% NaOH combined with 2.5% NH4OH).
Willow sawdust was pretreated with the white-rot fungi Leiotrametes menziesii and Abortiporus biennis for methane enhancement [113]. The authors concluded that L. menziesii was more efficient in delignification than A. biennis; removal of lignin after 30 days for L. menziesii was 30.5%, while for A. biennis was 17.1%. On the other hand, biochemical methane potential was increased for the A. biennis pretreatment by 43%, for 30 days cultivation, while fungal treatment with L. menziesii did not enhance methane potential. A combination of chemicals, that are a part of BP, led to high lignin decomposition for both fungi. The maximum biochemical methane potential was obtained for the alkaline combined with A. biennis pretreatment.
Physical (milling) and biological (incubation with Pleurotus ostreatus fungus) pretreatments were used for rice straw to enhance its decomposition and biogas generation during AD [114]. Combination of BP with Pleurotus ostreatus followed by milling prior to AD resulted in 30.4% lignin removal and the maximum methane yield of 258 L/kg VS.
3.3. Enzymatic Pretreatment
According to the literature, several pretreatment methods have been studied, such as physical, thermal, chemical, and biological pretreatment [16]. In recent years, one of the methods of biological pretreatment has begun to gain more and more interest due to its advantages, such as relatively short reaction time, but also because the inhibitors and microbial metabolism don’t affect the activity of enzymes [115], Ometto et al. [116] concluded that this type of pretreatment didn’t require expensive processing equipment, but the high cost of the enzyme it remains an economic challenge to develop industrial-scale biogas production [115,116].
Enzymatic pretreatment of LC biomass depends mainly on enzymes like hemicellulases and cellulases generated by microorganisms. In the first phase of AD, small and soluble monomers are produced through the decomposition of complex organic polymers, process performed using extracellular enzymes [7,16]. Specifically, enzymatic hydrolysis consists in the conversion of cellulose into glucose by fungal activity, and by enzymes, namely cellulases, which act by breaking the glycosidic bonds of cellulose microfibrils, generating the release of glucose, representing the ideal substrate for microorganism fermentation [117]. The influence of enzymes on LC biomass depends on the type of enzymes used, but also on the composition of the treated biomass [47].
The processes widely used for the production of microbial enzymes are solid state fermentation (SSF) and submerged fermentation (SF); these methods being used for the production of enzymes, such as proteases, pectinases, and cellulases. SF has advantages in easy enzyme recovery, in process control, and it has a well-established technological base to extend processes to industrial production efficiency [118].
3.3.1. Cellulase and Laccase
Out of more than forty enzymes tested in the biological pretreatment, cellulase is the most used one, followed by β-glucosidase and xylanase.
Cellulase is the essential enzyme of potential use for industrial saccharification of cellulosic materials into elementary sugars. It consists of an association of hydrolytic enzymes which hydrolyze the β-glycosidic bonds of native cellulose and related cello-oligosaccharides, as shown in Figure 3. Although a considerable number of microorganisms (fungi, bacteria, and actinomycetes) are able to degrade cellulose, only a few of them generate considerable volume of cell-free enzyme fractions able to achieve hydrolysis of cellulose in vitro. The most important cellulase producers include species such as Trichoderma, Aspergillus, T. viride, A. niger, and Penicillium [119]. It is demonstrated that cellulase obtained from Trichoderma reesei is reconized for its ability to reduce insoluble cellulose, which exists in considerable portions of LC biomass [16].
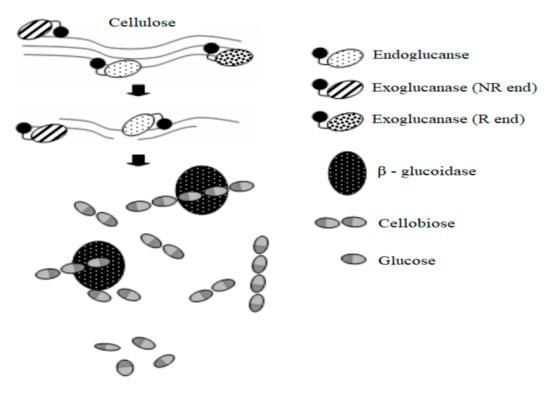
Figure 3.
Mechanism of cellulase action (data from [120]).
Enzyme pretreatment has proven to be an interesting approach in order to achieve increased biomethane yield. In this sense, considerable enzymatic pretreatment techniques have been studied. Zieminski et al. [121] studied the influence of enzymatic pretreatment of sugar beet pulp and spent hops prior to methane fermentation. Hydrolysis of the substrate for 24 h with a mixture of enzyme preparations Celustar XL and Agropect, with endoglucanase, xylanase, and pectinase activities proved to be the most effective, the source of the enzyme being Trichoderma longibrachiatum. The results showed that the biogas production was increased by the enzymatic treatment by 19% and 13%, respectively (versus relevant controls). The highest biogas yield was generated by sugar beet pulp. Silva et al. [122] investigated the cellulase production by Aspergillus japonicus URM5620 and its use as enzymatic pretreatment on passion fruit peel waste (Passiflora edulis) in order to simplify the AD of biogas production. The authors used SF as the method of cellulase production, analyzing the concentration of substrate and glucose. The highest activities were obtained at 3% substrate and 1% glucose. After 15 days, the biogas production generated methane with a concentration of over 64%. The literature describes the ranging of methane from 57–73% for fruit waste [123]. Applying Novozyme products, Wang et al. [124] showed that pretreatment of a mix of cow manure and corn straw by a cellulase blend at temperature of 55 °C for 18 h allowed a 103% increase in the methane yield.
Another enzyme used and known as laccase, is generated from phenoloxidase that contains numerous copper ions and works by catalyzing the oxidation of phenols, anilines, and aromatic thiols in the substrate. The efficiency of the AD process consists in improving the efficiency of fermentation and microbial multiplication [48]. Laccases are widely found in nature and are the first and most investigated enzymatic systems [125]. Laccases contain four copper atoms (Cu), that are dispersed in three redox sites (named T1, T2, and T3). The initial phase of the catalytic cycle (Figure 4) is the reduction of the lignin substrate electrons with Cu at the T1 site, producing a free radical. The electrons are next removed to the T2/T3 trinuclear site resulting in the conversion of the resting enzyme to a fully reduced state [126].
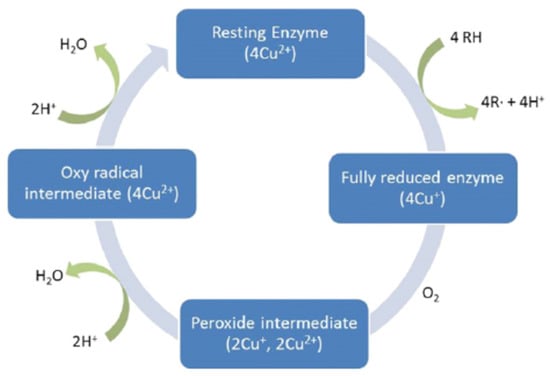
Figure 4.
Catalytic cycle of laccases (data from [126]).
Laccase is a part of the blue multicopper oxidases and catalyzes the cross-linking of monomers, reduction of polymers, and ring cleavage of aromatic compounds. It is located especially in plants (cabbages, turnip, potatoes, pears, apples), in some bacteria (S. lavendulae, S. cyaneus, Marinomonas mediterranea), in white-rot fungi like Phlebia radiata, Pleurotus ostreatus, and Trametes versicolor (which degrades lignin), but also in other fungi such as Ascomycetes, Deuteromycetes, and Basidiomycetes [125]. In plants, laccase is implicated in lignifications, while in fungi it is involved in degradation of lignin, sporulation, pigment biosynthesis, reproducing body formation and plant pathogenesis [127]. The laccases production depends on carbon source, nitrogen source, induction of laccase, pH, temperature, and cultivation type [128].
In recent decades, these enzymes have received special attention among researchers due to their efficiency to oxidize lignin-related phenolic and non-phenolic compounds and highly recalcitrant environmental pollutants, making them profitable for the utilization of different biotechnological processes [129]. Kudanga et al. [130] showed that laccase increase fermentability of lignocellulosic materials mainly through lignin degradation. Schroyen et al. [131] studied the influence of pretreatment with different enzymes (laccase, manganese peroxidase, and versatile peroxidase) at different incubation times (0, 6, and 24 h) on corn stover degradation and biogas production. The results showed that the pretreatments did not produce high concentrations of phenolic compounds—inhibitors of biogas production. The best results were obtained using laccase, which gave an increase of 25% compared after 24 h incubation, followed by pretreatment with peroxidase, which gave an increase in biomethane production by 17% [131].
3.3.2. Other Enzymes
Howard et al. [132] obtained that the oxidative enzymes generated by white-rot fungi are lignin peroxidase (LiP), manganese peroxidase (MnP) and laccase. The advantage of LiP, compared to the others, is that it has a great redox potential due to its heme pocket architecture where Fe (II) is pentacoordinated to the four heme tetrapyrrole nitrogens and a histidine residue. According to the catalytic cycle of LiP, as shown in Figure 5, compound I is produced by the oxidation reaction catalyzed by the enzyme with H2O2, where iron occure as Fe (IV) and a free radical is on the tetrapyrrole ring. After that, lignin substrate is oxidized by compound I with an electron to make compound II and a free radical substrate (there is no radical on the tetrapyrrole ring). Finally, compound II releases free radicals from lignin substrate and oxidizes another molecule [126].
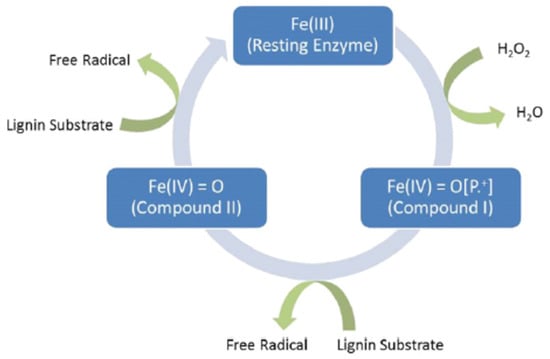
Figure 5.
Catalytic cycle of LiP (data from [126]).
Only a few white-rot fungi synthesize LiP, but most of them generate MnP. This enzyme is also a strong oxidant, and the only difference observed by Hammel and Cullen [133] is that the nonphenolic lignin-related compounds could not be oxidized by LiP. These catalytic reactions of MnP are similar as for LiP, except that Mn (II) is necessary to close the cycle, according to Figure 6 [126].
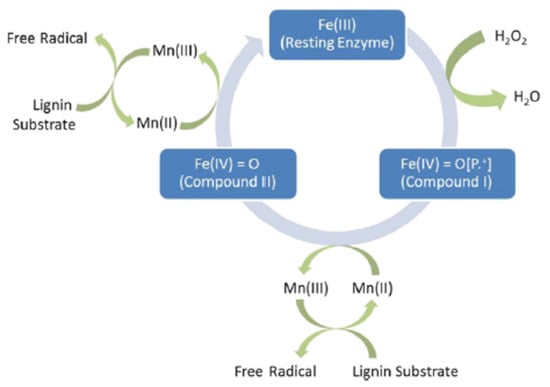
Figure 6.
Catalytic cycle of MnP (data from [126]).
In research made by Frigon et al. [11] with switch grass, LiP and MnP was used with loadings of 20 and 40 U/g, respectively, and obtained an increase of 29% and 42% in biomethane generation. Martinez et al. [134] described LiP and MnP as true degraders of lignin because of their increased redox potential. Another study made by Jayasinghe et al. [135] evaluated the efficiency of various peroxidase enzymes on methane production. The results showed that MnP presented the best efficiency in the methane yield obtained. The same results were reported by Frigon et al. [11]
Another enzyme used for enhanced biogas formation is lipase. Lipases are some enzymes that have the property to catalyze the hydrolysis of long chain triglycerides [136]. Lipases have been discovered since 1901 in Bacillus prodigiosus, B. pyocyaneus, and B. fluorescens, known later as Serratia marcescens, Pseudomonas aeruginosa, and Pseudomonas fluorescens, respectively [137]. Lipases extracted from microbial origin are recognized as thermostable and are known to perform biochemical conversions such as hydrolysis, esterification, alcoholysis, acidolysis, and aminolysis. According to studies [138,139], lipid elimination improved biogas formation by 15 to 93%. That can be associated with the increase in free fatty acids that can be metabolized by anaerobic bacteria. Kameswari et al. investigated the enhancement of biogas production using lipase in the co-digestion of tannery solid wastes. The optimal dose of lipase was set at 0.75 g, with the digestion period decreasing from 42 to 29 days. The results showed an increase of 15% compared to the process without adding lipase [140].
Amylase is the fundamental enzyme in catalyzing the hydrolysis of amylopectin and amylose. Xie et al. [141] studied the impact of amylase on biogas production and obtained a 21% increase at a temperature of 35 °C for 300 h.
The results of the studies showed that the addition of proteases and glycosidases lead to a significant increase in biogas production compared to an untreated control. Better results were obtained in the applicability of proteases from B. licheniformis and A. oryzae, the glycosidase SCO6604 and lysozyme compared to other enzymes such as (BCE_2078 and CTec 2) [142]. Muller et al. [143] studied the effect of some proteases (alkaline, serine, and aspartic types) on a mix of corn silage, chicken manure, and cow dung in a 2 L BMP (bio-methane potential) test. The results showed that the methane yield was increased from 9 to 52%. In another study, the authors added a mix of pectate lyases and cellulases in equal proportion (w/w) on dairy cattle manure. After three days of pretreatment at temperature of 50 °C followed by an AD, it was obtained a 4.5% increase in methane yield [144].
Table 2 reports the results of some researches on enzymatic hydrolysis conditions of LC biomass for improved biogas generation by the AD method.

Table 2.
Enzymatic pretreatment conditions of lignocellulosic (LC) biomass used for biogas enhancement (data from–see reference column).
4. Conclusions
BP using microorganisms and enzymes seems to have a higher effect and to be the least expensive treatment applied and tested to LC biomass in the biogas production process.
Microorganisms are known for their great ability to convert high molecular weight compounds in the substrate into lower mass compounds that can enter the AD process. This process is due to the synthesis of microbial extracellular enzymes capable of breaking down the recalcitrant polymeric structures in the substrate. Many bacteria and fungi produce cellulosolytic, lignolytic, amylolytic, pectinolytic, proteolytic, lipolytic, and other enzymes, which provide their necessary nutrients.
Bacterial consortia, single bacterial cultures, fluid rumen, and others can be used for BP of the substrate.
Fungal pretreatment with white-rot fungi is the most commonly investigated method considering their capacity to delignify biomass.
Enzymatic pretreatment of LC biomass depends mainly on enzymes like cellulases and hemicellulases synthesized by microorganisms. The action of enzymes on LC biomass depends on the class of enzymes used, but also on the composition of the treated biomass.
Thus, microbiological and enzymatic pretreatment represents a sustainable pretreatment strategy for clean energy production since they are the most effective and low cost methods for biogas enhancement.
Enzymes bring a wide range of processing benefits by improving the efficiency of biogas production through reduction of production time, replacing chemical or physical treatments, reducing energy consumption, and less waste.
In the future, increasing the efficiency of enzymatic preparations as well as the capacity of microorganisms to transform the substrate, could be achieved by selecting new highly productive microbial strains and using molecular genetic techniques. The microbial diversity can be explored to produce enzymes with higher catalytic efficiency, a large range of substrate profiles, and improved stability to higher temperature and inhibitors. For the industrially profitable production of enzymes and maximum product yield, new enzymes (including even artificial enzymes) can be studied and obtained through advanced biotechnologies.
Author Contributions
Conceptualization, M.F. and G.P.; methodology, M.F. and G.P.; validation, M.F.; formal analysis, G.M.; investigation, M.N.D.; data curation, B.Ș.Z.; writing—original draft preparation, M.N.D., G.M., and B.Ș.Z.; writing—review and editing, M.F., M.N.D., and B.Ș.Z.; visualization, G.P. and M.F.; supervision, G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sawatdeenarunat, C.; Surendra, K.C.; Takara, D.; Oechsner, H.; Kumar, S. Anaerobic digestion of lignocellulosic biomass: Challenges and opportunities. Bioresour. Technol. 2015, 178, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Calderon, C.; Colla, M.; Jossart, J.M. Statistical Report 2020–Report biogas. Bioenergy Eur. 2020, 1–17. Available online: https://bioenergyeurope.org/statistical-report.html (accessed on 18 July 2020).
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Karuppiah, T.; Azariah, V.E. Biomass pretreatment for enhancement of biogas production. In Anaerobic Digestion; Rajesh Banu, J., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Carrere, H.; Antonopoulou, G.; Affes, R.; Passos, F.; Battimelli, A.; Lyberatos, G.; Ferrer, I. Review of feedstock pretreatment strategies for improved anaerobic digestion: From lab-scale research to full-scale application. Bioresour. Technol. 2016, 199, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Suhag, M.; Dhaka, A. Augmented digestion of lignocellulose by steam explosion, acid and alkaline pretreatment methods: A review. Carbohydr. Polym. 2015, 117, 624–631. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy Combust. Sci. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Otto Wagner, A.; Lackner, N.; Mutschlechner, M.; Prem, E.M.; Markt, R.; Illmer, P. Biological pretreatment strategies for second-generation lignocellulosic resources to enhance biogas production. Energies 2018, 11, 1797. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, J.; Lee, D.J. Biogas from anaerobic digestion processes: Research updates. Renew. Energy 2016, 98, 108–119. [Google Scholar] [CrossRef]
- Luca, C.; Pilu, R.; Tambone, F.; Scaglia, B.; Adani, F. New energy crop giant cane (Arundo donax L.) can substitute traditional energy crops increasing biogas yield and reducing costs. Bioresour. Technol. 2015, 191, 197–204. [Google Scholar] [CrossRef]
- Frigon, J.C.; Mehta, P.; Guiot, S.R. Impact of mechanical, chemical and enzymatic pretreatments on the methane yield from the anaerobic digestion of switchgrass. Biomass Bioenerg. 2012, 36, 1–11. [Google Scholar] [CrossRef]
- Nanda, S.; Azargohar, R.; Dalai, A.K.; Kozinski, J.A. An assessment on the sustainability of lignocellulosic biomass for biorefining. Renew. Sustain. Energy Rev. 2015, 50, 925–941. [Google Scholar] [CrossRef]
- Hernández-Beltrán, J.U.; Hernández-De Lira, I.O.; Cruz-Santos, M.M.; Saucedo-Luevanos, A.; Hernández-Terán, F.; Balagurusamy, N. Insight into Pretreatment Methods of Lignocellulosic Biomass to Increase Biogas Yield: Current State, Challenges, and Opportunities. Appl. Sci. 2019, 9, 3721. [Google Scholar] [CrossRef]
- Hendriks, A.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Moglia, E.S. Enzymatic Pre-treatment of Cellulose Rich Biomasses for Use in the Biogas Process; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2008. [Google Scholar]
- Hosseini, K.E.; Dahadha, S.; Bazyar, L.A.A.; Azizi, A.; Elbeshbishy, E. Enzymatic pretreatment of lignocellulosic biomass for enhanced biomethane production-A review. J. Environ. Manag. 2019, 233, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Saini, J.K.; Saini, R.; Tewari, L. Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: Concepts and recent developments. 3 Biotech 2015, 5, 337–353. [Google Scholar] [CrossRef]
- Gao, J.; Chen, L.; Yuan, K.; Huang, H.; Yan, Z. Ionic liquid pretreatment to enhance the anaerobic digestion of lignocellulosic biomass. Bioresour. Technol. 2013, 150, 352–358. [Google Scholar] [CrossRef]
- Brandt, A.; Gräsvik, J.; Halletta, J.P.; Welton, T. Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem. 2013, 15, 550–583. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Ghanegaonkar, P.M. Pretreatment methods in anaerobic digestion for biogas generation: A review. Int. J. New Innov. Eng. Technol. 2015, 4, 14–18. [Google Scholar]
- Laureano-Perez, L.; Teymouri, F.; Alizadeh, H.; Dale, B.E. Understanding factors that limit enzymatic hydrolysis of biomass: Characterization of pretreated corn stover. Appl. Biochem. Biotechnol. 2005, 24, 1081–1099. [Google Scholar] [CrossRef]
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renew. Sustain. Energy Rev. 2012, 16, 1462–1476. [Google Scholar] [CrossRef]
- Muranaka, Y.; Nakagawa, H.; Hasegawa, I.; Maki, T.; Hosokawa, J.; Ikuta, J.; Mae, K. Lignin-based resin production from lignocellulosic biomass combining acidic saccharification and acetone-water treatment. Chem. Eng. J. 2017, 308, 754–759. [Google Scholar] [CrossRef]
- Yu, J.; Paterson, N.; Blamey, J.; Millan, M. Cellulose, xylan and lignin interactions during pyrolysis of lignocellulosic biomass. Fuel 2017, 191, 140–149. [Google Scholar] [CrossRef]
- Khan, A.S.; Man, Z.; Bustam, M.A.; Nasrullah, A.; Ullah, Z.; Sarwono, A.; Shah, F.U.; Muhammad, N. Efficient conversion of lignocellulosic biomass to levulinic acid using acidic ionic liquids. Carbohydr. Polym. 2017, 181, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, Y.; Yang, H.; Xia, M.; Li, K.; Chen, X.; Chen, H. Co-pyrolysis of lignocellulosic biomass and microalgae: Products characteristics and interaction effect. Bioresour. Technol. 2017, 245A, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-F.; Chiueh, P.-T.; Lo, S.-L. A review on microwave pyrolysis of lignocellulosic biomass. Sustain. Environ. Res. 2016, 26, 103–109. [Google Scholar] [CrossRef]
- Raud, M.; Tutt, M.; Olt, J.; Kikas, T. Dependence of the hydrolysis efficiency on the lignin content in lignocellulosic material. Int. J. Hydrogen Energy 2016, 41, 16338–16343. [Google Scholar] [CrossRef]
- De Caprariis, B.; De Filippis, P.; Petrullo, A.; Scarsella, M. Hydrothermal liquefaction of biomass: Influence of temperature and biomass composition on the bio-oil production. Fuel 2017, 208, 618–625. [Google Scholar] [CrossRef]
- Monlau, F.; Barakat, A.; Trably, E.; Dumas, C.; Steyer, J.P.; Carrère, H. Lignocellulosic materials into biohydrogen and biomethane: Impact of structural features and pretreatment. Crit. Rev. Environ. Sci. Technol. 2013, 43, 260–322. [Google Scholar] [CrossRef]
- Monlau, F.; Barakat, A.; Steyer, J.P.; Carrere, H. Comparison of seven types of thermo-chemical pretreatments on the structural features and anaerobic digestion of sunflower stalks. Bioresour. Technol. 2012, 120, 241–247. [Google Scholar] [CrossRef]
- Subhedar, P.B.; Ray, P.; Gogate, P.R. Intensification of delignification and subsequent hydrolysis for the fermentable sugar production from lignocellulosic biomass using ultrasonic irradiation. Ultrason. Sonochem. 2017, 40, 140–150. [Google Scholar] [CrossRef]
- Álvarez, A.; Cachero, S.; González-Sánchez, C.; Montejo-Bernardo, J.; Pizarro, C.; Bueno, J.L. Novel method for holocellulose analysis of non-woody biomass wastes. Carbohydr. Polym. 2018, 189, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Ahn, Y.; Kwak, S.-Y. Comparing the influence of acetate and chloride anions on the structure of ionic liquid pretreated lignocellulosic biomass. Biomass Bioenergy 2016, 93, 243–253. [Google Scholar] [CrossRef]
- Karthikeyan, O.P.; Visvanathan, C. Bio-energy recovery from high-solid organic substrates by dry anaerobic bio-conversion processes: A review. Rev. Environ. Sci. Bio. Technol. 2012, 12, 257–284. [Google Scholar] [CrossRef]
- Daza Serna, L.V.; Orrego Alzate, C.E.; Alzate, C.A.C. Supercritical fluids as a green technology for the pretreatment of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 113–120. [Google Scholar] [CrossRef]
- Liu, X.; Hiligsmann, S.; Gourdon, R.; Bayard, R. Anaerobic digestion of lignocellulosic biomasses pretreated with Ceriporiopsis subvermispora. J. Environ. Manag. 2017, 193, 154–162. [Google Scholar] [CrossRef]
- Ye, J.; Li, D.; Sun, Y.; Wang, G.; Yuan, Z.; Zhen, F.; Wang, Y. Improved biogas production from rice straw by co-digestion with kitchen waste and pig manure. Waste Manag. 2013, 33, 2653–2658. [Google Scholar] [CrossRef]
- Brown, D.; Shi, J.; Li, Y. Comparison of solid-state to liquid anaerobic digestion of lignocellulosic feedstocks for biogas production. Bioresour. Technol. 2012, 124, 379–386. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, R.; He, Y.; Zhang, C.; Liu, X.; Chen, C.; Liu, G. Anaerobic co-digestion of chicken manure and corn stover in batch and continuously stirred tank reactor (CSTR). Bioresour. Technol. 2014, 156, 342–347. [Google Scholar] [CrossRef]
- Romano, R.T.; Zhang, R.; Teter, S.; McGarvey, J.A. The effect of enzyme addition on anaerobic digestion of Jose Tall Wheat Grass. Bioresour. Technol. 2009, 100, 4564–4571. [Google Scholar] [CrossRef]
- Appels, L.; Baeyens, J.; Degreve, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Schroyen, M.; Vervaeren, H.; Vandepitte, H.; Van Hulle, S.W.; Raes, K. Effect of enzymatic pretreatment of various lignocellulosic substrates on production of phenolic compounds and biomethane potential. Bioresour. Technol. 2015, 192, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Teghammar, A.; Yngvesson, J.; Lundin, M.; Taherzadeh, M.J.; Horváth, I.S. Pretreatment of paper tube residuals for improved biogas production. Bioresour. Technol. 2010, 101, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, A.I., Jr.; de Carvalho, J.C.; de Melo Pereira, G.V.; Karp, S.G.; Câmara, M.C.; Medina, J.D.C.; Soccol, C. R-Lignocellulosic biomass from agro-industrial residues in South America: Current developments and perspectives. Biofuels Bioprod. Biorefin. 2019, 13, 1505–1519. [Google Scholar] [CrossRef]
- Nunes, C.S.; Kunamneni, A. Laccases—properties and applications. In Enzymes in Human and Animal Nutrition; Academic Press, Elsevier: Amsterdam, The Netherlands, 2018; pp. 133–161. [Google Scholar] [CrossRef]
- Michalska, K.; Bizukojć, M.; Ledakowicz, S. Pretreatment of energy crops with sodium hydroxide and cellulolytic enzymes to increase biogas production. Biomass Bioenergy 2015, 80, 213–221. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Dhar, B.R.; Nakhla, G.; Lee, H.S. A critical review on inhibition of dark biohydrogen fermentation. Renew. Sustain. Energy Rev. 2017, 79, 656–668. [Google Scholar] [CrossRef]
- Parawira, W. Enzyme research and applications in biotechnological intensification of biogas production. Crit. Rev. Biotechnol. 2012, 32, 172–186. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kumar, B.; Agarwal, K.; Chaturvedi, V.; Verma, P. Purification and characterization of a thermo-acid/alkali stable xylanases from Aspergillus oryzae LC1 and its application in Xylo-oligosaccharides production from lignocellulosic agricultural wastes. Int. J. Biol. Macrom. 2018, 122, 1191–1202. [Google Scholar] [CrossRef]
- Kumar, B.; Bhardwaj, N.; Agrawal, K.; Chaturvedi, V.; Verma, P. Current perspective on pretreatment technologies using lignocellulosic biomass: An emerging biorefinery concept. Fuel Process. Technol. 2020, 199, 106244. [Google Scholar] [CrossRef]
- Sindhu, R.; Binod, P.; Pandey, A. Biological pretreatment of lignocellulosic biomass–An overview. Bioresour. Technol. 2016, 199, 76–82. [Google Scholar] [CrossRef]
- Zhang, Q.; He, J.; Tian, M.; Mao, Z.; Tang, L.; Zhang, J.; Zhang, H. Enhancement of methane production from cassava residues by biological pretreatment using a constructed microbial consortium. Bioresour. Technol. 2011, 102, 8899–8906. [Google Scholar] [CrossRef]
- Singh, P.; Suman, A.; Tiwari, P.; Arya, N.; Gaur, A.; Shrivastava, A.K. Biological pretreatment of sugarcane trash for its conversion to fermentable sugars. World J. Microbiol. Biotechnol. 2008, 24, 667–673. [Google Scholar] [CrossRef]
- Lopez, M.J.; Vargas-Garcia, M.D.; Suarez-Estrella, F.; Nichols, N.N.; Dien, B.S.; Moreno, J.J. Lignocellulose-degrading enzymes produced by the ascomycete Coniochaeta ligniaria and related species: Application for a lignocellulosic substrate treatment. Enzyme Microb. Technol. 2007, 40, 794–800. [Google Scholar] [CrossRef]
- Galbe, M.; Zacchi, G. Pretreatment of lignocellulosic materials for efficient bioethanol production. Biofuels 2007, 108, 41–65. [Google Scholar] [CrossRef]
- Sankaran, R.; Parra Cruz, R.A.; Pakalapati, H.; Show, P.L.; Ling, T.C.; Chen, W.; Tao, Y. Recent advances in the pretreatment of microalgal and lignocellulosic biomass: A comprehensive review. Bioresour. Technol. 2020, 298, 122476. [Google Scholar] [CrossRef]
- Ali, S.S.; Mustafa, A.M.; Kornaros, M.; Manni, A.; Sun, J.; Khalil, M.A. Construction of novel microbial consortia CS-5 and BC-4 valued for the degradation of catalpa sawdust and chlorophenols simultaneously with enhancing methane production. Bioresour. Technol. 2020. [Google Scholar] [CrossRef]
- Kong, X.; Du, J.; Ye, X.; Xi, Y.; Jin, H.; Zhang, M.; Guo, D. Enhanced methane production from wheat straw with the assistance of lignocellulolytic microbial consortium TC-5. Bioresour. Technol. 2018, 263, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Abomohra, A.E.; Sun, J. Effective bio-pretreatment of sawdust waste with a novel microbial consortium for enhanced biomethanation. Bioresour. Technol. 2017, 238, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Al-Tohamy, R.; Manni, A.; Luz, F.C.; Elsamahy, T.; Sun, J. Enhanced digestion of bio-pretreated sawdust using a novel bacterial consortium: Microbial community structure and methane-producing pathways. Fuel 2019, 254, 115604. [Google Scholar] [CrossRef]
- Kato, S.; Haruta, S.; Cui, Z.J.; Ishii, M.; Igarashi, Y. Stable coexistence of five bacterial strains as a cellulose-degrading community. Appl. Environ. Microbiol. 2005, 71, 7099–7106. [Google Scholar] [CrossRef]
- Kato, S.; Haruta, S.; Cui, Z.J.; Ishii, M.; Igarashi, Y. Effective cellulose degradation by a mixed-culture system composed of a cellulolytic Clostridium and aerobic non-cellulolytic bacteria. FEMS Microbiol. Ecol. 2004, 51, 133–142. [Google Scholar] [CrossRef]
- Yuan, X.; Ma, L.; Wen, B.; Zhou, D.; Kuang, M.; Yang, W.; Cui, Z. Enhancing anaerobic digestion of cotton stalk by pretreatment with a microbial consortium (MC1). Bioresour. Technol. 2016, 207, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Hua, B.; Dai, J.; Liu, B.; Zhang, H.; Yuan, X.; Wang, X.; Cui, Z. Pretreatment of non-sterile, rotted silage maize straw by the microbial community MC1 increases biogas production. Bioresour. Technol. 2016, 216, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Zhang, Z.; Luo, Y.; Sun, S.; Qiao, W.; Xiao, M. Effect of biological pretreatments in enhancing corn straw biogas production. Bioresour. Technol. 2011, 102, 11174–11182. [Google Scholar] [CrossRef] [PubMed]
- Suksong, W.; Kongjan, P.; Prasertsan, P.; O-Thong, S. Thermotolerant cellulolytic Clostridiaceae and Lachnospiraceae rich consortium enhanced biogas production from oil palm empty fruit bunches by solid-state anaerobic digestion. Bioresour. Technol. 2019, 291, 121851. [Google Scholar] [CrossRef]
- Tantayotai, P.; Pornwongthong, P.; Muenmuang, C.; Phusantisampan, T.; Sriariyanun, M. Effect of Cellulase-producing Microbial Consortium on Biogas Production from Lignocellulosic Biomass. Energ. Proc. 2017, 141, 180–183. [Google Scholar] [CrossRef]
- Vervaeren, H.; Hostyn, K.; Ghekiere, G.; Willems, B. Biological ensilage additives as pretreatment for maize to increase the biogas production. Renew. Energ. 2010, 35, 2089–2093. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, C.; Ai, S.; Wang, H.; Gao, Y.; Yan, L.; Mei, Z.; Wang, W. Biological pretreatment enhances the activity of functional microorganisms and the ability of methanogenesis during anaerobic digestion. Bioresour. Technol. 2019, 290, 121660. [Google Scholar] [CrossRef]
- Xing, B.; Han, Y.; Wang, X.C.; Wen, J.; Cao, S.; Zhang, K.; Li, Q.; Yuan, H. Persistent action of cow rumen microorganisms in enhancing biodegradation of wheat straw by rumen fermentation. Sci. Total Environ. 2020, 715, 136529. [Google Scholar] [CrossRef]
- Li, F.; Zhang, P.; Zhang, G.; Tang, X.; Wang, S.; Jin, S. Enhancement of corn stover hydrolysis with rumen fluid pretreatment at different solid contents: Effect, structural changes and enzymes participation. Int. Biodeterior. Biodegrad. 2017, 119, 405–412. [Google Scholar] [CrossRef]
- Takizawa, S.; Baba, Y.; Tada, C.; Fukuda, Y.; Nakai, Y. Pretreatment with rumen fluid improves methane production in the anaerobic digestion of paper sludge. Waste Manag. 2018, 78, 379–384. [Google Scholar] [CrossRef]
- Jin, W.; Xu, X.; Yang, F.; Li, C.; Zhou, M. Performance enhancement by rumen cultures in anaerobic co-digestion of corn straw with pig manure. Biomass Bioenergy 2018, 115, 120–129. [Google Scholar] [CrossRef]
- Baba, Y.; Matsuki, Y.; Mori, Y.; Suyama, Y.; Tada, C.; Fukuda, Y.; Saito, M.; Nakai, Y. Pretreatment of lignocellulosic biomass by cattle rumen fluid for methane production: Bacterial flora and enzyme activity analysis. J. Biosci. Bioeng. 2017, 123, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.L.; Baba, Y.; Asano, R.; Fukuda, Y.; Tada, C.; Nakai, Y. Identification of bacteria involved in the decomposition of lignocellulosic biomass treated with cow rumen fluid by metagenomic analysis. J. Biosci. Bioeng. 2020, 130, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Nagler, M.; Kozjek, K.; Etemadi, M.; Insam, H.; Podmirseg, S.M. Simple yet effective: Microbial and biotechnological benefits of rumen liquid addition to lignocellulose-degrading biogas plants. J. Biotechnol. 2019, 300, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, S.; Baba, Y.; Tada, C.; Fukuda, Y.; Nakai, Y. Preservation of rumen fluid for the pretreatment of waste paper to improve methane production. Waste Manag. 2019, 87, 672–678. [Google Scholar] [CrossRef]
- Forgacs, G.; Alinezhad, S.; Mirabdollah, A.; Feuk-Lagerstedt, E.; Horvath, I.S. Biological treatment of chicken feather waste for improved biogas production. J. Environ. Sci. 2011, 23, 1747–1753. [Google Scholar] [CrossRef]
- Yadav, M.; Paritosh, K.; Pareek, N.; Vivekanand, V. Coupled treatment of lignocellulosic agricultural residues for augmented biomethanation. J. Clean. Prod. 2019, 213, 75–88. [Google Scholar] [CrossRef]
- Kavitha, S.; Subbulakshmi, P.; Rajesh Banu, J.; Gobi, M.; Yeom, I.T. Enhancement of biogas production from microalgal biomass through cellulolytic bacterial pretreatment. Bioresour. Technol. 2017, 233, 34–43. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. The potential of microalgae and their biopolymers as structuring ingredients in food: A review. Biotechnol. Adv. 2019, 107419. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Bai, M.-D.; Chang, J.-S. Improving microalgal oil collecting efficiency by pretreating the microalgal cell wall with destructive bacteria. Biochem. Eng. J. 2013, 81, 170–176. [Google Scholar] [CrossRef]
- He, S.; Fan, X.; Katukuri, N.R.; Yuan, X.; Wang, F.; Guo, R. Enhanced methane production from microalgal biomass by anaerobic bio-pretreatment. Bioresour. Technol. 2016, 204, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Barua, V.B.; Goud, V.V.; Kalamdhad, A.S. Microbial pretreatment of water hyacinth for enhanced hydrolysis followed by biogas production. Renew. Energy 2018, 126, 21–29. [Google Scholar] [CrossRef]
- Barua, V.B.; Kalamdhad, A.S. Anaerobic biodegradability test of water hyacinth after microbial pretreatment to optimise the ideal F/M ratio. Fuel 2018, 217, 91–97. [Google Scholar] [CrossRef]
- Tapadia-Maheshwari, S.; Pore, S.; Engineer, A.; Shetty, D.; Dagar, S.S.; Dhakephalkar, P.K. Illustration of the microbial community selected by optimized process and nutritional parameters resulting in enhanced biomethanation of rice straw without thermo-chemical pretreatment. Bioresour. Technol. 2019, 289, 121639. [Google Scholar] [CrossRef]
- Fu, S.F.; He, S.; Shi, X.S.; Katukuri, N.R.; Dai, M.; Guo, R.B. The chemical properties and microbial community characterization of the thermophilic microaerobic pretreatment process. Bioresour. Technol. 2015, 198, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Merlin Christy, P.; Gopinath, L.R.; Divya, D. A review on anaerobic decomposition and enhancement of biogas production through enzymes and microorganisms. Renew. Sust. Energ. Rev. 2014, 34, 167–173. [Google Scholar] [CrossRef]
- Najiah, N.; Nur Liyana, I.; Azlan Shah, H. Fungal pretreatment of lignocellulosic materials. In Biomass for Bioenergy-Recent Trends and Future Challenges; Abd El-Fatah, A., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Dashtban, M.; Schraft, H.; Syed, T.A.; Qin, W. Fungal biodegradation and enzymatic modification of lignin. Int. J. Biochem. Mol. Biol. 2010, 1, 36–50. [Google Scholar]
- Mishra, S.; Singh, P.K.; Dash, S.; Pattnaik, R. Microbial pretreatment of lignocellulosic biomass for enhanced biomethanation and waste management. 3 Biotec. 2018, 8, 458. [Google Scholar] [CrossRef]
- Sanchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef]
- Tian, S.Q.; Zhao, R.Y.; Chen, Z.C. Review of the pretreatment and bioconversion of lignocellulosic biomass from wheat straw materials. Renew. Sust. Energ. Rev. 2018, 91, 483–489. [Google Scholar] [CrossRef]
- Rouches, E.; Herpoel-Gimbert, I.; Steyer, J.P.; Carrere, H. Improvement of anaerobic degradation by white-rot fungi pretreatment of lignocellulosic biomass: A review. Renew. Sust. Energ. Rev. 2016, 59, 179–198. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: Challenges and opportunities. Bioresour Technol. 2016, 199, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, M.; Aghbashlo, M.; Valijanian, E.; Panahi, H.K.S.; Nizami, A.S.; Ghanavati, H.; Sulaiman, A.; Mirmohamadsadeghi, S.; Karimi, K. A comprehensive review on recent biological innovations to improve biogas production, Part 1: Upstream strategies. Renew. Energ. 2020, 146, 1204–1220. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Sulej, J.; Swiderska-Burek, U.; Jarosz-Wilkolazka, A.; Paszczynski, A. Lignin degradation: Microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zheng, Y.; Li, Y. Fungal pretreatment of yard trimmings for enhancement of methane yield from solid-state anaerobic digestion. Bioresour. Technol. 2014, 156, 176–181. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Poulsen, T.G.; Sheng, K. Fungal pretreatment of rice straw with Pleurotus ostreatus and Trichoderma reesei to enhance methane production under solid-state anaerobic digestion. Appl. Energy 2016, 180, 661–671. [Google Scholar] [CrossRef]
- Da Silva Machado, A.; Ferraz, A. Biological pretreatment of sugarcane bagasse with basidiomycetes producing varied patterns of biodegradation. Bioresour. Technol. 2017, 225, 17–22. [Google Scholar] [CrossRef]
- Nkemka, V.N.; Gilroyed, B.; Yanke, J.; Gruninger, R.; Vedres, D.; McAllister, T.; Hao, X. Bioaugmentation with an anaerobic fungus in a two-stage process for biohydrogen and biogas production using corn silage and cattail. Bioresour. Technol. 2015, 185, 79–88. [Google Scholar] [CrossRef]
- Lopez, M.J.; Suarez-Estrella, F.; Vargas-Garcia, M.C.; Lopez-Gonzales, J.A.; Verstichel, S.; Debeer, L.; Wierinck, I.; Moreno, J. Biodelignification of agricultural and forest wastes: Effect on anaerobic digestion. Biomass Bioenergy 2013, 58, 343–349. [Google Scholar] [CrossRef]
- Tisma, M.; Planinic, M.; Bucic-Kojic, A.; Panjicko, M.; Zupancic, G.D. Corn silage fungal-based solid-state pretreatment for enhanced biogas production in anaerobic co-digestion with cow manure. Bioresour. Technol. 2018, 253, 220–226. [Google Scholar] [CrossRef]
- Yadav, M.; Vivekanand, V. Biological treatment of lignocellulosic biomass by Curvularia lunata for biogas production. Bioresour. Technol. 2020, 306, 123151. [Google Scholar] [CrossRef] [PubMed]
- Akyol, C.; Ince, O.; Bozan, M.; Ozbayram, E.G.; Ince, B. Biological pretreatment with Trametes versicolor to enhance methane production from lignocellulosic biomass: A metagenomic approach. Ind. Crops Prod. 2019, 140, 111659. [Google Scholar] [CrossRef]
- Yildirim, E.; Ince, O.; Aydin, S.; Ince, B. Improvement of biogas potential of anaerobic digesters using rumen fungi. Renew. Energy 2017, 109, 346–353. [Google Scholar] [CrossRef]
- Kainthola, J.; Kalamdhad, A.S.; Goud, V.V.; Goel, R. Fungal pretreatment and associated kinetics of rice straw hydrolysis to accelerate methane yield from anaerobic digestion. Bioresour. Technol. 2019, 286, 121368. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zheng, Z.; Cai, Y.; Zhao, Y.; Zhang, Y.; Gao, Y.; Cui, Z.; Wang, X. Accelerated biomethane production from lignocellulosic biomass: Pretreated by mixed enzymes secreted by Trichoderma viride and Aspergillus sp. Bioresour. Technol. 2020, 309, 123378. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, X.; Zhang, P.; Morera, X.C.; van Lier, J.B.; Spanjers, H. Evaluation of white rot fungi pretreatment of mushroom residues for volatile fatty acid production by anaerobic fermentation: Feedstock applicability and fungal function. Bioresour. Technol. 2020, 297, 122447. [Google Scholar] [CrossRef]
- Shirkavand, E.; Baroutian, S.; Gapes, D.J.; Young, B.R. Combination of fungal and physicochemical processes for lignocellulosic biomass pretreatment–A review. Renew. Sustain. Energy Rev. 2016, 54, 217–234. [Google Scholar] [CrossRef]
- Ali, S.S.; Sun, J. Physico-chemical pretreatment and fungal biotreatment for park wastes and cattle dung for biogas production. SpringerPlus 2015, 4, 712. [Google Scholar] [CrossRef]
- Alexandropoulou, M.; Antonopoulou, G.; Fragkou, F.; Ntaikou, I.; Lyberatos, G. Fungal pretreatment of willow sawdust and its combination with alkaline treatment for enhancing biogas production. J. Environ. Manag. 2017, 203, 704–713. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Poulsen, T.G.; Xia, Y.; Sheng, K. Combinations of fungal and milling pretreatments for enhancing rice straw biogas production during solid-state anaerobic digestion. Bioresour. Technol. 2017, 224, 174–182. [Google Scholar] [CrossRef]
- Wei, S. The application of biotechnology on the enhancing of biogas production from lignocellulosic waste. Appl. Microbiol. Biotechnol. 2016, 100, 9821–9836. [Google Scholar] [CrossRef] [PubMed]
- Ometto, F.; Quiroga, G.; Pšenička, P.; Whitton, R.; Jefferson, B.; Villa, R. Impacts of microalgae pre-treatments for improved anaerobic digestion: Thermal treatment, thermal hydrolysis, ultrasound and enzymatic hydrolysis. Water Res. 2014, 65, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.E.F. Avaliacao Do Potencial De Uso De Residuos Do Processamento De Frutas Na Producao De Etanol 2G. Master’s Thesis, Universida de Federal De Alagoas, Maceio, Brazil, 2014. [Google Scholar]
- Hansen, G.H.; Lubeck, M.; Frisvad, J.C.; Lubeck, P.S.; Andersen, B. Production of cellulolytic enzymes from ascomycetes: Comparison of solid state and submerged fermentation. Process Biochem. 2015, 50, 1327–1341. [Google Scholar] [CrossRef]
- Chinedu, S.N.; Okochi, V.I.; Omidiji, O. Cellulase Production By Wild Strains Of Aspergillus Niger Penicillium Chrysogenum and Trichoderma Harzianum Grown On Waste Cellulosic Materials. J. Sci. 2011, 13, 57–62. [Google Scholar]
- Mathew, G.M.; Sukumaran, R.K.; Singhania, R.R.; Pandey, A. Progress in research on fungal cellulases for lignocellulose degradation. J. Sci. Ind. Res. 2008, 67, 898–907. [Google Scholar]
- Zieminski, K.; Romanowska, I.; Kowalska, M. Enzymatic pretreatment of lignocellulosic wastes to improve biogas production. Waste Manag. Res. 2012, 32, 1131–1137. [Google Scholar] [CrossRef]
- Silva, A.F.V.; Santos, L.A.; Valença, R.B.; Porto, T.S.; Da Motta Sobrinho, M.A.; Gomes, G.J.C.; Jucá, J.F.T.; Santos, A.F.M.S. Cellulase production to obtain biogas from passion fruit (Passiflora edulis) peel waste hydrolysate. J. Environ. Chem. Eng. 2019, 7, 103510. [Google Scholar] [CrossRef]
- Edwiges, T.; Frare, L.; Mayer, B.; Lins, L.; Triolo, J.M.; Flotats, X.; Costa, M.S.S.M. Influence of chemical composition on biochemical methane potential of fruit and vegetable waste. Waste Manag. 2018, 71, 618–625. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Zhou, X.; Wang, Q.; Wu, Y.; Saino, M.; Bai, X. Study on the bio-methane yield and microbial community structure in enzyme enhanced anaerobic co-digestion of cow manure and corn straw. Bioresour. Technol. 2016, 219, 150–157. [Google Scholar] [CrossRef]
- Shraddha; Shekher, R.; Sehgal, S.; Kamthania, M.; Kumar, A. Laccase: Microbial Sources, Production, Purification, and Potential Biotechnological Applications-Review Article. Enzyme Res. 2011, 11. [Google Scholar] [CrossRef]
- Liew, Y.X.; Chan, Y.J.; Manickam, S.; Chong, M.F.; Chong, S.; Tiong, T.J.; Lim, J.W.; Pan, G.T. Enzymatic pretreatment to enhance anaerobic bioconversion of high strength wastewater to biogas: A review. Sci. Total Environ. 2020, 713, 136373. [Google Scholar] [CrossRef] [PubMed]
- Yaver, D.S.; Berka, R.M.; Brown, S.H.; Xu, F. The Presymposium on Recent Advances in Lignin Biodegradation and Biosynthesis, Vikki Biocentre; University of Helsinki: Helsinki, Finland, 2001. [Google Scholar]
- Patel, N.; Shahane, S.; Shivam; Majumdar, R.; Mishra, U. Mode of Action, Properties, Production, and Application of Laccase: A Review. Recent Pat. Biotechnol. 2019, 13, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Couto, R.S.; Herrera, T.J.L. Industrial and biotechnological applications of laccases: A review. Biotechnol. Adv. 2006, 24, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Kudanga, T.; Le Roes-Hill, M. Laccase applications in biofuels production: Current status and future prospects. Appl. Microbiol. Biotechnol. 2014, 98, 6525–6542. [Google Scholar] [CrossRef]
- Schroyen, M.; Vervaeren, H.; Van Hulle, S.W.H.; Raes, K. Impact of enzymatic pretreatment on corn stover degradation and biogas production. Bioresour. Technol. 2014, 173, 59–66. [Google Scholar] [CrossRef]
- Howard, R.L.; Abotsi, E.L.J.R.; van Rensburg, E.J.; Howard, S. Lignocellulose biotechnology: Issues of bioconversion and enzyme production. Afr. J. Biotechnol. 2003, 2, 602–619. [Google Scholar] [CrossRef]
- Hammel, K.; Cullen, D. Role of fungal peroxidases in biological ligninolysis. Curr. Opin. Plant Biol. 2008, 11, 349–355. [Google Scholar] [CrossRef]
- Martínez, A.T.; Speranza, M.; Ruiz-Dueñas, F.J.; Ferreira, P.; Camarero, S.; Guillén, F.; Martínez, M.J.; Gutiérrez, A.; del Río, J.C. Biodegradation of lignocellulosics: Microbial, chemical, and enzymatic aspects of the fungal attack of lignin. Int. Microbiol. 2005, 8, 195–204. [Google Scholar]
- Jayasinghe, P.A.; Hettiaratchi, J.P.A.; Mehrotra, A.K.; Kumar, S. Effect of enzyme additions onmethane production and lignin degradation of landfilled sample of municipal solid waste. Bioresour. Technol. 2011, 102, 4633–4637. [Google Scholar] [CrossRef]
- Hasan, F.; Ali Shah, A.; Hameed, A. Industrial applications of microbial lipases. Enzym. Microb. Technol. 2006, 39, 235–251. [Google Scholar] [CrossRef]
- Eijkman, C.U. Ber Enzyme bei bakterien und Schimmelpilzen. Cbl Bakt Parasitenk Infektionskr 1901, 29, 841–848. [Google Scholar]
- Donoso-Bravo, A.; Fdz-Polanco, M. Anaerobic co-digestion of sewage sludge and grease trap: Assessment of enzyme addition. Process Biochem. 2013, 48, 936–940. [Google Scholar] [CrossRef]
- Dors, G.; Mendes, A.A.; Pereira, E.B.; Castro, H.F.; Furigo, A. Simultaneous enzymatic hydrolysis and anaerobic biodegradation of lipid-rich wastewater from poultry industry. Appl. Water Sci. 2013, 3, 343–349. [Google Scholar] [CrossRef]
- Kameswari, K.S.B.; Kalyanaraman, C.; Porselvam, S.; Thanasekaran, K. Enhancement of Biogas Generation by Addition of Lipase in the Co-Digestion of Tannery Solid Wastes. Clean (Weinh) 2011, 39, 781–786. [Google Scholar] [CrossRef]
- Xie, B.; Cheng, J.; Zhou, J.; Song, W.; Liu, J.; Cen, K. Production of hydrogen andmethane from potatoes by two-phase anaerobic fermentation. Bioresour. Technol. 2008, 99, 5942–5946. [Google Scholar] [CrossRef]
- Bonilla, S.; Choolaei, Z.; Meyer, T.; Edwards, E.A.; Yakunin, A.F.; Grant, A.D. Evaluating the effect of enzymatic pretreatment on the anaerobic digestibility of pulp and paper biosludge. Biotechnol. Rep. 2018, 17, 77–85. [Google Scholar] [CrossRef]
- Müller, L.; Kretzschmar, J.; Pröter, J.; Liebetrau, J.; Nelles, M.; Scholwin, F. Does the addition of proteases affect the biogas yield from organic material in anaerobic. Bioresour. Technol. 2016, 203, 267–271. [Google Scholar] [CrossRef]
- Sutaryo, S.; Ward, A.J.; Moller, H.B. The effect of mixed-enzyme addition in anaerobic digestion on methane yield of dairy cattle manure. Environ. Technol. 2014, 35, 2476–2482. [Google Scholar] [CrossRef]
- Lin, Y.; Liang, J.; Zeng, C.; Wang, D.; Lin, H. Anaerobic digestion of pulp and paper mill sludge pretreated by microbial consortium OEM1 with simultaneous degradation of lignocellulose and chlorophenols. Renew. Energy 2017, 108, 108–115. [Google Scholar] [CrossRef]
- Passos, F.; Hom-Diaz, A.; Blanquez, P.; Vicent, T.; Ferrer, I. Improving biogas production from microalgae by enzymatic pretreatment. Bioresour. Technol. 2016, 199, 347–351. [Google Scholar] [CrossRef]
- Al-Zuhair, S.; Ahmed, K.; Abdulrazak, A.; El-Naas, M.H. Synergistic effect of pretreatment and hydrolysis enzymes on the production of fermentable sugars from date palm lignocellulosic waste. J. Ind. Eng. Chem. 2013, 19, 413–415. [Google Scholar] [CrossRef]
- Lee, J.-W.; Gwak, K.-S.; Park, J.-Y.; Park, M.-J.; Choi, D.-H.; Kwon, M.; Choi, I.-G. Biological pretreatment of softwood Pinus densiflora by three white rot fungi. J. Microbiol. 2007, 45, 485–491. [Google Scholar] [PubMed]
- Pérez-Rodríguez, N.; García-bernet, D.; Domínguez, J.M. Extrusion and enzymatic hydrolysis as pretreatments on corn cob for biogas production. Renew. Energy 2017, 107, 597–603. [Google Scholar] [CrossRef]
- Domingues, R.F.; Sanches, T.; Silva, G.S.; Bueno, B.E.; Ribeiro, R.; Kamimura, E.S.; Franzolin Neto, R.; Tommaso, G. Effect of enzymatic pretreatment on the anaerobic digestion of milk fat for biogas production. Food Res. Int. 2015, 73, 26–30. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).