The Effect of Chemical Sulfide Oxidation on the Oxygenic Activity of an Alkaliphilic Microalgae Consortium Deployed for Biogas Upgrading
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Culture Conditions
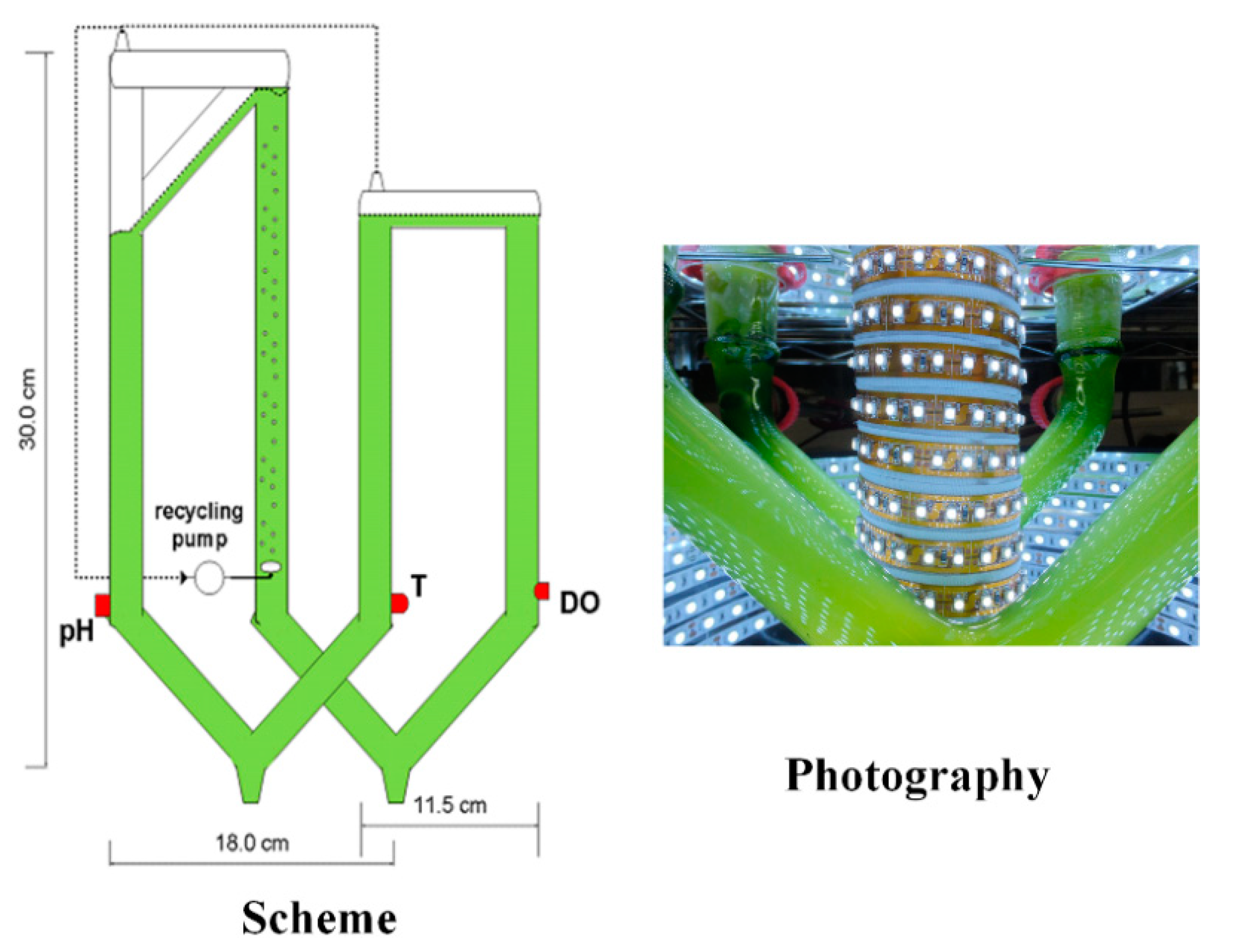
2.2. Experimental System
Kinetic Assays Approach
2.3. Oxygenic Photosynthetic Activity Evaluation
2.4. Analytical Methods
3. Results and Discussion
3.1. Kinetic Assays: Single Sulfide Addition
3.2. Consecutive Additions of Sulfide
3.3. Thiosulfate Production and Oxygenic Photosynthetic Activity in Biotic and Abiotic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMC | alkaliphilic microalgae consortium |
| TCP | tubular closed photobioreactor |
| HRAP | high-rate algal pond |
| HRT | hydraulic retention time |
| dO2/dt | slope of experimental DO concentration profile (µmolO2/L min) |
| CSO | chemical oxidation reaction (µmolO2/L min) |
| OPA | oxygenic photosynthetic activity (µmolO2/L min) |
| DO | dissolved oxygen (mg/L) |
References
- González-Sánchez, A.; Revah, S. The effect of chemical oxidation on the biological sulfide oxidation by an alkaliphilic sulfoxidizing bacterial consortium. Enzym. Microb. Technol. 2007, 40, 292–298. [Google Scholar] [CrossRef]
- Khan, I.U.; Othman, M.H.D.; Hashim, H.; Matsuura, T.; Ismail, A.F.; Rezaei-DashtArzhandi, M.; Azelee, I.W. Biogas as a renewable energy fuel—A review of biogas upgrading, utilisation and storage. Energy Convers. Manag. 2017, 150, 277–294. [Google Scholar] [CrossRef]
- Adnan, A.I.; Ong, M.Y.; Nomanbhay, S.; Chew, K.W.; Show, P.L. Technologies for biogas upgrading to biomethane: A review. Bioengineering 2019, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, R.; Meier, L.; Diaz, I.; Jeison, D. A review on the state-of-the-art of physical/chemical and biological technologies for biogas upgrading. Rev. Environ. Sci. Biol. 2015, 14, 727–759. [Google Scholar] [CrossRef]
- Toledo-Cervantes, A.; Estrada, J.M.; Lebrero, R.; Muñoz, R. A comparative analysis of biogas upgrading technologies: Photosynthetic vs physical/chemical processes. Algal. Res. 2017, 25, 237–243. [Google Scholar] [CrossRef]
- Cline, C.; Hoksberg, A.; Abry, R.; Janssen, A. Biological process for H2S removal from gas streams: The shell-paques/thiopaqTM gas desulfurization process. In Proceedings of the Laurance Reid Gas Conditioning Conference, Norman, OK, USA, 23–26 February 2003; pp. 1–18. [Google Scholar]
- Bahr, M.; Díaz, I.; Dominguez, A.; González Sánchez, A.; Muñoz, R. Microalgal-biotechnology as a platform for an integral biogas upgrading and nutrient removal from anaerobic effluents. Environ. Sci. Technol. 2014, 48, 573–581. [Google Scholar] [CrossRef]
- Bona, D.; Papurello, D.; Flaim, G.; Cerasino, L.; Biasioli, F.; Silvestri, S. Management of digestate and exhausts from solid oxide fuel cells produced in the dry anaerobic digestion pilot plant: Microalgae cultivation approach. Waste Biomass Valori. 2020, 1–16. [Google Scholar] [CrossRef]
- Santarelli, M.; Briesemeister, L.; Gandiglio, M.; Herrmann, S.; Kuczynski, P.; Kupecki, J.; Swiatkowski, B. Carbon recovery and re-utilization (CRR) from the exhaust of a solid oxide fuel cell (SOFC): Analysis through a proof-of-concept. J. CO2 Util. 2017, 18, 206–221. [Google Scholar] [CrossRef]
- Franco-Morgado, M.; Toledo-Cervantes, A.; González-Sánchez, A.; Lebrero, R.; Muñoz, R. Integral (VOCs, CO2, mercaptans and H2S) photosynthetic biogas upgrading using innovative biogas and digestate supply strategies. Chem. Eng. J. 2018, 354, 363–369. [Google Scholar] [CrossRef]
- Toledo-Cervantes, A.; Serejo, M.L.; Blanco, S.; Pérez, R.; Lebrero, R.; Muñoz, R. Photosynthetic biogas upgrading to bio-methane: Boosting nutrient recovery via biomass productivity control. Algal. Res. 2016, 17, 46–52. [Google Scholar] [CrossRef]
- Del Rosario Rodero, M.; Lebrero, R.; Serrano, E.; Lara, E.; Arbib, Z.; García-Encina, P.A.; Muñoz, R. Technology validation of photosynthetic biogas upgrading in a semi-industrial scale algal-bacterial photobioreactor. Bioresour. Technol. 2019, 279, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Grobbelaar, J.U. Algal Nutrition. In Handbook of Microalgal Culture: Biotechnology of Applied Phycology, 1st ed.; Amos, R., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2003; pp. 95–115. [Google Scholar]
- González-Sánchez, A.; Posten, C. Fate of H2S during the cultivation of Chlorella sp. deployed for biogas upgrading. J. Environ. Manag. 2017, 191, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.R.; Bebout, B.M. Variation in sulfide tolerance of photosystem II in phylogenetically diverse cyanobacteria from sulfidic habitats. Appl. Environ. Microb. 2004, 70, 736–744. [Google Scholar] [CrossRef]
- Theissen, U.; Hoffmeister, M.; Grieshaber, M.; Martin, W. Single eubacterial origin of eukaryotic sulfide: Quinone oxidoreductase, a mitochondrial enzyme conserved from the early evolution of eukaryotes during anoxic and sulfidic times. Mol. Bio. Evol. 2003, 20, 1564–1574. [Google Scholar] [CrossRef] [PubMed]
- Küster, E.; Dorusch, F.; Altenburger, R. Effects of hydrogen sulfide to Vibrio fischeri, Scenedesmus vacuolatus, and Daphnia magna. Environ. Toxicol. Chem. 2005, 24, 2621–2629. [Google Scholar] [CrossRef]
- González-Camejo, J.; Serna-García, R.; Viruela, A.; Pachés, M.; Durán, F.; Robles, A.; Ruano, M.V.; Barat, R.; Seco, A. Short and long-term experiments on the effect of sulphide on microalgae cultivation in tertiary sewage treatment. Bioresour. Technol. 2017, 244, 15–22. [Google Scholar] [CrossRef]
- Nielsen, A.H.; Vollertsen, J.; Hvitved-Jacobsen, T. Determination of kinetics and stoichiometry of chemical sulfide oxidation in wastewater of sewer networks. Environ. Sci. Technol. 2003, 37, 3853–3858. [Google Scholar] [CrossRef]
- Toro-Huertas, E.I.; Franco-Morgado, M.; de Los Cobos Vasconcelos, D.; González-Sánchez, A. Photorespiration in an outdoor alkaline open-photobioreactor used for biogas upgrading. Sci. Total Environ. 2019, 667, 613–621. [Google Scholar] [CrossRef]
- Cabello, J.; Toledo-Cervantes, A.; Sánchez, L.; Revah, S.; Morales, M. Effect of the temperature, pH and irradiance on the photosynthetic activity by Scenedesmus obtusiusculus under nitrogen replete and deplete conditions. Bioresour. Technol. 2015, 181, 128–135. [Google Scholar] [CrossRef]
- De los Cobos-Vasconcelos, D.; García-Cruz, E.L.; Franco-Morgado, M.; González-Sánchez, A. Short-term evaluation of the photosynthetic activity of an alkaliphilic microalgae consortium in a novel tubular closed photobioreactor. J. Appl. Phycol. 2016, 28, 795–802. [Google Scholar] [CrossRef]
- Sorokin, D.Y.; Lysenko, A.M.; Mityushina, L.L.; Tourova, T.P.; Jones, B.E.; Rainey, F.A.; Robertson, L.A.; Kuenen, G.J. Thioalkalimicrobium aerophilum gen. nov., sp. nov. and Thioalkalimicrobium sibericum sp. nov., and Thioalkalivibrio versutus gen. nov., sp. nov., Thioalkalivibrio nitratis sp.nov., novel and Thioalkalivibrio denitrificancs sp. nov., novel obligately alkaliphilic and obligately chemolithoautotrophic sulfur-oxidizing bacteria from soda lakes. Int. J. Syst. Evol. Microbiol. 2001, 51, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Granada-Moreno, C.I.; Aburto-Medina, A.; de los Cobos Vasconcelos, D.; González-Sánchez, A. Microalgae community shifts during the biogas upgrading in an alkaline open photobioreactor. J. Appl. Microbiol. 2017, 123, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Escalante-Vázquez, E.J. Diseño y Análisis de Experimentos, 1st ed.; Limusa: Distrito Federal, Mexico, 2014; p. 523. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; Rice, E.W., Baird, R.B., Eaton, A.D., Clesceri, L.S., Eds.; American Public Health Association (APHA), American Water Works Association (AWWA), Water Environment Federation (WEF): Washington, DC, USA, 2012; p. 724. [Google Scholar]
- HACH. Sulfide Methylene Blue Method: Water Analysis Handbook Procedures. Available online: https://www.hach.com/asset-get.download.jsa?id=11122402600 (accessed on 8 June 2020).
- Torzillo, G.; Vonshak, A. Environmental stress physiology with reference to mass cultures. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; Amos, R., Hu, Q., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2013; pp. 90–113. [Google Scholar]
- Benavente-Valdés, J.R.; Aguilar, C.; Contreras-Esquivel, J.C.; Méndez-Zavala, A.; Montañez, J. Strategies to enhance the production of photosynthetic pigments and lipids in chlorophycae species. Biotechnol. Rep. 2016, 10, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, C.N.; Nayaka, S. Comprehensive screening of micro-and macroalgal species for bioenergy. In Algal Biofuels, 1st ed.; Gupta, S.K., Malik, A., Bux, F., Eds.; Springer: Cham, Switzerland, 2017; pp. 39–56. [Google Scholar]
- Cheng, J.; Wang, Z.; Lu, H.; Xu, J.; He, Y.; Cen, K. Hydrogen sulfide promotes cell division and photosynthesis of Nannochloropsis oceanica with 15% carbon dioxide. ACS Sustain. Chem. Eng. 2019, 7, 16344–16354. [Google Scholar] [CrossRef]
- Gun, J.; Goifman, A.; Shkrob, I.; Kamyshny, A.; Ginzburg, B.; Hadas, O.; Dor, I.; Modestov, A.D.; Lev, O. Formation of polysulfides in an oxygen rich freshwater lake and their role in the production of volatile sulfur compounds in aquatic systems. Environ. Sci. Technol. 2000, 34, 4741–4746. [Google Scholar] [CrossRef]
- Klatt, J.M.; Haas, S.; Yilmaz, P.; de Beer, D.; Polerecky, L. Hydrogen sulfide can inhibit and enhance oxygenic photosynthesis in a Cyanobacterium from sulfidic springs. Environ. Microbial. 2015, 17, 3301–3313. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wang, Z.; Lu, H.; Yang, W.; Fan, Z. Hydrogen sulfide improves lipid accumulation in Nannochloropsis oceanica through metabolic regulation of carbon allocation and energy supply. ACS Sustain. Chem. Eng. 2020, 8, 2481–2489. [Google Scholar] [CrossRef]
- Krauss, F.; Schmidt, A. Sulphur sources for growth of Chlorella fusca and their influence on key enzymes of sulphur metabolism. Microbiology 1987, 133, 1209–1219. [Google Scholar] [CrossRef][Green Version]
- Pootakham, W.; Gonzalez-Ballester, D.; Grossman, A.R. Identification and regulation of plasma membrane sulfate transporters in Chlamydomonas. Plant. Physiol. 2010, 153, 1653–1668. [Google Scholar] [CrossRef]
- Wang, S.K.; Stiles, A.R.; Guo, C.; Liu, C.Z. Microalgae cultivation in photobioreactors: An overview of light characteristics. Eng. Life Sci. 2014, 14, 550–559. [Google Scholar] [CrossRef]
- Costache, T.A.; Acién Fernández, F.G.; Morales, M.M.; Fernández-Sevilla, J.M.; Stamatin, I.; Molina, E. Comprehensive model of microalgae photosynthesis rate as a function of culture conditions in photobioreactors. Appl. Microbiol. Biotechnol. 2013, 97, 7627–7637. [Google Scholar] [CrossRef] [PubMed]
- Kwon, G.; Le, L.T.; Jeon, J.; Noh, J.; Jang, Y.; Kang, D.; Jahng, D. Effects of light and mass ratio of microalgae and nitrifiers on the rates of ammonia oxidation and nitrate production. Biochem. Eng. J. 2020, 107656. [Google Scholar] [CrossRef]


 ) and 9.2 (
) and 9.2 ( ).
).
 ) and 9.2 (
) and 9.2 ( ).
).
| Treatment No. | Variable Irradiance (μE/m2 s); pH (-); Sulfide (mg/L) | Response Parameter |
|---|---|---|
| 1 | 15; 7.3; 0 | DO (mg/L) |
| 2 | 15; 8.5; 3.2 | |
| 3 | 15; 9.2; 16 | |
| 4 | 50; 8.5; 16 | |
| 5 | 50; 9.2; 0 | |
| 6 | 50; 7.3; 3.2 | |
| 7 | 120; 9.2;3.2 | |
| 8 | 120; 7.3; 16 | |
| 9 | 120; 8.5; 0 |
| Sulfide Conc. (mg/L) | Experimental Stage (min) | Treatment No. 10 | Treatment No. 11 | Response Parameter |
|---|---|---|---|---|
| pH | pH | |||
| 0 | From 0 to 90 | 8.5 | 9.2 | DO (mg/L) |
| 3.2 | From 91 to 170 | |||
| 16 | From 171 to 240 |
| Biotic Test | ||||||
|---|---|---|---|---|---|---|
| Sulfide Conc. (mg/L) | pH Value | S2O32− (mg/L) | O2/S Molar Consumption Ratio | dO2/dt (µmolO2/L min) | CSO (µmolO2/L min) | OPA (µmolO2/L min) |
| 3.2 | 8.5 | 4.7 ± 0.4 | 0.84 ± 0.02 | 5.66 ± 1.05 | −1.39 ± 0.1 | 7.05 ± 0.94 (25.3) * |
| 9.2 | 4.0 ± 0.0 | 0.73 ± 0.07 | 6.59 ± 0.01 | −1.18 ± 0.0 | 7.77 ± 0.01 (27.8) * | |
| 16 | 8.5 | 16.7 ± 2.6 | 0.60 ± 0.1 | 3.90 ± 0.05 | −4.98 ± 0.8 | 8.88 ± 0.84 (31.8) * |
| 9.2 | 15.7 ± 2.8 | 0.56 ± 0.1 | 3.60 ± 0.35 | −4.68 ± 0.8 | 8.28 ± 1.19 (29.7) * | |
| Abiotic Test | ||||||
| 3.2 | 8.5 | 2.8 ± 0.4 | 0.50 ± 0.07 | −0.38 ± 0.06 | −0.83 ± 0.1 | N.A. |
| 9.2 | 2.6 ± 0.2 | 0.47 ± 0.04 | −0.65 ± 0.05 | −0.77 ± 0.3 | N.A. | |
| 16 | 8.5 | 6.5 ± 0.3 | 0.23 ± 0.1 | −1.41 ± 0.4 | −1.93 ± 0.4 | N.A. |
| 9.2 | 6.2 ± 0.2 | 0.22 ± 0.1 | −1.36 ± 0.1 | −1.84 ± 0.5 | N.A. | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Rueda, A.; Velasco, A.; González-Sánchez, A. The Effect of Chemical Sulfide Oxidation on the Oxygenic Activity of an Alkaliphilic Microalgae Consortium Deployed for Biogas Upgrading. Sustainability 2020, 12, 6610. https://doi.org/10.3390/su12166610
Ramírez-Rueda A, Velasco A, González-Sánchez A. The Effect of Chemical Sulfide Oxidation on the Oxygenic Activity of an Alkaliphilic Microalgae Consortium Deployed for Biogas Upgrading. Sustainability. 2020; 12(16):6610. https://doi.org/10.3390/su12166610
Chicago/Turabian StyleRamírez-Rueda, Arnold, Antonio Velasco, and Armando González-Sánchez. 2020. "The Effect of Chemical Sulfide Oxidation on the Oxygenic Activity of an Alkaliphilic Microalgae Consortium Deployed for Biogas Upgrading" Sustainability 12, no. 16: 6610. https://doi.org/10.3390/su12166610
APA StyleRamírez-Rueda, A., Velasco, A., & González-Sánchez, A. (2020). The Effect of Chemical Sulfide Oxidation on the Oxygenic Activity of an Alkaliphilic Microalgae Consortium Deployed for Biogas Upgrading. Sustainability, 12(16), 6610. https://doi.org/10.3390/su12166610






