Evaluation of Parallel-Series Configurations of Two-Phase Partitioning Biotrickling Filtration and Biotrickling Filtration for Treating Styrene Gas-Phase Emissions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
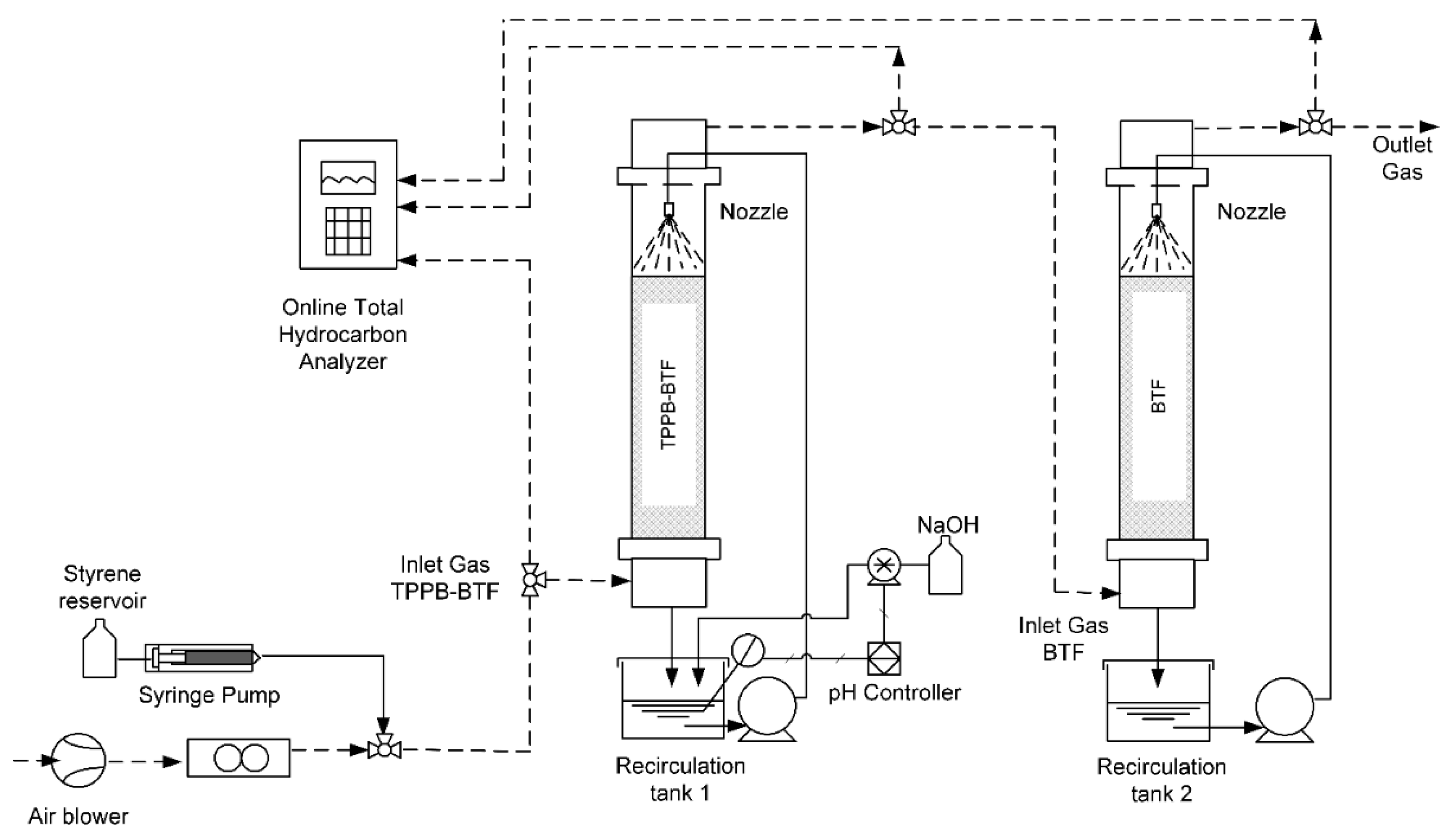
2.2. Experimental set-up
2.3. Experimental Plan
2.3.1. TPPB-BTF + BTF Series Configuration: Influence of Spraying Frequency and Transient Loading of Styrene
2.3.2. Alternative Parallel-Series Configurations
2.4. Microbial Community Analysis
2.5. Analytical methods
3. Results and Discussion
3.1. TPPB-BTF + BTF Series Configuration: Influence of Spraying Frequency
3.2. TPPB-BTF + BTF Series Configuration: Influence of Transient Styrene Loading
3.3. Alternative Series and Parallel Configurations
3.4. Microbial Community Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kogevinas, M.; Gwinn, W.M.; Kriebel, D.; Phillips, D.H.; Sim, M.; Bertke, S.J.; Calaf, G.M.; Colosio, C.; Fritz, J.M.; Fukushima, S.; et al. Carcinogenicity of quinoline, styrene, and styrene-7,8-oxide. Lancet Oncol. 2018, 19, 728–729. [Google Scholar] [CrossRef]
- Derwent, R.G.; Jenkin, M.E.; Saunders, S.M.; Pilling, M.J. Photochemical ozone creation potentials for organic compounds in northwest Europe calculated with a master chemical mechanism. Atmos. Environ. 1998, 32, 2429–2441. [Google Scholar] [CrossRef]
- Di Tomasso, C.; József Gombos, Z.; Summerscales, J. Styrene emissions during gel-coating of composites. J. Clean. Prod. 2014, 83, 317–328. [Google Scholar] [CrossRef]
- Álvarez-Hornos, F.J.; Martínez-Soria, V.; Marzal, P.; Izquierdo, M.; Gabaldón, C. Performance and feasibility of biotrickling filtration in the control of styrene industrial air emissions. Int. Biodeterior. Biodegrad. 2017, 119, 329–335. [Google Scholar] [CrossRef]
- Parnian, P.; Zamir, S.M.; Shojaosadati, S.A. Styrene vapor mass transfer in a biotrickling filter: Effects of silicone oil volume fraction, gas-to-liquid flow ratio, and operating temperature. Chem. Eng. J. 2016, 284, 926–933. [Google Scholar] [CrossRef]
- Dobslaw, D.; Schöller, J.; Krivak, D.; Helbich, S.; Engesser, K.H. Performance of different biological waste air purification processes in treatment of a waste gas mix containing tert-butyl alcohol and acetone: A comparative study. Chem. Eng. J. 2019, 355, 572–585. [Google Scholar] [CrossRef]
- Tepe, O.; Dursun, A.Y. Combined effects of external mass transfer and biodegradation rates on removal of phenol by immobilized Ralstonia eutropha in a packed bed reactor. J. Hazard. Mater. 2008, 151, 9–16. [Google Scholar] [CrossRef]
- Dumont, E.; Andrès, Y.; Cloirec, P. Le Mass transfer coefficients of styrene into water/silicone oil mixtures: New interpretation using the “equivalent absorption capacity” concept. Chem. Eng. J. 2014, 237, 236–241. [Google Scholar] [CrossRef]
- Wu, C.; Xu, P.; Xu, B.; Li, W.; Li, S.; Wang, X. o-Xylene removal using one- and two-phase partitioning biotrickling filters: Steady/transient-state performance and microbial community. Environ. Technol. 2018, 39, 109–119. [Google Scholar] [CrossRef]
- Patel, M.J.; Popat, S.C.; Deshusses, M.A. Determination and correlation of the partition coefficients of 48 volatile organic and environmentally relevant compounds between air and silicone oil. Chem. Eng. J. 2017, 310, 72–78. [Google Scholar] [CrossRef]
- Rene, E.R.; Montes, M.; Veiga, M.C.; Kennes, C. Styrene removal from polluted air in one and two-liquid phase biotrickling filter: Steady and transient-state performance and pressure drop control. Bioresour. Technol. 2011, 102, 6791–6800. [Google Scholar] [CrossRef] [PubMed]
- San-Valero, P.; Gabaldón, C.; Penya-roja, J.M.; Quijano, G. Enhanced styrene removal in a two-phase partitioning bioreactor operated as a biotrickling filter: Towards full-scale applications. Chem. Eng. J. 2017, 309, 588–595. [Google Scholar] [CrossRef]
- Devinny, J.S.; Ramesh, J. A phenomenological review of biofilter models. Chem. Eng. J. 2005, 113, 187–196. [Google Scholar] [CrossRef]
- Sempere, F.; Gabaldón, C.; Martínez-Soria, V.; Marzal, P.; Penya-roja, J.M.; Javier Álvarez-Hornos, F. Performance evaluation of a biotrickling filter treating a mixture of oxygenated VOCs during intermittent loading. Chemosphere 2008, 73. [Google Scholar] [CrossRef]
- Webster, T.S.; Cox, H.H.J.; Deshusses, M.A. Resolving operational and performance problems encountered in the use of a pilot/full-scale biotrickling fiber reactor. Environ. Prog. 1999, 18, 162–172. [Google Scholar] [CrossRef]
- Portune, K.J.; Pérez, M.C.; Álvarez-Hornos, J.; Gabaldón, C. Contribution of bacterial biodiversity on the operational performance of a styrene biotrickling filter. Chemosphere 2020, 247, 125800. [Google Scholar] [CrossRef]
- Li, J.; Ye, G.; Sun, D.; An, T.; Sun, G.; Liang, S. Performance of a biotrickling filter in the removal of waste gases containing low concentrations of mixed VOCs from a paint and coating plant. Biodegradation 2012, 23, 177–187. [Google Scholar] [CrossRef]
- Liao, D.; Li, E.; Li, J.; Zeng, P.; Feng, R.; Xu, M.; Sun, G. Removal of benzene, toluene, xylene and styrene by biotrickling filters and identification of their interactions. PLoS ONE 2018, 13, e0189927. [Google Scholar] [CrossRef]
- Timmis, K.N. Handbook of Hydrocarbon and Lipid Microbiology; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 9783540775874. [Google Scholar]
- Révész, F.; Farkas, M.; Kriszt, B.; Szoboszlay, S.; Benedek, T.; Táncsics, A. Effect of oxygen limitation on the enrichment of bacteria degrading either benzene or toluene and the identification of Malikia spinosa (Comamonadaceae) as prominent aerobic benzene-, toluene-, and ethylbenzene-degrading bacterium: Enrichment, isolation and whole-genome analysis. Environ. Sci. Pollut. Res. 2020, 1–13. [Google Scholar] [CrossRef]
- Warhurst, A.M.; Clarke, K.F.; Hill, R.A.; Holt, R.A.; Fewson, C.A. Metabolism of styrene by Rhodococcus rhodochrous NCIMB 13259. Appl. Environ. Microbiol. 1994, 60, 1137–1145. [Google Scholar] [CrossRef]
- Jung, I.G.; Park, C.H. Characteristics of styrene degradation by Rhodococcus pyridinovorans isolated from a biofilter. Chemosphere 2005, 61, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Tischler, D.; Eulberg, D.; Lakner, S.; Kaschabek, S.R.; Van Berkel, W.J.H.; Schlömann, M. Identification of a novel self-sufficient styrene monooxygenase from Rhodococcus opacus 1CP. J. Bacteriol. 2009, 191, 4996–5009. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Izawa, M.; Yanase, H. Isolation and application of a styrene-degrading strain of Pseudomonas putida to biofiltration. J. Biosci. Bioeng. 2003, 95, 633–636. [Google Scholar] [CrossRef]
- Jang, J.H.; Hirai, M.; Shoda, M. Enhancement of styrene removal efficiency in biofilter by mixed cultures of Pseudomonas sp. SR-5. J. Biosci. Bioeng. 2006, 102, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.C.; Álvarez-Hornos, F.J.; Portune, K.; Gabaldón, C. Abatement of styrene waste gas emission by biofilter and biotrickling filter: Comparison of packing materials and inoculation procedures. Appl. Microbiol. Biotechnol. 2015, 99, 19–32. [Google Scholar] [CrossRef]
- Vanek, T.; Silva, A.; Halecky, M.; Paca, J.; Ruzickova, I.; Kozliak, E.; Jones, K. Biodegradation of airborne acetone/styrene mixtures in a bubble column reactor. J. Environ. Sci. Health. Part A Toxic Hazard. Subst. Environ. Eng. 2017, 52, 905–915. [Google Scholar] [CrossRef]
- Gąszczak, A.; Bartelmus, G.; Burghardt, A.; Rotkegel, A.; Sarzyński, R. Experiments and modelling of a biotrickling filter (BTF) for removal of styrene from airstreams. J. Chem. Technol. Biotechnol. 2018, 93, 2659–2670. [Google Scholar] [CrossRef]
- Song, Y.; Qiu, R.; Hu, J.; Li, X.; Zhang, X.; Chen, Y.; Wu, W.M.; He, D. Biodegradation and disintegration of expanded polystyrene by land snails Achatina fulica. Sci. Total Environ. 2020, 746, 141289. [Google Scholar] [CrossRef]
- Arnold, M.; Reittu, A.; von Wright, A.; Martikainen, P.J.; Suihko, M.L. Bacterial degradation of styrene in waste gases using a peat filter. Appl. Microbiol. Biotechnol. 1997, 48, 738–744. [Google Scholar] [CrossRef]
- Portune, K.J.; Pérez, M.C.; Álvarez-Hornos, F.J.; Gabaldón, C. Investigating bacterial populations in styrene-degrading biofilters by 16S rDNA tag pyrosequencing. Appl. Microbiol. Biotechnol. 2015, 99, 3–18. [Google Scholar] [CrossRef]
- Zhong, F.; Wu, J.; Dai, Y.; Yang, L.; Zhang, Z.; Cheng, S.; Zhang, Q. Bacterial community analysis by PCR-DGGE and 454-pyrosequencing of horizontal subsurface flow constructed wetlands with front aeration. Appl. Microbiol. Biotechnol. 2015, 99, 1499–1512. [Google Scholar] [CrossRef] [PubMed]
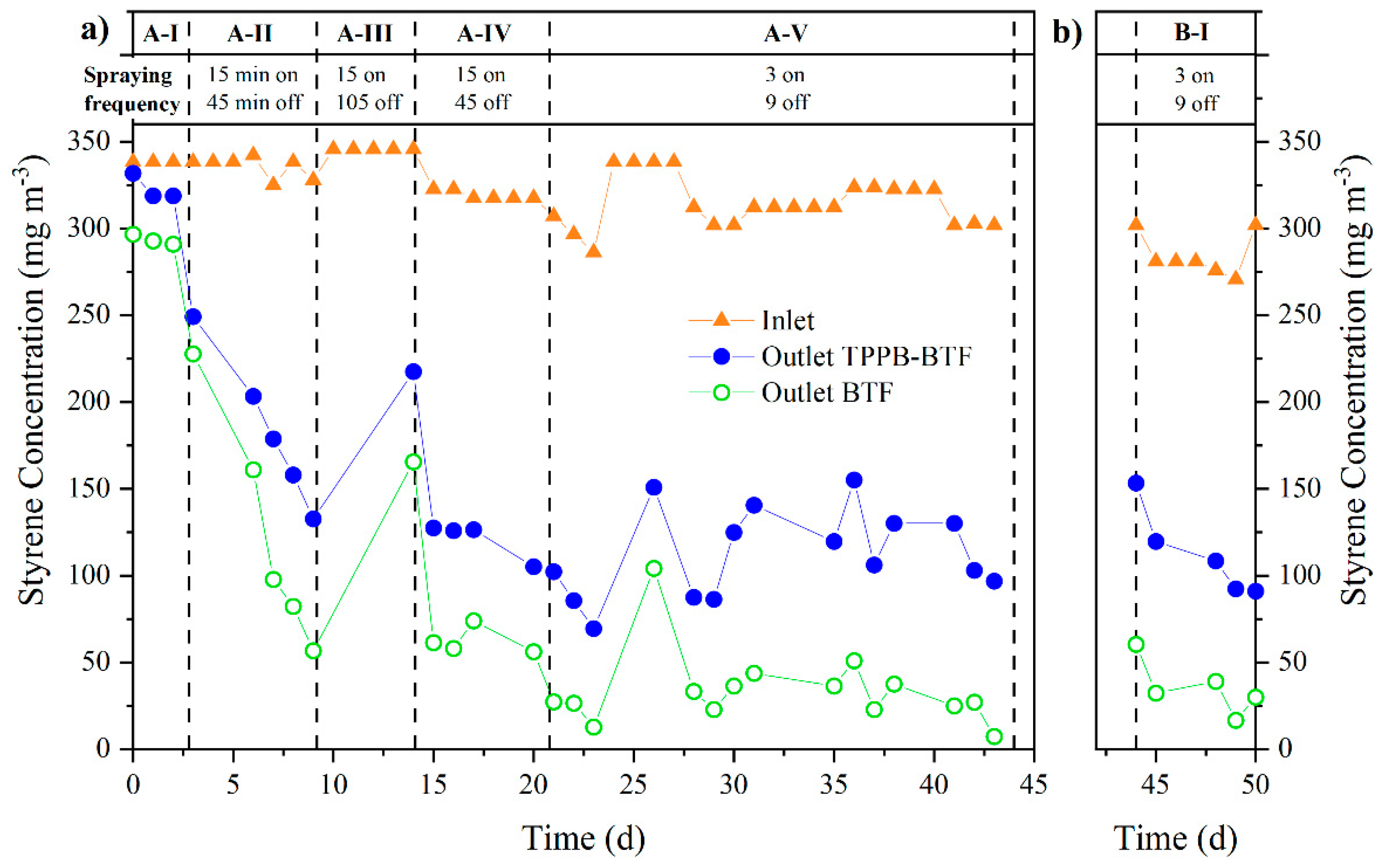
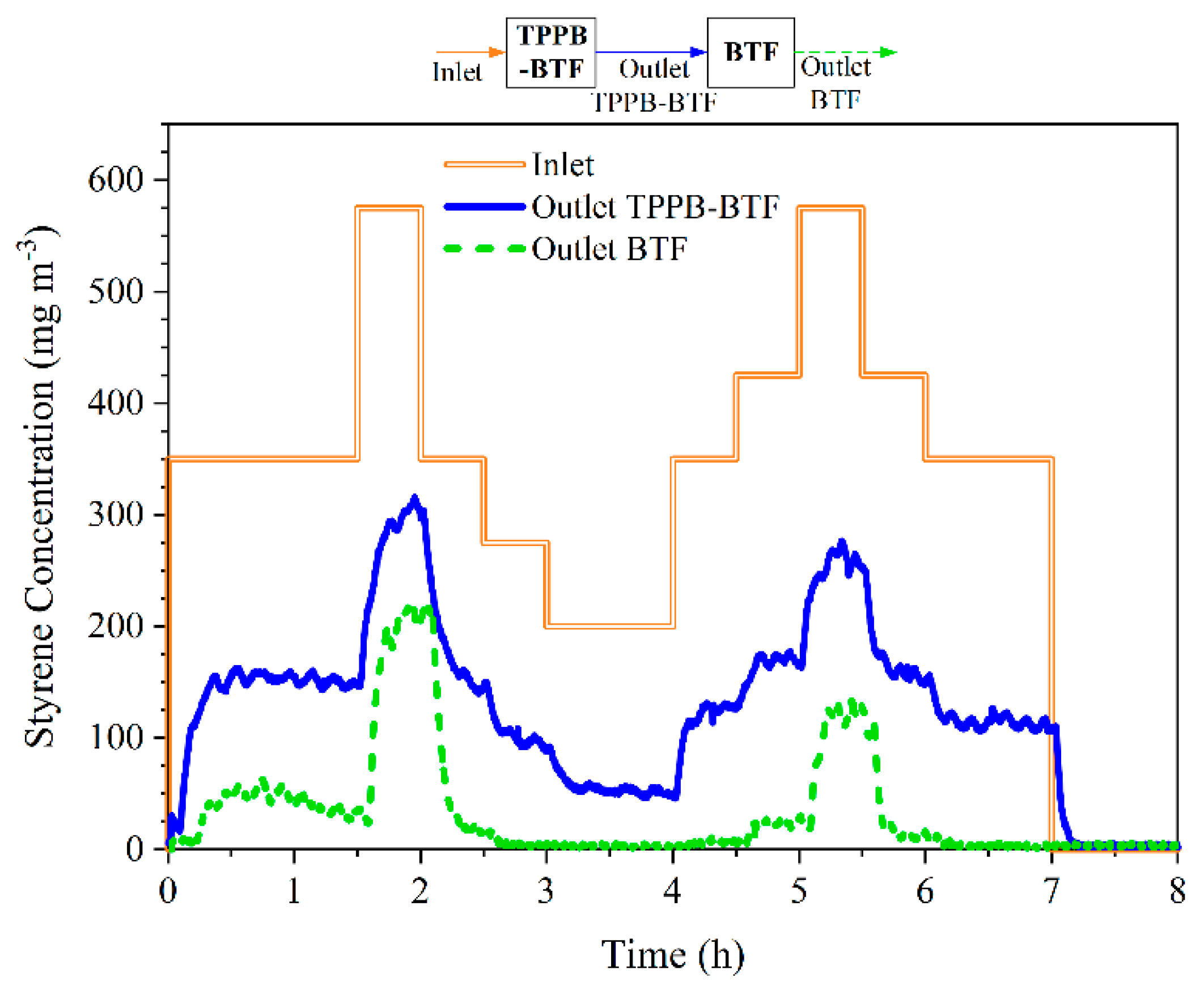
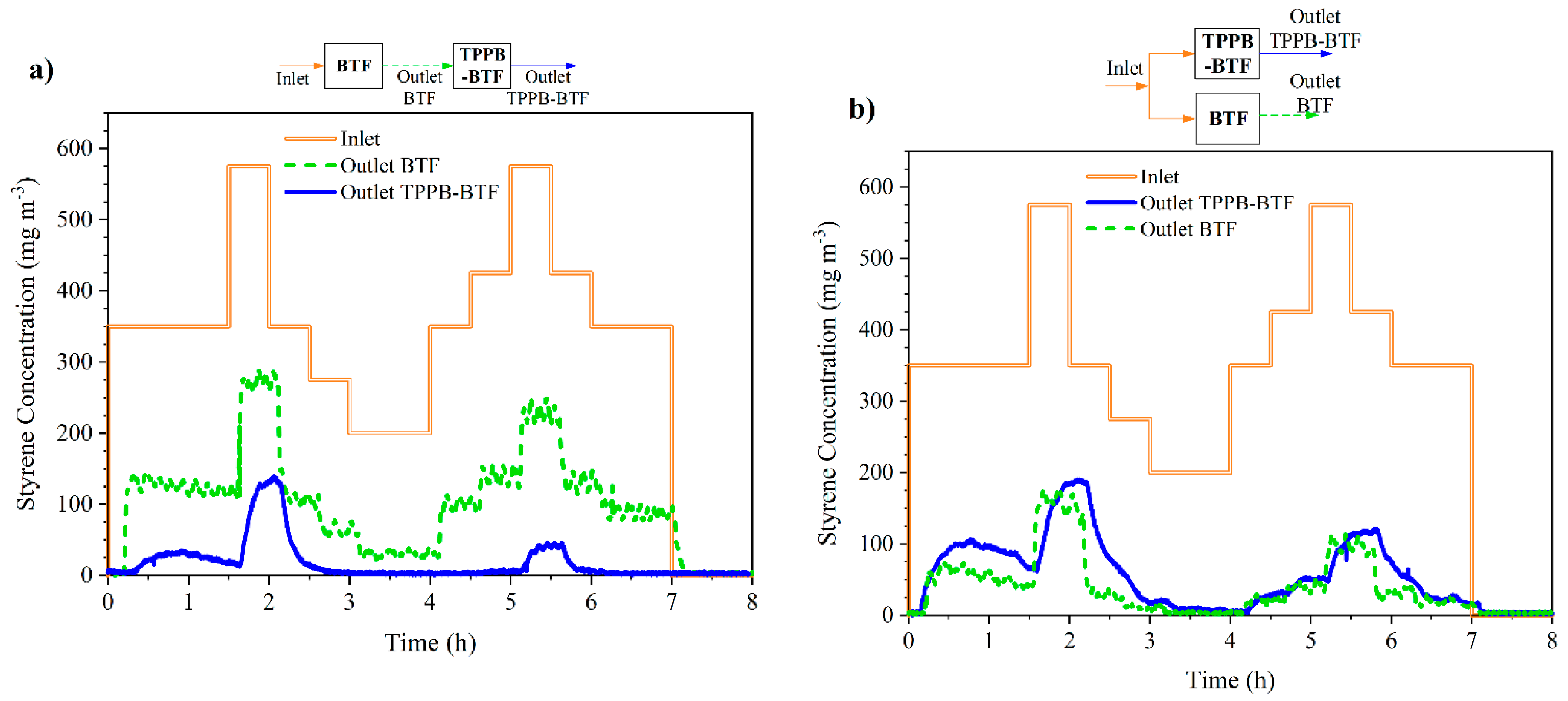
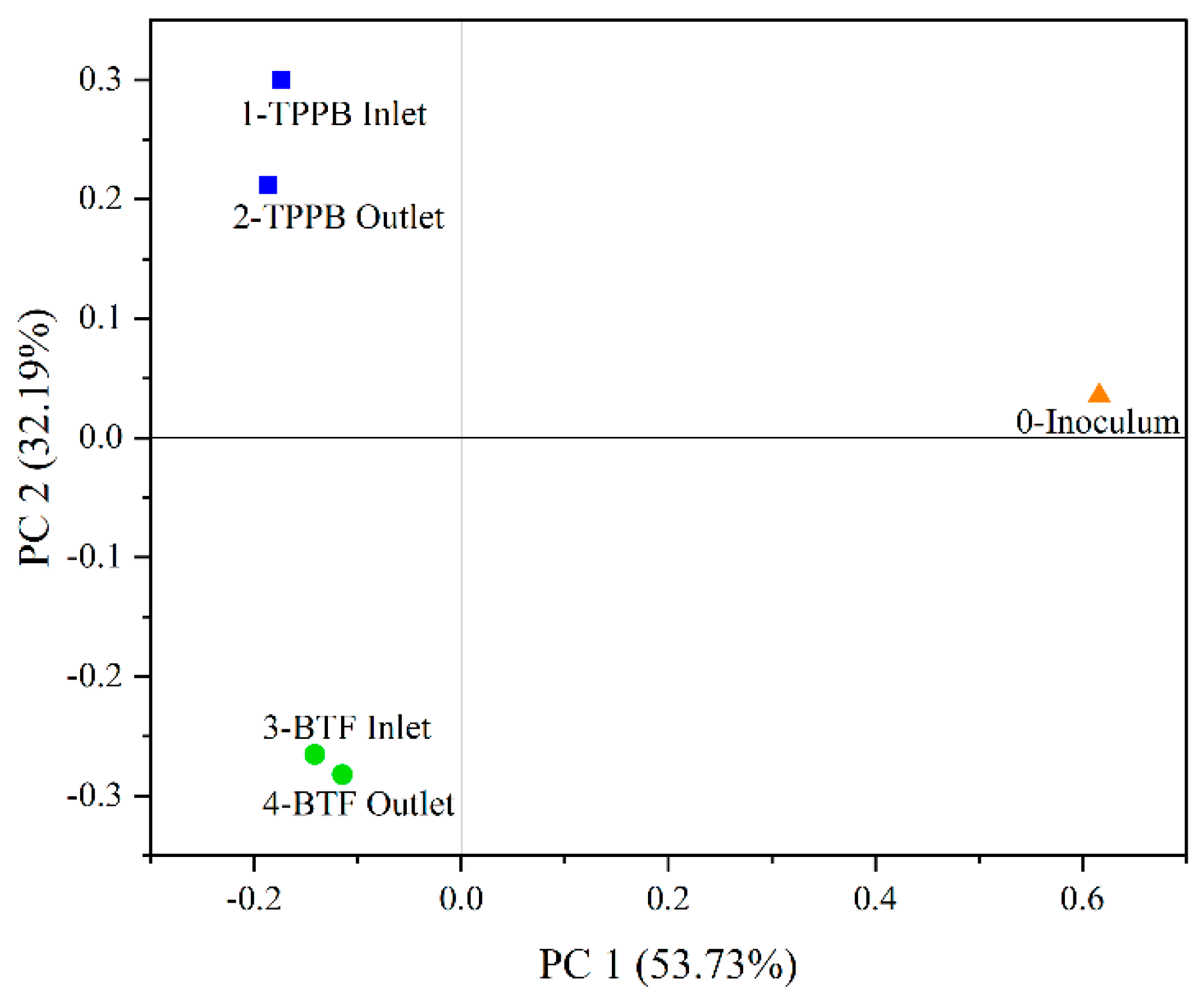
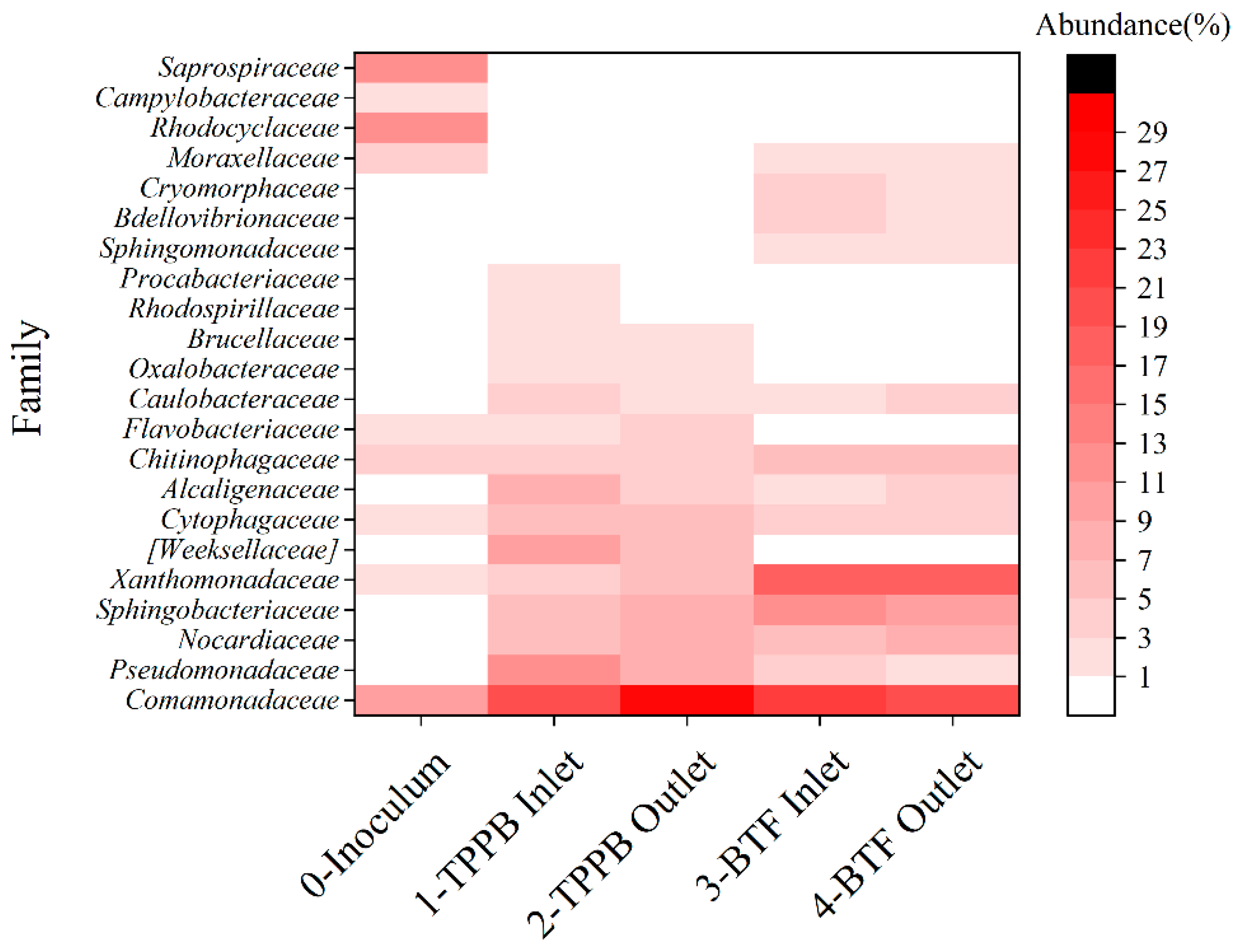
| Phase | Days | Spraying | Styrene Loading |
|---|---|---|---|
| A-I (Start-up) | 0–3 | Continuous | Continuous Inlet conc. = 330 mg m−3 |
| A-II | 4–9 | 15 min on/45 min off | |
| A-III | 10–14 | 15 min on/105 min off | |
| A-IV | 15–21 | 15 min on/45 min off | |
| A-V | 21–44 | 3 min on/9 min off | |
| B-I | 45–51 | 3 min on/9 min off | Discontinuous: 16 h d−1 Inlet conc. = 330 mg m−3 |
| B-II | 52–62 | 3 min on/9 min off | Discontinuous: 16 h d−1 Oscillating inlet conc. |
| B-III | 63–65 | 3 min on/9 min off | Discontinuous: 16 h d−1 Inlet conc. = 330 mg m−3 |
| Sample | Sampling Day | OTUs 1 | Chao1 | Shannon Index | |
|---|---|---|---|---|---|
| 0 | Inoculum | 0 | 1565 | 1655 (1575–1734) 2 | 8.25 (8.20–8.31) 2 |
| 1 | TPPB-BTF—Inlet 3 | 51 | 545 | 566 (506–627) | 5.83 (5.81–5.85) |
| 2 | TPPB-BTF—Outlet 4 | 51 | 616 | 736 (667–805) | 5.94 (5.92–5.96) |
| 3 | BTF—Inlet 3 | 51 | 653 | 702 (622–781) | 6.39 (6.36–6.42) |
| 4 | BTF—Outlet 4 | 51 | 754 | 915 (828–1002) | 6.58 (6.55–6.60) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
San-Valero, P.; Álvarez-Hornos, J.; Ferrero, P.; Penya-Roja, J.M.; Marzal, P.; Gabaldón, C. Evaluation of Parallel-Series Configurations of Two-Phase Partitioning Biotrickling Filtration and Biotrickling Filtration for Treating Styrene Gas-Phase Emissions. Sustainability 2020, 12, 6740. https://doi.org/10.3390/su12176740
San-Valero P, Álvarez-Hornos J, Ferrero P, Penya-Roja JM, Marzal P, Gabaldón C. Evaluation of Parallel-Series Configurations of Two-Phase Partitioning Biotrickling Filtration and Biotrickling Filtration for Treating Styrene Gas-Phase Emissions. Sustainability. 2020; 12(17):6740. https://doi.org/10.3390/su12176740
Chicago/Turabian StyleSan-Valero, Pau, Javier Álvarez-Hornos, Pablo Ferrero, Josep M. Penya-Roja, Paula Marzal, and Carmen Gabaldón. 2020. "Evaluation of Parallel-Series Configurations of Two-Phase Partitioning Biotrickling Filtration and Biotrickling Filtration for Treating Styrene Gas-Phase Emissions" Sustainability 12, no. 17: 6740. https://doi.org/10.3390/su12176740
APA StyleSan-Valero, P., Álvarez-Hornos, J., Ferrero, P., Penya-Roja, J. M., Marzal, P., & Gabaldón, C. (2020). Evaluation of Parallel-Series Configurations of Two-Phase Partitioning Biotrickling Filtration and Biotrickling Filtration for Treating Styrene Gas-Phase Emissions. Sustainability, 12(17), 6740. https://doi.org/10.3390/su12176740





