“What a Waste”—Can We Improve Sustainability of Food Animal Production Systems by Recycling Food Waste Streams into Animal Feed in an Era of Health, Climate, and Economic Crises?
Abstract
1. Introduction
2. Maximizing Resource Recovery and Value of Waste Streams
2.1. Food Waste
2.2. Carcass Rendering
3. Comparison of Environmental Impacts of Alternative Disposal Methods
3.1. Food Waste
3.2. Animal Mortalities and Carcass Residuals
4. Potential Amounts of Food Waste Streams That Can Be Used as Animal Feed Ingredients
5. Urgent Need to Achieve Greater Global Nitrogen, Phosphorus, Carbon Resource Recovery
5.1. Nitrogen
5.2. Phosphorus
6. Most Food Waste and Rendered Animal By-Products Are Concentrated Sources of Energy, Nitrogen, and Phosphorus
6.1. Energy, Protein, and Phosphorus in Animal Nutrition
6.2. Nutritional Composition of Food Waste Sources
6.3. Nutritional Efficiency of Food Waste Sources Can be Equivalent or Greater Than Corn and Soybean Meal
6.4. Nutrition and Technical Challenges Limiting Use of Food Waste in Animal Feed
6.5. Nutritional Efficiency of Rendered Animal By-Products Can be Equivalent or Greater Than Corn and Soybean Meal
7. Using Food Waste and Rendered Animal By-Products as Animal Feed Ingredients Can Substantially Reduce Several Environmental Impacts of Food Animal Production
8. Real and Perceived Biosafety Risks of Rendered Animal By-Products and Food Waste
8.1. Comparison of Biosafety Risks of Carcass Disposal Methods
8.2. Potential Biosafety Risks of Feeding Rendered Animal By-Products to Food-Producing Animals
8.2.1. Adequate Thermal Processing Minimizes Feed Safety Risks of Rendered Animal By-Products
8.2.2. Salmonella
8.2.3. Bovine Spongiform Encephalitis
8.2.4. Swine Corona Viruses
8.2.5. African Swine Fever Virus
8.3. Different Perspectives of Potential Biosafety Risks of Recycling Food Waste into Animal Feed
Adequate Thermal Processing Minimizes Feed Safety Risks of Food Waste
8.4. Solutions to Overcome Biosafety Concerns of Using Rendered Animal By-Products and Thermally Treated Food Waste in Animal Feed
9. Next Steps
Funding
Conflicts of Interest
References
- Kuzmanova, M.; Ivanov, I. Relation between change management and crisis management: Survey evidence. Int. Conf. Knowl. Based Organ. 2015, 25, 255–260. [Google Scholar] [CrossRef]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; Global Food Losses and Food Waste. Rome (Italy): Food and Agriculture Organization of the United Nations. 2011. Available online: http://www.fao.org/docrep/014/mb060e/mb060e00.pdf (accessed on 6 July 2020).
- Lipinski, B.; Hanson, C.; Lomax, J.; Kitinoja, L.; Waite, R.; Searchinger, T. Reducing Food Loss and Waste. Available online: http://pdf.wri.org/reducing_food_loss_and_waste.pdf (accessed on 15 August 2020).
- United Nations. Transforming our world: The 2030 agenda for sustainable development. 2015. Available online: http://www.un.org/ga/search/view_doc.asp?symbol=A/RES/70/1&Lang=E (accessed on 6 July 2020).
- Hakovirta, M.; Denuwara, N. How COVID-19 redefines the concept of sustainability. Sustainability 2020, 12, 3727. [Google Scholar] [CrossRef]
- ReFED 2020 COVID-19, U.S. Food System Review. Available online: https://www.refed.com/content-hub/refeds-covid-19-u-s-food-system-review/ (accessed on 16 August 2020).
- Jámbor, A.; Czine, P.; Balogh, P. The impact of the Coronavirus on agriculture: First evidence based on global newspapers. Sustainability 2020, 12, 4535. [Google Scholar] [CrossRef]
- Aldaco, R.; Hoehn, D.; Laso, J.; Margallo, M.; Ruiz-Salmón, J.; Cristobal, J.; Kahhat, R.; Villanueva-Rey, P.; Bala, A.; Batlle-Bayer, L.; et al. Food waste management during the COVID-19 outbreak: A holistic climate, economic and nutritional approach. Sci. Total Environ. 2020, 742, 140524. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Response Team. Update: COVID-19 Among Workers in Meat and Poultry Processing Facilities—United States, April–May 2020; Morbidity and Mortality Weekly Report; US Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2020; Volume 69, pp. 887–892. [Google Scholar]
- Hadrich, J.; Roberts, M.; Tuck, B. The role of hog farmers in Minnesota’s rural economy. In Hog Economic Report 2020; University of Minnesota Extension: St. Paul, MN, USA, 2020; pp. 1–6. [Google Scholar]
- McEwan, K.; Marchand, L.; Shang, M.; Bucknell, D. Potential implications of COVID-19 on the Canadian pork industry. Can. J. Agric. Econ. 2020, 68, 201–206. [Google Scholar] [CrossRef]
- Roembke, J. 7 Ways COVID-19 has Impacted the US Feed Industry. Feed Strategy. June 2020. pp. 5–9. Available online: www.FeedStrategy.com (accessed on 6 July 2020).
- Rogers, E.; Rozeboom, D.; Zangaro, C. In Times of Supply Chain Disruption, how do I Appropriately Dispose of My Livestock Mortalities? Michigan State University Extension Newsletter. 2020. Available online: https://www.canr.msu.edu/news/in-times-of-supply-chain-disruption-how-do-i-appropriately-dispose-of-my-livestock-mortalities (accessed on 15 August 2020).
- Penrod, E. How COVID-19 will Reshape Feed Industry Supply Chains. Feed Strategy. June 2020. pp. 14–17. Available online: www.FeedStrategy.com (accessed on 6 July 2020).
- Galanakis, C.M. The food systems in the era of the coronavirus (COVID-19) pandemic crisis. Foods 2020, 9, 523. [Google Scholar] [CrossRef]
- Gu, H.; Daly, T. China has Shown ‘Shortcomings’ in Bid to Contain African Swine Fever. Reuters. 3 July 2019. Available online: https://uk.reuters.com/article/us-china-swinefever-policy/china-has-shown-shortcomings-in-bid-to-contain-african-swine-fever-cabinet-idUKKCN1TY15E (accessed on 15 August 2020).
- Pan, C. African Swine Fever Affects China’s Pork Consumption. Rabobank. June 2019. Available online: https://research.rabobank.com/far/en/sectors/animal-protein/african-swine-fever-affects-china-s-pork-consumption.html (accessed on 15 August 2020).
- Daly, J.; Birtles, B. China Struggles to Contain African Swine Fever, Resorts to Mass Live-Pig Burials, Millions of Culls. ABC Rural News, 29 May 2019. Available online: https://www.abc.net.au/news/rural/rural-news/2019-05-30/mass-live-pig-burials-millions-culled-china-african-swine-fever/11146642 (accessed on 6 July 2020).
- Capua, I.; Marangon, S. Control of avian influenza in poultry. Emerg. Infect. Dis. 2006, 12, 1319–1324. [Google Scholar] [CrossRef]
- Morse, S.S. Factors in the emergence of infectious diseases. Emerg. Infect. Dis. 1995, 1, 7–15. [Google Scholar] [CrossRef]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef]
- Madhav, N.; Oppenheim, B.; Gallivan, M.; Mulembakani, P.; Rubin, E.; Wolfe, N. Pandemics: Risks, impacts, and mitigation. In Disease Control Priorities, 3rd ed.; Improving Health and Reducing Poverty; International Bank for Reconstruction and Development; The World Bank: Washington, DC, USA, 2017; Volume 9, pp. 315–345. [Google Scholar]
- Pudenz, C.C.; Schulz, L.L.; Tonsor, G.T. Adoption of secure pork supply plan biosecurity by U.S. swine producers. Front. Vet. Sci. 2019, 6, 146. [Google Scholar] [CrossRef]
- Herrero, M.; Thornton, P.K.; Gerber, P.; Reid, R.S. Livestock, livelihoods and the environment: Understanding the trade-offs. Curr. Opin. Environ. Sustain. 2009, 1, 111–120. [Google Scholar] [CrossRef]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change Through Livestock—A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013. [Google Scholar]
- Papargyropoulou, E.; Lozano, R.; Steinberger, J.K.; Wright, J.; Ujang, N.; Bin, Z. The food waste hierarchy as a framework for the management of food surplus and food waste. J. Clean. Prod. 2014, 76, 106–115. [Google Scholar] [CrossRef]
- Zu Ermgassen, E.K.H.J.; Phalen, B.; Green, R.E.; Balmford, A. Reducing the land use of EU pork production: Where there’s swill, there’s a way. Food Policy 2016, 58, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Gardner, G.; Assadourian, E.; Sarin, R. The State of Consumption Today. State of the World. 2004. Available online: http://erikassadourian.com/wp-content/uploads/2013/07/SOW-04-Chap-1.pdf (accessed on 6 July 2020).
- Gandenberger, C.; Garrelts, H.; Wehlau, D. Assessing the effects of certification networks on sustainable production and consumption: The cases of FLO and FSC. J. Consum. Policy 2011, 34, 107–126. [Google Scholar] [CrossRef]
- Grunert, K.G.; Hieke, S.; Wills, J. Sustainability labels on food products: Consumer motivation, understanding and use. Food Policy 2014, 44, 177–189. [Google Scholar] [CrossRef]
- USDA-APHIS. Carcass Management during a Mass Animal Health Emergency; Final Programmatic Environmental Impact Statement, December 2015; National Center for Animal Health Emergency Management, Veterinary Services, Animal and Plant Health Inspection Service; U.S. Department of Agriculture: Riverdale, MD, USA, 2015; p. 252. [Google Scholar]
- European Union Regulation 1069/2009. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02009R1069-20191214&from=EN (accessed on 6 July 2020).
- Gwyther, C.L.; Williams, A.P.; Golyshin, P.N.; Edwards-Jones, G.; Jones, D.L. The environmental and biosecurity characteristics of livestock carcass disposal methods: A review. Waste Manag. 2011, 31, 767–778. [Google Scholar] [CrossRef]
- Eriksson, M.; Strid, I.; Hansson, P. Carbon footprint of food waste management options in the waste hierarchy—A swedish case study. J. Clean. Prod. 2015, 93, 115–125. [Google Scholar] [CrossRef]
- Lee, S.-H.; Choi, K.-I.; Osako, M.; Dong, J.-I. Evaluation of environmental burdens caused by changes of food waste management systems in Seoul, Korea. Sci. Total Environ. 2007, 387, 42–53. [Google Scholar] [CrossRef]
- Ogino, A.; Hirooka, H.; Ikeguchi, A.; Tanaka, Y.; Waki, M.; Yokoyama, H.; Kawashima, T. Environmental impact evaluation of feeds prepared from food residues using life cycle assessment. J. Environ. Qual. 2007, 36, 1061–1068. [Google Scholar] [CrossRef]
- Kim, M.-H.; Kim, J.-W. Comparison through a LCA evaluation analysis of food waste disposal options from the perspective of global warming and resource recovery. Sci. Total Environ. 2010, 408, 3998–4006. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-H.; Song, Y.-E.; Song, H.-B.; Kim, J.-W.; Hwang, S.-J. Evaluation of food waste disposal options by LCC analysis from the perspective of global warming: Jungnang case, South Korea. Waste Manag. 2011, 31, 2112–2120. [Google Scholar] [CrossRef] [PubMed]
- Takata, M.; Fukushima, K.; Kino-Kimata, N.; Nagao, N.; Niwa, C.; Toda, T. The effects of recycling loops in food waste management in Japan: Based on the environmental and economic evaluation of food recycling. Sci. Total Environ. 2012, 432, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Tufvesson, L.M.; Lantz, M.; Borjesson, P. Environmental performance of biogas produced from industrial residues including competition with animal feed—life cycle calculations according to different methodologies and standards. J. Clean Prod. 2013, 53, 214–223. [Google Scholar] [CrossRef]
- Vandermeersch, T.; Alvarenga, R.A.F.; Ragaert, P.; Dewulf, J. Environmental sustainability assessment of food waste valorization options. Resour. Conserv. Recycl. 2014, 87, 57–64. [Google Scholar] [CrossRef]
- Salemdeeb, R.; zu Ermgassen, E.K.H.J.; Kim, M.H.; Balmford, A.; Al-Tabbaa, A. Environmental and health impacts of using food waste as animal feed: A comparative analysis of food waste management options. J. Clean. Prod. 2017, 140, 871–880. [Google Scholar] [CrossRef]
- Gooding, C.H.; Meeker, D.L. Review: Comparison of 3 alternatives for large-scale processing of animal carcasses and meat by-products. Prof. Anim. Sci. 2016, 32, 259–270. [Google Scholar] [CrossRef]
- Mottet, A.; de Haan, C.; Falcucci, A.; Tempio, G.; Opio, C.; Gerber, P. Livestock: On our plates or eating at the table? A new analysis of the feed/food debate. Global Food Secur. 2017, 14, 1–8. [Google Scholar] [CrossRef]
- Hamilton, C.R. Real and perceived issues involving animal protein. In Protein Sources for the Animal Feed Industry, Proc. Expert Consultation and Workshop, Bangkok, 28 April–3 May, 2002; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004. [Google Scholar]
- FAO. FAOSTAT: Statistical Databases. 2020. Available online: http://www.fao.org/faostat/en/#data/ (accessed on 6 July 2020).
- Dou, Z.; Toth, J.D.; Westendorf, M.L. Food waste for livestock feeding: Feasibility, safety, and sustainability. Global Food Secur. 2018, 17, 154–161. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Nutrient Flows and Associated Environmental Impacts in Livestock Supply Chains: Guidelines for Assessment (Version 1); Livestock Environmental Assessment and performance (LEAP) Partnership; FAO: Rome, Italy, 2018; p. 196. [Google Scholar]
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; de Vries, W.; de Wit, C.A. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 1259855. [Google Scholar] [CrossRef]
- Bouwman, L.; Goldewijk, K.K.; Van Der Hoek, K.W.; Beusen, A.H.W.; Van Vuuren, D.P.; Willems, J.; Rufino, M.C.; Stehfest, E. Exploring global changes in nitrogen and phosphorus cycles in agriculture induced by livestock production over the 1900–2050 period. Proc. Natl. Acad. Sci. USA 2013, 110, 20882–20887. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.A.; Bleeker, A.; Howard, C.; Bekunda, M.; Grizzetti, B.; de Vries, W.; van Grinsven, H.; Abrol, Y.; Adhya, T.; Billen, G.; et al. Our nutrient world: The challenge to produce more food and energy with less pollution. In Global Overview of Nutrient Management; Sutton, M.A., Bleeker, A., Howard, C.M., Bekunda, M., Grizzetti, B., de Vries, W., van Grinsven, H.J.M., Abrol, Y.P., Adhya, T.K., Billen, G., et al., Eds.; CEH/UNEP: Edinburgh, UK, 2013. [Google Scholar]
- Meena, V.S.; Verma, J.P.; Meena, S.K. Towards the current scenario of nutrient use efficiency in crop species. J. Clean. Prod. 2015, 102, 556–557. [Google Scholar] [CrossRef]
- Oenema, O.; Ju, X.; de Klein, C.; Alfaro, M.; del Prado, A.; Lesschen, J.P.; Zheng, X.; Velthof, G.; Ma, L.; Gao, B.; et al. Reducing nitrous oxide emissions from the global food system. SI Syst. Dyn. Sustain. 2014, 9–10, 55–64. [Google Scholar] [CrossRef]
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision; ESA Workshop Paper 12-03; Agricultural Development Economics Division; FAO: Rome, Italy, 2012. [Google Scholar]
- Kim, S.W.; Less, J.F.; Wang, L.; Yan, Y.; Kiron, V.; Kaushik, S.J.; Lei, Z.G. Meeting global feed protein demand: Challenge, opportunity, and strategy. Annu. Rev. Anim. Biosci. 2019, 7, 221–243. [Google Scholar] [CrossRef] [PubMed]
- Cordell, D.; White, S. Life’s bottleneck: Sustaining the world’s phosphorus for a food secure future. Ann. Rev. Environ. Resour. 2014, 39, 161–188. [Google Scholar] [CrossRef]
- Smil, V. Phosphorus in the environment: Natural flows and human interferences. Annu. Rev. Energy Environ. 2000, 25, 53–88. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.-O.; White, S. The story of phosphorus: Global food security and food for thought. Global Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Fixen, P. Phosphorus: Worldwide supplies and efficiency. In Proceedings of the Presented at the Manitoba Agronomists Conference, Winnipeg, MB, Canada, 16 December 2009. [Google Scholar]
- van Vuuren, D.P.; Bouwman, A.F.; Beusen, A.H.W. Phosphorus demand for the 1970–2100 period: A scenario analysis of resource depletion. Global Environ. Chang. 2010, 20, 428–439. [Google Scholar] [CrossRef]
- van Kauwenbergh, S.J. World Phosphorus Rock Reserves and Resources; Tech. Bull. T-75; International Fertilizer Development Center (IFDC): Muscle Shoals, AL, USA, 2010. [Google Scholar]
- Cordell, D.; White, S.; Lindström, T. Peak Phosphorus: The crunch time for humanity? Sustain. Rev. 2011, 2, 1. Available online: https://www.thesustainabilityreview.org/articles/peak-phosphorus-the-crunch-time-for-humanity (accessed on 29 August 2020).
- Mohr, S.; Evans, G. Projections of Future Phosphorus Production. PHILICA.COM, Article 380, 9 July 2013. Available online: http://www.resilience.org/wp-content/uploads/articles/General/2013/09_Sep/peak-phosphorus/Phosphorus%20Projections.pdf (accessed on 6 July 2020).
- Walan, P. Modeling of Peak Phosphorus a Study of Bottlenecks and Implications. Master’s Thesis, Department of Earth Sciences, Uppsala University, Uppsala, Sweden, 2013. [Google Scholar]
- Carpenter, S.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Smith, V.; Schindler, D. Eutrophication science: Where do we go from here? Trends Ecol. Evol. 2009, 24, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Leinweber, P.; Bathmann, U.; Buczko, U.; Douhaire, C.; Eichler-Löbermann, B.; Frossard, E.; Ekardt, F.; Jarvie, H.; Krämer, I.; Kabbe, C.; et al. Handling the phosphorus paradox in agriculture and natural ecosystems: Scarcity, necessity, and burden of P. Ambio 2018, 47 (Suppl. 1), S3–S19. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Swine, 11th ed.; Natl. Acad. Press: Washington, DC, USA, 2012. [Google Scholar]
- García, A.J.; Esteban, M.B.; Márquez, M.C.; Ramos, P. Biodegradable municipal solid waste: Characterization and potential use as animal feedstuffs. Waste Manag. 2005, 25, 780–787. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, T.; Li, H. Hydrothermal treatment for inactivating some hygienic microbial indicators from food waste-amended animal feed. J. Air Waste Manag. Assoc. 2012, 62, 810–816. [Google Scholar] [CrossRef][Green Version]
- Chen, T.; Jin, Y.; Shen, D. A safety analysis of food waste-derived animal feeds from three typical conversion techniques in China. Waste Manag. 2015, 45, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Truong, L.; Morash, D.; Liu, Y.; King, A. Food waste in animal feed with a focus on use for broilers. Int. J. Recycl. Organic Waste Agric. 2019, 8, 417–429. [Google Scholar] [CrossRef]
- Pomar, C.; Remus, A. Precision pig feeding: A breakthrough toward sustainability. Anim. Front. 2019, 9, 52–59. [Google Scholar] [CrossRef]
- Fung, L.; Urriola, P.E.; Baker, L.; Shurson, G.C. Estimated energy and nutrient composition of difference sources of food waste and their potential use in sustainable swine feeding programs. Transl. Anim. Sci. 2019, 3, 143–152. [Google Scholar] [CrossRef]
- Fung, L.; Urriola, P.E.; Shurson, G.C. Energy, amino acid, and phosphorus digestibility and energy prediction of thermally processed food waste sources for swine. Transl. Anim. Sci. 2019, 3, 676–691. [Google Scholar] [CrossRef]
- Angulo, J.; Mahecha, L.; Yepes, S.A.; Yepes, A.M.; Bustamente, G.; Jaramillo, H.; Valencia, E.; Villamil, T.; Gallo, J. Nutritional evaluation of fruit and vegetable waste as a feedstuff for diets of lactating Holstein cows. J. Environ. Manage. Environ. Risks Probl. Strateg. Reduce Biotechnol. Eng. 2012, 95, S210–S214. [Google Scholar] [CrossRef]
- Ishida, K.; Yani, S.; Kitagawa, M.; Oishi, K.; Hirooka, H.; Kumagai, H. Effects of adding food by-products mainly including noodle waste to total mixed ration silage on fermentation quality, feed intake, digestibility, nitrogen utilization and ruminal fermentation in wethers. Anim. Sci. J. 2012, 83, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Paek, B.H.; Kang, S.W.; Chi, Y.M.; Cho, W.M.; Yang, C.J.; Yun, S.G. Effects of substituting concentrate with dried leftover food on growth and carcass characteristics of Hanwoo steers. Asian Australas. J. Anim. Sci. 2005, 18, 209–213. [Google Scholar] [CrossRef]
- Summers, J.D.; Macleod, G.K.; Warner, W.C. Chemical composition of culinary wastes and their potential as a feed for ruminants. Anim. Feed Sci. Technol. 1980, 5, 205–214. [Google Scholar] [CrossRef]
- Cheng, Z.; Mo, W.Y.; Man, Y.B.; Lam, C.L.; Choi, W.M.; Nie, X.P.; Liu, Y.H.; Wong, M.H. Environmental mercury concentrations in cultured low-trophic-level fish using food waste-based diets. Environ. Sci. Pollut. Res. 2015, 22, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Damron, B.; Waldroup, P.; Harms, R. Evaluation of dried bakery products for use in broiler diets. Poult. Sci. 1965, 44, 1122–1126. [Google Scholar] [CrossRef]
- Farhat, A.; Normand, L.; Chavez, E.R.; Touchburn, S.P. Comparison of growth performance, carcass yield and composition, and fatty acid profiles of Pekin and Muscovy ducklings fed diets based on food wastes. Can. J. Anim. Sci. 2001, 81, 107–114. [Google Scholar] [CrossRef]
- Al-Tulaihan, A.; Najib, H.; Al-Eid, S. The nutritional evaluation of locally produced dried bakery waste (DBW) in the broiler diets. Pak. J. Nutr. 2004, 3, 294–299. [Google Scholar]
- Ayanwale, B.; Aya, V. Nutritional evaluation of cornflakes waste in diets of broilers. Pak. J. Nutr. 2006, 5, 485–489. [Google Scholar]
- Stefanello, C.; Vieira, S.; Xue, P.; Ajuwon, K.; Adeola, O. Age-related energy values of bakery meal for broiler chickens determined using the regression method. Poult. Sci. 2016, 95, 1582–1590. [Google Scholar] [CrossRef]
- Kwak, W.S.; Kang, J. Effect of feeding food waste-broiler litter and bakery by-product mixture to pigs. Bioresour. Technol. 2006, 97, 243–249. [Google Scholar] [CrossRef]
- Almeida, F.N.; Petersen, G.I.; Stein, H.H. Digestibility of amino acids in corn, corn coproducts, and bakery meal fed to growing pigs. J. Anim. Sci. 2011, 89, 4109–4115. [Google Scholar] [CrossRef] [PubMed]
- Rojas, O.J.; Liu, Y.; Stein, H.H. Phosphorus digestibility and concentration of digestible and metabolizable energy in corn, corn coproducts, and bakery meal fed to growing pigs. J. Anim. Sci. 2013, 91, 5326–5335. [Google Scholar] [CrossRef] [PubMed]
- Hammoumi, A.; Faid, M.; El Yachioui, M.; Amarouch, H. Characterization of fermented fish waste used in feeding trials with broilers. Process. Biochem. 1997, 33, 423–427. [Google Scholar] [CrossRef]
- Joshi, V.; Gupta, K.; Devrajan, A.; Lal, B.; Arya, S. Production and evaluation of fermented apple pomace in the feed for broilers. J. Food Sci. Technol. 2000, 37, 609–612. [Google Scholar]
- Wadhwa, W.; Bakshi, M.; Makkar, H. Utilization of Fruit and Vegetable Wastes as Livestock Feed and as Substrates for Generations of other Value-Added Products; FAO: Rome, Italy, 2013. [Google Scholar]
- Fard, S.; Toghyani, M.; Tabeidian, S. Effect of oyster mushroom wastes on performance, immune responses and intestinal morphology of broiler chickens. Int. J. Recycl. Org. Waste Agric. 2014, 3, 141–146. [Google Scholar] [CrossRef]
- Bakshi, M.; Wadhwa, M.; Makkar, P. Waste to worth: Vegetable wastes as animal feed. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2016, 11, 1–26. [Google Scholar] [CrossRef]
- Cho, Y.; Lee, G.; Jang, J.; Shin, I.; Myung, K.; Choi, K.; Bae, I.; Yang, C. Effects of feeding dried leftover food on growth and body composition of broiler chicks. Asian-Aust. J. Anim. Sci. 2004, 17, 386–393. [Google Scholar] [CrossRef]
- Kojima, S. Dehydrated kitchen waste as a feedstuff for laying hens. Int. J. Poult. Sci. 2005, 4, 689–694. [Google Scholar]
- Asar, E.; Raheem, H.; Daoud, J. Using dried leftover food as nontraditional feed for Muscovy duck diet. Assiut. Vet. Med. J. 2018, 64, 107–114. [Google Scholar]
- Navidshad, B.; Adibmoradi, M.; Seifdavati, J. Effect of dietary levels of a modified meat meal on performance and small intestine morphology of broiler chicken. Afr. J. Biotechnol. 2009, 8, 5620–5626. [Google Scholar]
- Chen, H.-L.; Chang, H.-J.; Yang, C.-K.; You, S.-H.; Jeng, H.-D.; Yu, B. Effect of dietary inclusion of dehydrated food waste products on Taiwan native chicken (Taishi No.13). Asian Australas. J. Anim. Sci. 2007, 20, 754–760. [Google Scholar] [CrossRef]
- Kornegay, E.T.; Vander Noot, G.W.; Barth, K.M.; McGrath, W.S.; Welch, J.G.; Purkhiser, E.D. Nutritive value of garbage as a feed for swine. I. Chemical composition, digestibility and nitrogen utilization of various types of garbage. J. Anim. Sci. 1965, 24, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Hossein, S. Growth Performances, Carcass Yield and Meat Quality of Free-Range Village Chickens Fed on Diet Containing Dehydrated Processed Food Waste. Master’s Thesis, Universiti Putra Malaysia, Seri Kembangan, Malaysia, 2015. [Google Scholar]
- Westendorf, M.L.; Schuler, T.; Zirkle, E.W. Nutritional quality of recycled food plate waste in diets fed to swine. Prof. Anim. Sci. 1999, 15, 106–111. [Google Scholar] [CrossRef]
- Westendorf, M.L.; Zirkle, E.W.; Gordon, R. Feeding food or table waste to livestock. Prof. Anim. Sci. 1996, 12, 129–137. [Google Scholar] [CrossRef]
- Myer, R.O.; Brendemuhl, J.H.; Johnson, D.D. Evaluation of dehydrate restaurant food waste products as feedstuffs for finishing pigs. J. Anim. Sci. 1999, 77, 685–692. [Google Scholar] [CrossRef]
- Chae, B.J.; Choi, S.C.; Kim, Y.G.; Kim, C.H.; Sohn, K.S. Effects of feeding dried food waste on growth and nutrient digestibility of growing-finishing pigs. Asian Australas. J. Anim. Sci. 2000, 13, 1304–1308. [Google Scholar] [CrossRef]
- Choe, J.; Moyo, K.M.; Park, K.; Jeong, J.; Kim, H.; Ryu, Y.; Kim, J.; Kim, J.-M.; Lee, S.; Go, G.-W. Meat quality traits of pigs finished on food waste. Korean J. Food Sci. Anim. Resour. 2017, 37, 690–697. [Google Scholar] [CrossRef]
- Ruttanavut, J.; Yamauchi, K.; Thongwittaya, N. Utilization of eco-feed containing mugwort microorganism compounds as a feed ingredient source for layer hens. Am. J. Anim. Vet. Sci. 2011, 6, 35–39. [Google Scholar] [CrossRef][Green Version]
- Food and Agriculture Organization. 1. Basic Foodstuffs. Available online: http://www.fao.org/3/Y4343E/y4343e02.htm (accessed on 6 July 2020).
- Noblet, J.; Perez, J.M. Prediction of digestibility of nutrients and energy values of pig diets from chemical analysis. J. Anim. Sci. 1993, 71, 3389–3398. [Google Scholar] [CrossRef]
- Kerr, B.J.; Jha, R.; Urriola, P.E.; Shurson, G.C. Nutrient composition, digestible and metabolizable energy content, and prediction of energy for animal protein byproducts in finishing pig diets. J. Anim. Sci. 2017, 95, 2614–2626. [Google Scholar] [CrossRef]
- Ferguson, N. Commercial application of integrated models to improve performance and profitability in pigs and poultry. In Nutritional Modelling in Pigs and Poultry; Sakmoura, N., Gous, R., Kyriazakis, I., Hauschild, L., Eds.; CABI: Wallingford, Oxfordshire, UK, 2014; pp. 141–156. [Google Scholar]
- Saddoris-Clemons, K.; Schneider, J.; Feoli, C.; Cook, D.; Newton, B. Cost-effective feeding strategies for grow-finish pigs. Adv. Pork Prod. 2011, 22, 187–194. [Google Scholar]
- FAO. Livestock’s Long Shadow—Environmental Issues and Options; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006. [Google Scholar]
- LEAP. Environmental Performance of Animal Feed Supply Chains: Guidelines for Assessment; Livest. Environ. Assess. Perform. Partnership; Food and Agriculture Organization: Rome, Italy, 2015. [Google Scholar]
- Notarnicola, B.; Sala, S.; Anton, A.; McLaren, S.J.; Saouter, E.; Sonesson, U. The role of life cycle assessment in supporting sustainable agri-food systems: A review of the challenges. J. Clean. Prod. 2017, 140, 399–409. [Google Scholar] [CrossRef]
- Sala, S.; Anton, A.; McLaren, S.J.; Notarnicola, B.; Saouter, E.; Sonesson, U. In quest of reducing the environmental impacts of food production and consumption. J. Clean. Prod. 2017, 140, 387–398. [Google Scholar] [CrossRef]
- Feed Print Database. Available online: http://webapplicaties.wur.nl/software/feedprintNL/index.asp (accessed on 6 July 2020).
- FAO. Executive Summary: Expert Consultation and Workshop on Protein Sources for the Animal Feed Industry; Food and Agriculture Organization in association with the International Feed Industry Federation: Bangkok, Thailand, 2002. [Google Scholar]
- Gooding, C.H. Data for the carbon footprinting of rendering operations. J. Indust. Ecol. 2012, 16, 223–230. [Google Scholar] [CrossRef]
- Taylor, D.; Woodgate, S.; Atkinson, M. Inactivation of the bovine spongiform encephalopathy agent by rendering procedures. Vet. Rec. 1995, 137, 605–610. [Google Scholar] [PubMed]
- Wichuk, K.; McCartney, D. A review of the effectiveness of current time-temperature regulations on pathogen inactivation during composting. J. Environ. Eng. Sci. 2007, 6, 573–586. [Google Scholar] [CrossRef]
- Franke-Whittle, I.; Insam, H. Treatment alternatives of slaughterhouse wastes, and their effect on the inactivation of different pathogens: A review. Crit. Rev. Microbiol. 2013, 39, 139–151. [Google Scholar] [CrossRef]
- Masse, D.; Talbot, G.; Gilbert, Y. On farm biogas production: A method to reduce GHG emissions and develop more sustainable livestock operation. Anim. Feed Sci. Technol. 2011, 166–167, 436–445. [Google Scholar] [CrossRef]
- Dee, S.A.; Niederwerder, M.C.; Patterson, G.; Cochrane, R.; Jones, C.; Diel, D.; Brockhoff, E.; Nelson, E.; Spronk, G.; Sundberg, P. The risk of viral transmission in feed: What do we know, what do we do? Transbound. Emerg. Dis. 2020, 1–7. [Google Scholar] [CrossRef]
- Auvermann, B.; Kalbasi, A.; Ahmed, A. Rendering, Chapter 4. In Carcass Disposal: A Comprehensive Review; National Agricultural Biosecurity Center Consortium; USDA APHIS Cooperative Agreement Project; Carcass Disposal Working Group; Kansas State University: Manhattan, KS, USA, 2004; p. 76. [Google Scholar]
- Kotula, A.W.; Murrell, K.D.; Acosta-Stein, L.; Lamb, L.; Douglass, L. Trichinella spiralis: Effect of high temperature on infectivity in pork. Exp. Parasitol. 1983, 56, 15–19. [Google Scholar] [CrossRef]
- Alizadeh, A.M.; Jazaeri, S.; Shemshadi, B.; Hashempour-Baltork, F.; Sarlak, Z.; Pilevar, Z.; Hosseini, H. A review on inactivation methods of Toxoplasma gondii in foods. Pathogens Glob. Health 2018, 112, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Duong, T.M. Qualitative Assessment of Pig Health Risks Related to the Use of Food Waste for Pig Production in Sub-Urban Area of Hanoi Capital. Master’s Thesis, Kasetsart University, Bangkok, Thailand, 2016. [Google Scholar]
- Knight, A.I.; Haines, J.; Zuber, S. Thermal inactivation of animal virus pathogens. Curr. Top. Virol. 2013, 11, 103–119. [Google Scholar]
- Taylor, D.M. Inactivation of transmissible degenerative encephalopathy agents: A review. Vet. J. 2000, 159, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Burr, W.E.; Helmboldt, C.F. Salmonella species contaminants in three animal by-products. Avian Dis. 1962, 6, 441–443. [Google Scholar] [CrossRef]
- Veldman, A.; Vahl, H.A.; Borggreve, G.J.; Fuller, D.C. A survey of the incidence of Salmonella species and Enterobacteriaceae in poultry feeds and feed components. Vet. Rec. 1995, 136, 169–172. [Google Scholar] [CrossRef]
- Edwards, P.R.; Bruner, D.W.; Moran, A.B. The genus Salmonella: Its occurrence and distribution in the United States. Kentucky Agric. Exp. Sta. Bull. 1948, 525, 1–60. [Google Scholar]
- Crump, J.A.; Griffin, P.M.; Angulo, F.J. Bacterial contamination of animal feed and its relationship to human foodborne illness. Clin. Infect. Dis. 2002, 35, 859–865. [Google Scholar] [CrossRef]
- Davies, P.R. The Role of Contaminated Feed in the Epidemiology of Salmonella in Modern Swine Production; Proc. CDC Animal Feeds Workshop/Symposium: Atlanta, GA, USA, 2004. [Google Scholar]
- Sapkota, A.R.; Lefferts, L.Y.; McKenzie, S.; Walker, P. What do we feed to food-production animals? A review of animal feed ingredients and their potential impacts on human health. Environ. Health Perspect. 2007, 115, 663–670. [Google Scholar] [CrossRef]
- Boyer, C.I.; Bruner, D.W.; Brown, J.A. Salmonella organisms isolated from poultry feed. J. Avian Dis. 1958, 2, 396. [Google Scholar] [CrossRef]
- Watkins, J.R.; Flowers, A.I.; Grumbles, L.C. Salmonella organisms in animal products used in poultry feeds. Avian Dis. 1959, 3, 290. [Google Scholar] [CrossRef]
- Pomeroy, B.S.; Grady, M.K. Salmonella Organisms Isolated from Feed Ingredients; Proc. 65th Annual Meeting of the U.S. Livestock Sanitation Association; MacCrellish & Quigley Co.: Trenton, NJ, USA, 1962; Volume 65, pp. 449–452. [Google Scholar]
- Harris, I.T.; Fedorka-Cray, P.J.; Gary, J.T.; Thomas, L.A. Prevalence of Salmonella organisms in swine feed. J. Am. Vet. Med. Assoc. 1997, 210, 382–385. [Google Scholar] [PubMed]
- Franco, D.A. A survey of Salmonella serovars and most probable numbers in rendered animal protein meals: Inferences for animal and human health. J. Environ. Health 2005, 67, 18–22. [Google Scholar]
- Franco, D.A. The genus salmonella. In Proceedings of the Animal Protein Producers Industry; Inst. Contin. Education: Washington, DC, USA, 1999; pp. 1–22. [Google Scholar]
- Canadian Food Inspection Agency. 2005. Available online: https://www.inspection.gc.ca/food-safety-for-industry/archived-food-guidance/fish-and-seafood/manuals/standards-and-methods/eng/1348608971859/1348609209602?chap=7#s19c7 (accessed on 6 July 2020).
- Sreenivas, P.T. Salmonella-control strategies for the feed industry. Feed Mix. 1988, 6, 8. Available online: https://agris.fao.org/agris-search/search.do?recordID=US201302898369 (accessed on 6 July 2020).
- Beumer, H.; van der Poel, A.F.B. Effects on hygienic quality of feeds examined. Feedstuffs 1997, 13, 13–15. [Google Scholar]
- Brooks, P. Technical Service Publication, 1989; National Renderers Association, Inc.; Canadian Food Inspection Agency: Ottawa, ON, Canada, 1989. [Google Scholar]
- Isa, J.M.; Boycott, B.R.; Broughton, E. A survey of Salmonella contamination in animal feeds and feed constituents. Can. Vet. J. 1963, 4, 41–43. [Google Scholar] [PubMed]
- Troutt, H.F.; Schaeffer, D.; Kakoma, I.; Pearl, G.G. Prevalence of Selected Foodborne Pathogens in Final Rendered Products; Fats and Proteins Research Foundation, Inc.: Alexandria, VA, USA, 2001. [Google Scholar]
- Davies, P.R.; Funk, J.A. Proc. 3rd International Symposium on the Epidemiology and Control of Salmonella in Pork; Safe Pork Conference, Iowa State University: Ames, IA, USA, 1999; pp. 1–11. [Google Scholar]
- Wilesmith, J.W. 1998. Manual on Bovine Spongiform Encephalopathy. FAO, Rome. Available online: http://www.fao.org/3/W8656E/W8656E00.htm (accessed on 6 July 2020).
- Prince, M.J.; Bailey, J.A.; Barrowman, P.R.; Bishop, K.J.; Campbell, G.R.; Wood, J.M. Bovine spongiform encephalopathy. Rev. Sci. Tech. Off. Int. Épizooties 2003, 22, 37–60. [Google Scholar] [CrossRef]
- Hörnlimann, B.; Bachmann, J.; Bradley, R. Portrait of bovine spongiform encephalopathy in cattle and other ungulates. In Prions in Humans and Animals; Hörnlimann, B., Riesner, D., Kretschmar, H., Eds.; De Gruyter: Berlin, Germany, 2007; pp. 233–249. [Google Scholar]
- Kimberlin, R.H. Scrapie and possible relationships with viroids. Se. Virol. 1990, 1, 153–162. [Google Scholar]
- Jahns, H.; Callanan, J.J.; Sammin, D.J.; McElroy, M.C.; Bassett, H.F. Survey for transmissible spongiform encephalopathies in Irish pigs fed meat and bone meal. Vet. Rec. 2006, 159, 137–142. [Google Scholar] [CrossRef]
- Wells, G.A.; Hawkins, S.A.; Austin, A.R.; Ryder, S.J.; Done, S.H.; Green, R.B.; Dexter, I.; Dawson, M.; Kimberlin, R.H. Studies of the transmissibility of the agent of bovine spongiform encephalopathy to pigs. J. Gen. Virol. 2003, 84, 1021–1031. [Google Scholar] [CrossRef]
- Cutlip, R.C.; Miller, J.M.; Hamir, A.N.; Peters, J.; Robinson, M.M.; Jenny, A.L.; Lehmkuhl, H.D.; Taylor, W.D.; Bisplinghoff, F.D. Resistance of cattle to scrapie by the oral route. J. Infect. Dis. 2001, 169, 814–820. [Google Scholar] [CrossRef]
- Franco, D.A. An Introduction to the Prion Diseases of Animals: Assessing the History, Risk Inferences, and Public Health Implications in the United States; National Renderers Association: Alexandria, VA, USA, 2005; pp. 1–32. [Google Scholar]
- Hill, A.F.; Desbruslais, M.; Joiner, S.; Sidle, K.C.; Gowland, I.; Collinge, J.; Doey, L.J.; Lantos, P. The same prion strain causes vCJD and BSE. Nature 1997, 389, 448–450. [Google Scholar] [CrossRef] [PubMed]
- Chesboro, B. A fresh look at BSE. Science 2004, 305, 1918–1921. [Google Scholar] [CrossRef] [PubMed]
- Richt, J.A.; Hall, S.M. BSE case associated with prion protein gene mutation. PLoS Pathog. 2008, 4, e1000156. [Google Scholar] [CrossRef]
- Kamisato, T. BSE crisis in Japan: A chronological overview. Environ. Health Prevent. Med. 2005, 10, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Food Standards Australia and New Zealand. 2020. Available online: http://www.foodstandards.gov.au/industry/bse/beefexport/pages/default.aspx (accessed on 6 July 2020).
- Beltran-Alcrudo, D.; Falco, J.R.; Raizman, E.; Dietze, K. Transboundary spread of pig diseases: The role of international trade and travel. BMC Vet. Res. 2019, 15, 64. [Google Scholar] [CrossRef]
- Dee, S.; Clement, T.; Schelkopf, A.; Nerem, J.; Knudsen, D.; Christopher-Hennings, J.; Nelson, E. An evaluation of contaminated complete feed as a vehicle for porcine epidemic diarrhea virus infection of naïve pigs following consumption via natural feeding behavior: Proof of concept. BMC Vet. Res. 2014, 10, 176. [Google Scholar] [CrossRef]
- Pasick, J.; Berhane, Y.; Ojkic, D.; Maxie, G.; Embury-Hyatt, C.; Swekla, K.; Handel, K.; Fairles, J.; Alexandersen, S. Investigation into the role of potentially contaminated feed as a source of the first-detected outbreaks of Porcine Epidemic Diarrhea in Canada. Transbound. Emerg. Dis. 2014, 61, 397–410. [Google Scholar] [CrossRef]
- Pasma, T.; Furness, M.C.; Alves, D.; Aubry, P. Outbreak investigation of porcine epidemic diarrhea in swine in Ontario. Can. Vet. J. 2016, 57, 84–88. [Google Scholar]
- Aubry, P.; Thompson, J.L.; Pasma, T.; Furness, M.C.; Tataryn, J. Weight of the evidence linking feed to an outbreak of porcine epidemic diarrhea in Canadian swine herds. J. Swine Health Prod. 2017, 25, 69–72. [Google Scholar]
- Perri, A.M.; Poljak, Z.; Dewey, C.; Harding, J.C.S.; O’Sullivan, T.L. An epidemiological investigation of the early phase of the porcine epidemic diarrhea (PED) outbreak in Canadian swine herds in 2014: A case-control study. Prev. Vet. Med. 2018, 150, 101–109. [Google Scholar] [CrossRef]
- Scott, A.; McCluskey, B.; Brown-Reid, M.; Grear, D.; Pitcher, P.; Ramos, G.; Spencer, D.; Singrey, A. Porcine epidemic diarrhea virus introduction into the United States: Root cause investigation. Preventive Vet. Med. 2016, 123, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Schulz, L.L.; Tonsor, G.T. Assessment of the economic impacts of porcine epidemic diarrhea virus in the United States. J. Anim. Sci. 2015, 93, 5111–5118. [Google Scholar] [CrossRef] [PubMed]
- Trudeau, M.P.; Verma, H.; Sampedro, F.; Urriola, P.E.; Shurson, G.C.; Goyal, S.M. Environmental persistence of porcine coronaviruses in feed and feed ingredients. PLoS ONE 2017, 12, e0178094. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.K.; Stahl, K.; Jori, F.; Vial, L.; Pfeiffer, D.U. African swine fever epidemiology and control. Annu. Rev. Anim. Biosci. 2020, 8, 221–246. [Google Scholar] [PubMed]
- Dee, S.A.; Bauermann, F.V.; Niederwerder, M.C.; Singrey, A.; Clement, T.; de Lima, M.; Long, C.; Patterson, G.; Sheahan, M.A.; Stolan, A.M.M.; et al. Survival of viral pathogens in animal feed ingredients under transboundary shipping models. PLoS ONE 2018, 13, e0194509. [Google Scholar] [CrossRef] [PubMed]
- USDA-APHIS-VS-Center for Epidemiology and Animal Health, Risk Assessment Team. Qualitative Assessment of the Likelihood of African Swine Fever Virus Entry to the United States: Entry Assessment. Fort Collins, CO, 8 pp. Available online: https://www.aphis.usda.gov/animal_health/downloads/animal_diseases/swine/asf-entry.pdf (accessed on 6 July 2020).
- Westendorf, M.L. Food waste as animal feed: An introduction. In Food Waste to Animal Feed; Westendorf, M.L., Ed.; Iowa State University Press: Ames, IA, USA, 2000; pp. 91–111. [Google Scholar]
- Menikpura, S.N.M.; Sang-Arun, J.; Bengtsson, M. Integrated solid waste management: An approach for enhancing climate co-benefits through resource recovery. J. Clean. Prod. 2013, 58, 34–42. [Google Scholar] [CrossRef]
- Liu, C.; Hotta, Y.; Santo, A.; Hengesbaugh, M.; Watabe, A.; Totoki, Y.; Allen, D.; Bengstsson, M. Food waste in Japan: Trends, current practices and key challenges. J. Clean. Prod. 2016, 133, 557–564. [Google Scholar] [CrossRef]
- Sugiura, K.; Yamatani, S.; Watahara, M.; Onodera, T. Ecofeed, animal feed produced from recycled food waste. Vet. Ital. 2009, 45, 397–404. [Google Scholar]
- European Commission. Regulation (EC) No. 1774/2002 of the European Parliament and of the Council of 3 October 2002 Laying down Health Rules Concerning Animal By-Products not Intended for Human Consumption. Available online: https://op.europa.eu/en/publication-detail/-/publication/28ab554e-8e93-4976-89a9-8b6c9d17dfb4/language-en (accessed on 29 August 2020).
- Muroga, N.; Hayama, Y.; Yamamoto, T.; Kurogi, A.; Tsuda, T.; Tstsui, T. The 2010 foot-and-mouth disease epidemic in Japan. J. Vet. Med. Sci. 2012, 74, 399–404. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, K.-N.; Ko, Y.-J.; Kim, S.-M.; Lee, H.-S.; Shin, Y.-K.; Sohn, H.-J.; Park, J.-Y.; Yeh, J.-Y.; Lee, Y.-H.; et al. Control of foot-and-mouth disease during 2010-2011 Epidemic, South Korea. Emerg. Infect. Dis. 2013, 19, 655–659. [Google Scholar]
- European Commission. Regulation (EC) No. 999/2001 of the European Parliament and of the Council of 22 May 2001 Laying down Rules for the Prevention, Control and Eradication of Certain Transmissible Spongiform Encephalopathies. Available online: https://www.legislation.gov.uk/eur/2001/999/contents# (accessed on 6 July 2020).
- Andreoletti, O.; Budka, H.; Buncic, S.; Colin, P.; Collins, J.D.; De Koeijer, A.; Griffin, J.; Havelaar, A.; Hope, J.; Klein, G. Opinion of the scientific panel on biological hazards on a request from the European parliament on certain aspects related to the feeding of animal proteins to farm animals. EFSA J. 2007, 576, 1–41. [Google Scholar]
- Whitehead, M.L.; Roberts, V. Backyard poultry: Legislation, zoonoses and disease prevention. J. Small Anim. Pract. 2014, 55, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Raymundo, D.; Carloto Gomes, D.; Boabaid, F.; Colodel, E.; Schmitz, M.; Correa, A.; Dutra, I.; Driemeier, D. Type C botulism in swine fed restaurant waste. Pesqui. Vet. Brasil. 2012, 32, 1145–1147. [Google Scholar] [CrossRef]
- Zimmerman, J.J.; Karriker, L.A.; Ramirez, A.; Schwartz, K.J.; Stevenson, G.W. Diseases of Swine, 10th ed.; Wiley-Blackwell: Ames, IA, USA, 2012; p. 1008. [Google Scholar]
- Gamble, H.R.; Murrell, K.D. Trichinellosis. In Laboratory Diagnosis of Infectious Disease: Principles and Practice; Balows, A., Hausler, W.J., Lennette, E.H., Eds.; Springer: New York, NY, USA, 1998; p. 1018. [Google Scholar]
- U.S. Congressional Record. In Proceedings of the Swine Health Protection Act. H.R. 6593. Public Lay 96-468, 96th Congress, 17 October 1980. Available online: https://www.congress.gov/public-laws/96th-congress (accessed on 11 July 2020).
- U.S. Food and Drug Administration. 2017. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=589&showFR=1 (accessed on 6 July 2020).
- Broad Leib, E.; Blakus, O.; Rice, C.; Maley, M.; Taneja, R.; Cheng, R.; Civita, N.; Alvoid, T. Leftovers for Livestock: A Legal Guide for Using Excess Food as Animal Feed; Harvard Food Law and Policy Clinic: Cambridge, MA, USA, 2016. [Google Scholar]
- Rethink Food Waste through Economics and Data (ReFED). Roadmap to Reduce, U.S. Food Waste by 20 Percent. 2016. Available online: https://www.refed.com/downloads/Executive-Summary.pdf (accessed on 6 July 2020).
- Sanchez-Vizcaino, J.M. African swine fever. In Diseases of Swine, 8th ed.; Mengeling, W.L., Ed.; Iowa State University Press: Ames, IA, USA, 1999; pp. 93–112. [Google Scholar]
- House, J.A.; House, C.A. Vesicular diseases. In Diseases of Swine, 8th ed.; Mengeling, W.L., Ed.; Iowa State University Press: Ames, IA, USA, 1999; pp. 327–340. [Google Scholar]
- van Oirschot, J.T. Classical swine fever (hog cholera). In Diseases of Swine, 8th ed.; Mengeling, W.L., Ed.; Iowa State University Press: Ames, IA, USA, 1999; pp. 159–172. [Google Scholar]
- Costard, S.; Porphyre, V.; Messad, S.; Rakotondrahanta, S.; Vidon, H.; Roger, F.; Pfeiffer, D. Multivariate analysis of management and biosecurity practices in smallholder pig farms in Madagascar. Prev. Vet. Med. 2009, 92, 199–209. [Google Scholar] [CrossRef]
- Kagira, J.M.; Kanyari, P.W.; Maingi, N.; Githigia, S.M.; Ng’ang’a, J.C.; Karuga, J.W. Characteristics of the smallholder free-range pig production system in western Kenya. Trop. Anim. Health Prod. 2010, 42, 865–873. [Google Scholar] [CrossRef]
- Phengsavanh, P.; Ogle, B.; Stür, W.; Frankow-Lindberg, B.E.; Lindberg, J.E. Feeding and performance of pigs in smallholder production systems in Northern Lao PDR. Trop. Anim. Health Prod. 2010, 42, 1627–1633. [Google Scholar] [CrossRef]
- Mazur-Panasiuk, N.; Żmudzki, J.; Woźniakowski, G. African swine fever virus -persistence in different environmental conditions and the possibility of its indirect transmission. J. Vet. Res. 2019, 63, 303–310. [Google Scholar] [CrossRef]
- Karunasagar, I. International risk assessment leading to development of food safety standards. Procedia Food Sci. 2016, 6, 34–36. [Google Scholar] [CrossRef]
- Dennis, S.B.; Miliotis, M.D.; Buchanan, R.L. Hazard Characterization/Dose-Response Assessment. Microbiological Risk Assessment in Food Processing; CRC Press: Boca Raton, FL, USA, 2002; Volume 301, pp. 77–99. [Google Scholar]
- FDA. 2018. Available online: https://www.fda.gov/files/animal%20&%20veterinary/published/CVM-GFI--246-Hazard-Analysis-and-Risk-Based-Preventive-Controls-for-Food-for-Animals--Supply-Chain-Program.pdf (accessed on 6 July 2020).
- Dalmasso, A.; Fontanella, E.; Piatti, P.; Civera, T.; Rosati, S.; Bottero, M.T. A multiplex PCR assay for the identification of animal species in feedstuffs. Molec. Cell. Probes 2004, 18, 81–87. [Google Scholar] [CrossRef]
- Lahiff, S.; Glennon, M.O.B.L.; Lyng, J.; Smith, T.; Maher, M.; Shelton, N. Species-specific PCR for the identification of ovine, porcine and chicken species in meat and bone meal (MBM). Molec. Cell. Probes 2001, 15, 27–35. [Google Scholar] [CrossRef]
- Schmidt, G.R.; Hossner, K.L.; Yemm, R.S.; Gould, D.H.; O’Callaghan, J.P. An enzyme-linked immunosorbent assay for glial fibrillary acidic protein as an indicator of the presence of brain or spinal cord in meat. J. Food Protect. 1999, 62, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Gray, P.; Piltch, M.; Bulgin, M.S.; Sorensen-Melson, S.; Miller, M.W.; Davies, P.; Brown, D.R.; Coughlin, D.R.; Rubenstein, R. Surround optical fiber immunoassay (SOFIA): An ultra-sensitive assay for prion protein detection. J. Virol. Methods 2009, 159, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Jinno, C.; He, Y.; Morash, D.; McNamara, E.; Zicari, S.; King, A.; Stein, H.H.; Liu, Y. Enzymatic digestion turns food waste into feed for growing pigs. Anim. Feed Sci. Technol. 2018, 242, 48–58. [Google Scholar] [CrossRef]
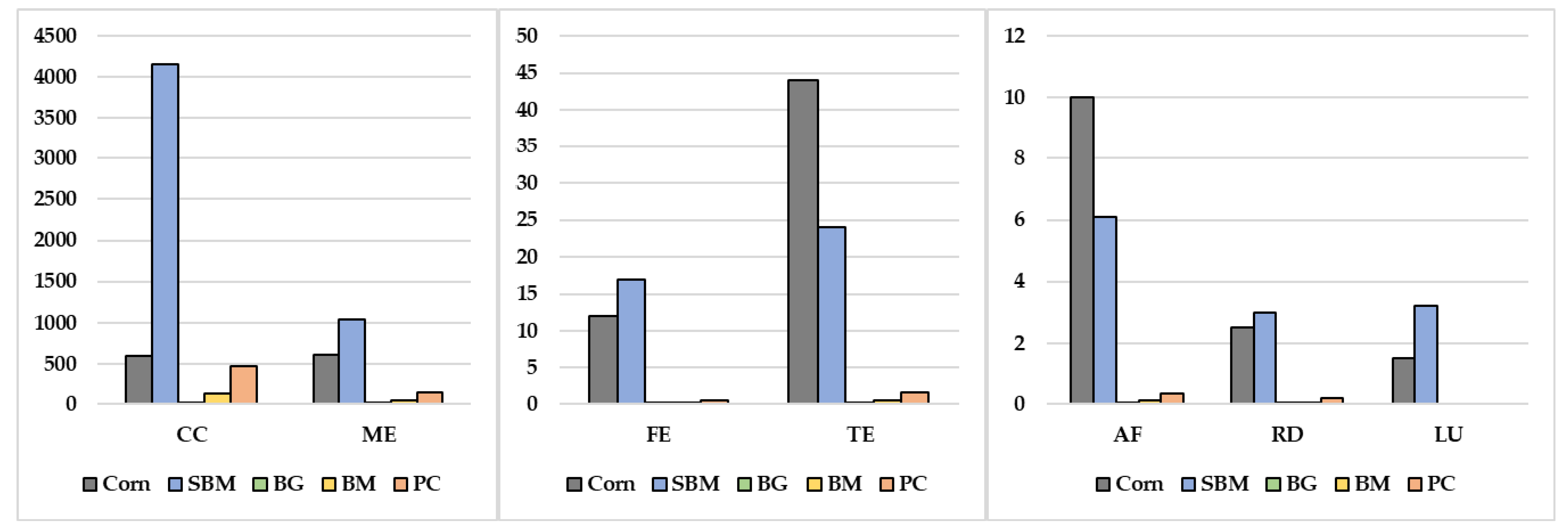
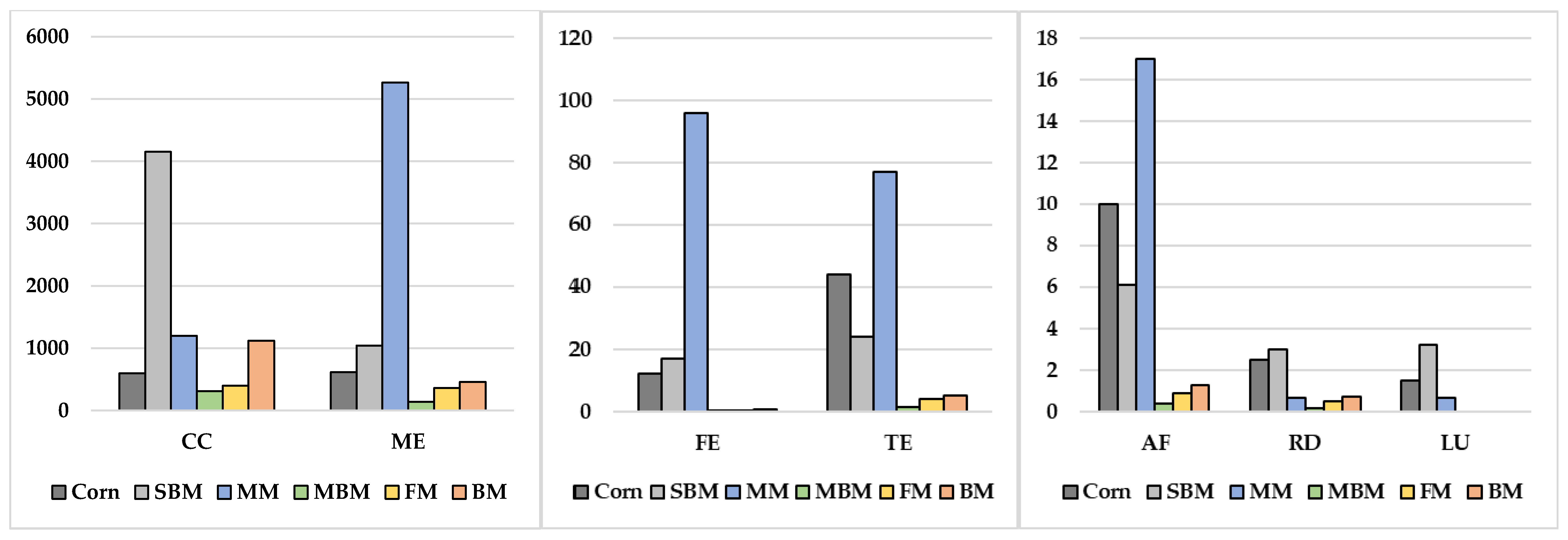
| Food Waste Source/Reference | Disposal Methods Compared | Environmental Indicators | Key Results |
|---|---|---|---|
| Household and catering food waste [36] | Composting Incineration Landfill 1 Dry animal feed | Global warming Human toxicity Acidification Eutrophication Ecotoxicity | Feed manufacturing had:
|
| Kitchen and food factory waste [37] | Wet animal feed (sterilization) Dry animal feed Incineration | Global warming Energy consumption Water use |
|
| Household and catering food waste [38] | Composting Dry animal feed Wet animal feed Landfill | Global warming | 1 tonne of food waste contributed:
|
| Household and catering food waste [39] | Anaerobic digestion Composting Dry animal feed Wet animal feed Incineration Landfill | Global warming Energy/resource recovery | Environmental benefit/cost ratio was:
|
| Catering, retail, and manufacturing food waste [40] | Anaerobic digestion Machine composting Windrow composting Dry animal feed Wet animal feed | Global warming Economics | 1 tonne of food waste contributed:
|
| Evaluated by-products used in animal feeds including distiller’s waste, rapeseed cake, whey permeate, fodder milk, and bakery residues [41] | Anaerobic digestion Anaerobic digestion with portion diverted to animal feed | Global warming | All industrial organic by-products evaluated are suitable for biogas production, provide substantial reduction in GHG compared with fossil fuels, but contribute to eutrophication and acidification potential. If these by-products are used as animal feed, the reduction would be significantly less. |
| Retail food waste [42] | Anaerobic digestion Dry animal feed (bread) fraction combined with anaerobic digestion of remaining fraction | 18 environmental and health impact categories | More environmental benefits were obtained by converting the bread waste portion into animal feed than by using only anaerobic digestion to produce heat and electricity. |
| Compared banana, chicken, lettuce, beef, bread [35] | Anaerobic digestion Dry animal feed Composting Donations Incineration Landfill | Global warming |
|
| Household and catering food waste [43] | Anaerobic digestion Composting Dry animal feed Wet animal feed | 14 environmental and health impact categories |
|
| Disposal Method | Pollution and Contamination | |||||
|---|---|---|---|---|---|---|
| Odor | GHG Emission | Air | Soil and Vegetation | Water | Land Application of Waste | |
| Burial | - | - | Very low | High | Moderate | NA |
| Burning | Very high | ?? | ?? | ?? | ?? | ?? |
| Incineration, on-farm a | Low | High | Low b | Low b | Low b | ?? |
| Incineration, large central facility | Very low | High | Moderate b | Moderate b | Moderate b | ?? |
| Rendering | Moderate | Low | ?? | Very low | Moderate | ?? |
| Composting c | Low | Low | ?? | Moderate | ?? | Low |
| Anaerobic digestion | Low | Very low | Very low | ?? | ?? | Low |
| Alkaline hydrolysis | Moderate | ?? | ?? | Low | Moderate | Moderate |
| Food Waste Source | Poultry Feeding Value References | Swine Feeding Value References |
|---|---|---|
| Bakery by-product/breakfast cereal | [82,83,84,85,86] | [87,88,89] |
| Fish waste | [90] | [76] |
| Fruit and vegetable waste | [91,92,93,94] | [76] |
| Household waste, dried | [95,96,97] | [75] |
| Meat meal | [98] | - |
| Municipal waste | [99] | [75,100] |
| Restaurant and cafeteria waste, dried | [101] | [75,102,103,104,105,106] |
| Supermarket waste | [107] | [75,76] |
| Ingredient | GE, kcal/kg | ME 1, kcal/kg | ME:GE | CP 2, % | Digestible N3, g/kg | P 4, % | Digestible P 5, g/kg |
|---|---|---|---|---|---|---|---|
| Corn a | 4454 | 3844 | 0.86 | 9.33 (80) | 11.9 | 0.29 (34) | 0.99 |
| Dehulled soybean meal b | 4730 | 3660 | 0.77 | 53.05 (87) | 73.8 | 0.79 (48) | 3.79 |
| Food waste source | |||||||
| Supermarket c | 5909 | 4832 | 0.82 | 25.51 | - | 0.64 | - |
| University dining hall c | 5419 | 4188 | 0.77 | 18.90 | - | 0.30 | - |
| Transfer station c | 4829 | 3198 | 0.66 | 17.71 | - | 0.46 | - |
| Household source separated organics c | 4455 | 4114 | 0.92 | 13.53 | - | 0.31 | - |
| Fish waste d | 6376 | 4820 | 0.76 | 62.49 (95) | 95.0 | 2.95 (59) | 17.4 |
| Supermarket d | 6316 | 4922 | 0.78 | 29.42 (89) | 41.9 | 0.37 (82) | 3.03 |
| Fruit and vegetable d | 4123 | 2460 | 0.60 | 10.13 (11) | 1.78 | 0.27 (74) | 2.00 |
| Ingredient | GE, kcal/kg | ME, kcal/kg | ME:GE | CP, % | Digestible N 3, g/kg | P, % | Digestible P 4, g/kg |
|---|---|---|---|---|---|---|---|
| Corn 1 | 4454 | 3844 | 0.86 | 9.33 (65) | 9.70 | 0.29 (26) | 0.75 |
| Dehulled soybean meal 1 | 4730 | 3660 | 0.77 | 53.05 (82) | 69.6 | 0.79 (39) | 3.08 |
| Animal protein by-product 2 | |||||||
| Blood meal | 5789 | 4618 | 0.80 | 97.09 (93) | 144 | 0.20 (99) | 1.98 |
| Chicken meal | 5015 | 3719 | 0.74 | 69.52 (91) | 101 | 3.26 (42) | 13.7 |
| Chicken by-product meal | 5521 | 4204 | 0.76 | 69.20 (87) | 96.3 | 1.84 (63) | 11.6 |
| Feather meal | 5809 | 4031 | 0.69 | 88.86 (80) | 114 | 0.32 (59) | 1.89 |
| Meat meal | 4732 | 3034 | 0.64 | 57.97 (81) | 75.1 | 3.49 (38) | 13.6 |
| Meat and bone meal | 4469 | 2620 | 0.59 | 56.14 (82) | 73.7 | 4.46 (33) | 14.7 |
| Poultry meal | 4183 | 2508 | 0.60 | 49.26 (80) | 63.1 | 4.51 (37) | 16.7 |
| Poultry by-product meal | 4,381 | 3,038 | 0.69 | 58.04 (87) | 80.7 | 4.67 (34) | 15.9 |
| Disposal Method | Human Health | Pathogen Contamination | |||||
|---|---|---|---|---|---|---|---|
| Dioxins/Furans | Air | Soil and Vegetation | Water | Land Application of Waste | Transport of Animals Off-Farm | Prion Destruction | |
| Burial | Very low | Low | Moderate | ?? | NA | Very low | Very high |
| Burning | High | ?? | ?? | ?? | ?? | Very low | Moderate |
| Incineration, on-farm a | Low | Very low b | Very low b | Very low b | ?? | Very low | Very low |
| Incineration, large central facility | Moderate | Very low b | Very low b | Very low b | ?? | Very high | Very low |
| Rendering | ?? | Very low | NA | ?? | NA | Very high | Low |
| Composting c | ?? | Moderate | Moderate | ?? | ?? | Very low | Moderate |
| Anaerobic digestion | ?? | Low | Moderate | Moderate | ?? | Very low | High |
| Alkaline hydrolysis | ?? | Very low | Very low | Very low | Very low | Very low | Very low |
| Hazardous Agent | Rendering | Incineration | Landfill | Pyre | Burial |
|---|---|---|---|---|---|
| Campylobacter, Escherichia coli, Listeria, Salmonella, Bacillus anthracis, Clostridium botulinum, Leptospira, Mycobaterium tuberculosis var bovis, Yersinia | Low | Low | Some | Low | High |
| Cryptosporidium, Giardia | Low | Low | Some | Low | High |
| Clostridium tetani | Low | Low | Some | Low | High |
| Prions for transmissible spongiform encephalopathies | Some | Low | Some | Some | High |
| Methane, Carbon dioxide | Low | Low | Some | Low | High |
| Fuel-specific chemicals, Metal salts | Low | Low | Low | High | Low |
| Particulates, sulfur dioxide, nitrous oxide, nitrous particles | Low | Some | Low | High | Low |
| Polycyclic aromatic hydrocarbons, Dioxins | Low | Some | Low | High | Low |
| Disinfectants, Detergents | Low | Low | Some | Some | High |
| Hydrogen sulfide | Low | Low | Some | Low | High |
| Radiation | Low | Some | Low | Some | Some |
| Biological Agent | Temperature and Time for Inactivation | Reference |
|---|---|---|
| Prions | ||
| Bovine spongiform encephalitis | 136–138 °C for 18 min at 2 bar (29.4 psi) | [125] |
| Parasites | ||
| Trichinella spiralis | 55 °C for 6 min 60 °C for 2 min | [126] |
| Toxoplasma gondii | 60 °C for 1 min | [127] |
| Bacteria | ||
| Salmonella | 80 °C for 30 min | [128] |
| Escherichia coli | 65 °C for 20 min | [128] |
| Viruses | ||
| African swine fever virus | 56 °C for 70 min or 60 °C for 20 min | [129] |
| Classical swine fever virus | 65.5 °C for 30 min or 71 °C for 1 min | [129] |
| Highly pathogenic avian influenza virus H5 and H7 | 74 °C for 3.5 s | [129] |
| Newcastle disease virus | 56 °C for 3 h or 60 °C for 30 min | [129] |
| Foot-and-mouth disease virus | 70 °C for 30 min | [129] |
| Country | Animal Proteins | Plant Proteins | Grains | Fishmeal | Reference |
|---|---|---|---|---|---|
| Canada | 20 | 18 | 5 | 22 | [143] |
| Germany | 6 | 26 | 3 | - | [144] |
| Netherlands | 6 | 3 | - | - | [145] |
| United Kingdom | 3 | 7 | 1 | 22 | [146] |
| United States | 33 | 10 | 0 | 10 | [147] |
| Percentage of Positive Samples a, % | ||
|---|---|---|
| Pathogen | Unprocessed Raw Material | Rendered Final Product |
| Campylobacter jejuni | 20.0 | 0 |
| Campylobacter spp. | 29.8 | 0 |
| Clostridium perfringens | 71.4 | 0 |
| Listeria monocytogenes | 8.3 | 0 |
| Listeria spp. | 76.2 | 0 |
| Salmonella spp. | 84.5 | 0 |
| Feed Ingredient | PEDV | PDCoV | TGEV |
|---|---|---|---|
| Spray dried porcine plasma | 1.14 | 3.25 a | 19.18 a |
| Blood meal | 2.84 | 1.23 a | 2.15 a |
| Meat meal | 3.87 | 2.82 a | 1.04 a |
| Meat and bone meal | 4.90 | 6.22 a | 0.99 a |
| Corn | 2.25 | 25.60 b | 11.78 a |
| Soybean meal | 7.50 | 42.04 c | 41.94 b |
| Low oil DDGS 1 | 0.70 | 6.23 a | 1.04 a |
| Medium oil DDGS | 7.32 | 3.76 a | 1.66 a |
| High oil DDGS | 0.56 | 8.80 a | 0.78 a |
| Complete feed | 1.12 | 2.29 a | 3.20 a |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shurson, G.C. “What a Waste”—Can We Improve Sustainability of Food Animal Production Systems by Recycling Food Waste Streams into Animal Feed in an Era of Health, Climate, and Economic Crises? Sustainability 2020, 12, 7071. https://doi.org/10.3390/su12177071
Shurson GC. “What a Waste”—Can We Improve Sustainability of Food Animal Production Systems by Recycling Food Waste Streams into Animal Feed in an Era of Health, Climate, and Economic Crises? Sustainability. 2020; 12(17):7071. https://doi.org/10.3390/su12177071
Chicago/Turabian StyleShurson, Gerald C. 2020. "“What a Waste”—Can We Improve Sustainability of Food Animal Production Systems by Recycling Food Waste Streams into Animal Feed in an Era of Health, Climate, and Economic Crises?" Sustainability 12, no. 17: 7071. https://doi.org/10.3390/su12177071
APA StyleShurson, G. C. (2020). “What a Waste”—Can We Improve Sustainability of Food Animal Production Systems by Recycling Food Waste Streams into Animal Feed in an Era of Health, Climate, and Economic Crises? Sustainability, 12(17), 7071. https://doi.org/10.3390/su12177071





