Effect of Irrigation Systems and Soil Conditioners on the Growth and Essential Oil Composition of Rosmarinus officinalis L. Cultivated in Egypt
Abstract
1. Introduction
2. Materials and Methods
2.1. Location of the Experiments
2.2. Experimental Design
2.3. Statistical Analysis
2.4. Irrigation Setup
2.5. Plant Materials
2.6. Cultivation
2.7. EO Production
2.8. Qualitative and Quantitative Analyses of EOs
3. Results and Discussion
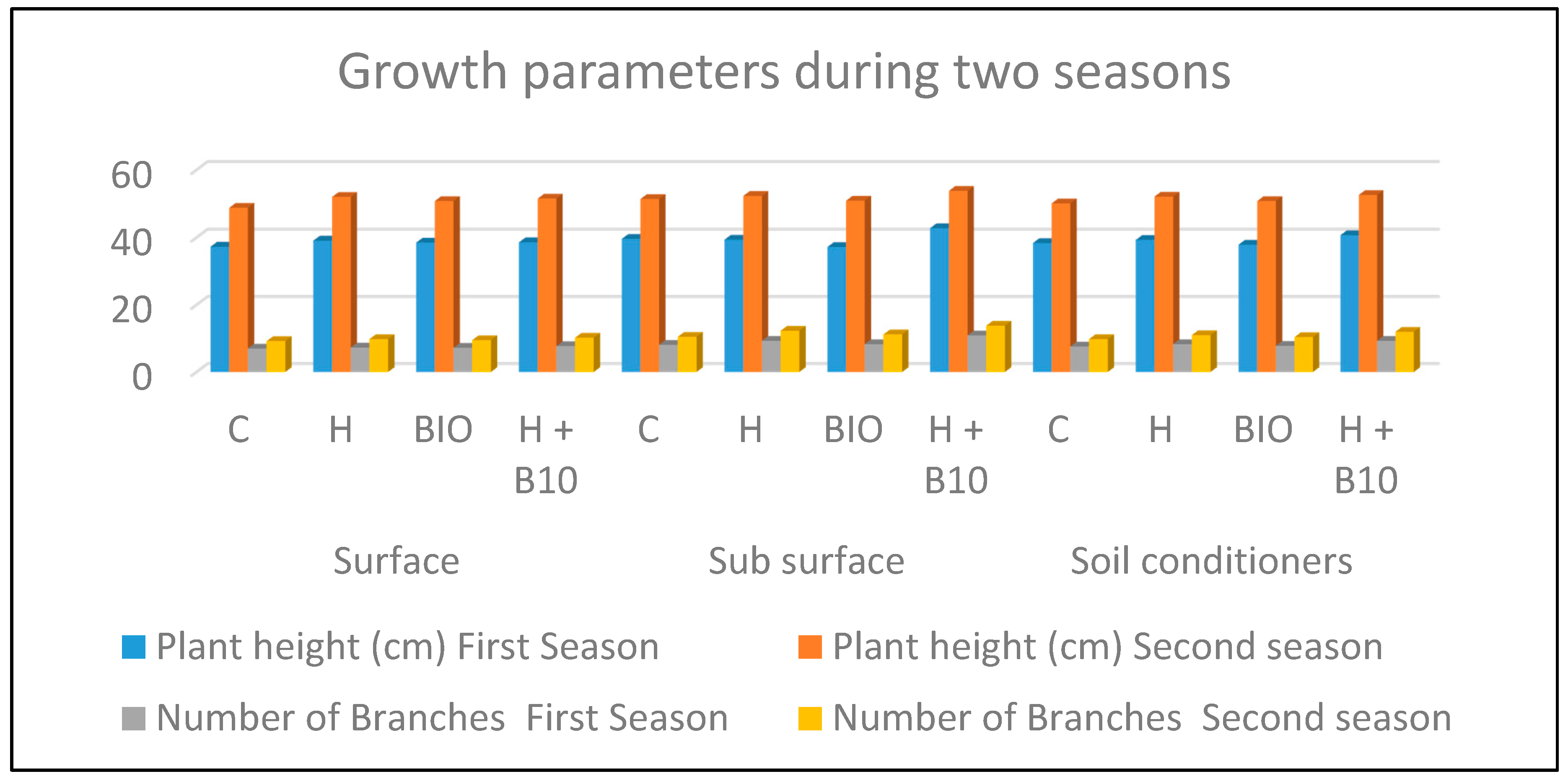
3.1. Plant Height (cm) and Branch/Plant Number
3.2. Fresh Herb and Dry Weights (g/Plant and kg/Fad.)
3.3. EO Content (%) and Yield (mL/Plant)
3.4. Composition of the EO
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Falkenmark, M.; Lindh, G. Water For a Starving World, 1st ed.; Routledge: Abingdon, UK, 2019; ISBN -13: 978-0367213183. [Google Scholar]
- Fahmy, S.; Ezzat, M.; Shalby, A.; Kandil, H.; Sharkawy, M.; Allam, M.; Assiouty, I.; Tczap, A. Water Policy Review and Integration Study; Report No. 65; Ministry of Water Resources and Irrigation: Cairo, Egypt, 2002. [Google Scholar]
- Kang, S.Z.; Shi, P.; Pan, Y.H.; Liang, Z.S.; Hu, X.T.; Zhang, J. Soil water distribution, uniformity and water-use efficiency under alternate furrow irrigation in arid areas. Agric. Water Manag. 2000, 19, 181–190. [Google Scholar] [CrossRef]
- Molden, D.J.; El-Kady, M.; Zhu, Z. Use and productivity of Egypt’s Nile water. In Contemporary Challenges for Irrigation and Drainage, Proceedings of the USCID 14th Technical Conference on Irrigation, Drainage and Flood Control, Phoenix, AZ, USA, 3–6 June 1998; Burns, I.J., Anderson, S.S., Eds.; USCID: Denver, CO, USA, 1998. [Google Scholar]
- Petretto, G.L.; Urgeghe, P.P.; Massa, D.; Melito, S. Effect of salinity (NaCl) on plant growth, nutrient content, and glucosinolate hydrolysis products trends in rocket genotypes. Plant. Physiol. Biochem. 2019, 141, 30–39. [Google Scholar] [CrossRef]
- Ashour, A.; El Attar, S.T.; Rafaat, Y.M.; Mohamed, M.N. Water Resources Management in Egypt. J. Eng. Sci. Assiut Univ. 2009, 37, 269–279. [Google Scholar]
- Annex I: Irrigation Efficiencies. Available online: http://www.fao.org/3/t7202e/t7202e08.htm (accessed on 28 April 2020).
- Phene, C.J.; Davis, K.R.; Hutmacher, R.B.; Yosef, B.; Meek, D.W. Effect of high frequency surface and subsurface drip irrigation on root distribution of sweet corn. Irr. Sci. 1991, 12, 135–140. [Google Scholar] [CrossRef]
- Al-Jamal, M.S.; Ball, S.; Sammis, T.W. Comparison of sprinkler, trickle and furrow irrigation efficiencies for onion production. Agric. Water Manag. 2001, 46, 253–266. [Google Scholar] [CrossRef]
- Zhao, R.H.; He, W.-Q.; Lou, Z.-K.; Nie, W.-B.; Ma, X.-Y. Synchronization Optimization of Pipeline Layout and Pipe Diameter Selection in a Self-Pressurized Drip Irrigation Network System Based on the Genetic Algorithm. Water 2019, 11, 489. [Google Scholar] [CrossRef]
- Kumar, R.R.; Sriram, K.; Narayanan, I.S. Self Optimizing Drip Irrigation System Using Data Acquisition and Virtual Instrumentation to Enhance the Usage of Irrigation Water, Lecture Notes in Networks and Systems. In Cyber-Physical Systems and Digital Twins; REV2019; Auer, M., Ram, B.K., Eds.; Springer: Cham, Germany, 2019; Volume 80. [Google Scholar]
- Hanson, B.; May, D. Effect of subsurface drip irrigation on processing tomato yield, water table depth, soil salinity, and profitability. Agric. Water Manag. 2004, 68, 1–17. [Google Scholar] [CrossRef]
- Suganya, S.; Sivasamy, R. Moisture retention and cation exchange capacity of sandy soil as influenced by soil additives. J. Appl. Sci. Res. 2006, 2, 949–951. [Google Scholar]
- Mansour, H.A.; Gaballah, M.S.; Abd El-Hady, M.; Ebtisam, I. Influence of different localized irrigation systems and treated agricultural wastewater on distribution uniformities, potato growth, tuber yield and water use efficiency. J. Agric. Sci. 2014, 2, 143–150. [Google Scholar]
- Mansour, H.A.-G.; Tayel, M.Y.; Abd El-Hady, M.A.; Lightfoot, D.A.; El-Gindy, A.M. Modification of water application uniformity among closed circuit trickle irrigation systems. Agric. Sci. 2010, 1, 1–9. [Google Scholar] [CrossRef][Green Version]
- Ahmed, E.M.; Barakat, M.M.A.; Ragheb, H.M.; Rushdi, M.K. Impact of Surface and Subsurface Drip Irrigation Systems and Fertigation Managements on Yield and Water Use Efficiencies of Two Squash Varieties. Assiut J. Agric. Sci. 2017, 48, 303–318. [Google Scholar]
- Oron, G.; DeMalach, Y.; Gillerman, L.; David, I.; Rao, V.P. Improved saline—Water use under subsurface drip irrigation. Agric. Water Manag. 1998, 39, 19–33. [Google Scholar] [CrossRef]
- Oliveira, M.R.G.; Calado, A.M.; Portas, C.A.M. Tomato root distribution under drip irrigation. J. Am. Soc. Hort. Sci. 1996, 121, 644–648. [Google Scholar] [CrossRef]
- Grabow, G.L.; Huffman, R.L.; Edmisten, K. Automated Control of Subsurface Drip Irrigation Using Rainfall and Soil Water Data; ASAE Paper No. 042190; ASAE: St. Joseph, MI, USA, 2004. [Google Scholar]
- Gengoglan, C.; Altunbey, H.; Gengoglan, S. Response of green bean (phaseolus vulgaris L.) to subsurface drip irrigation and partial rootzone drying irrigation. Agric. Water Manag. 2006, 84, 274–280. [Google Scholar] [CrossRef]
- El-Shawadfy, M. Influence of Different Irrigation Systems and Treatments on Productivity and Fruit Quality of Some Bean Varieties. Master’s Thesis, Ain Shams University, Cairo, Egypt, 2008. [Google Scholar]
- Camp, C.R. Subsurface drip irrigation: A review. Trans. ASAE 1998, 41, 1353–1367. [Google Scholar] [CrossRef]
- Singh, D.K.; Rajput, T.B.S. Response of lateral placement depths of subsurface drip irrigation on okra (Abelmoschus esculentus). Int. J. Plant. Prod. 2007, 1, 73–84. [Google Scholar]
- Lamm, F.R.; Manges, H.L.; Stone, L.R.; Khan, A.H.; Rogers, D.H. Water requirement of subsurface drip-irrigated corn in northwest Kansas. Trans. ASAE 1995, 38, 441–448. [Google Scholar] [CrossRef]
- Maldini, M.; Montoro, P.; Addis, R.; Toniolo, C.; Petretto, G.L.; Foddai, M.; Nicoletti, M.; Pintore, G. A new approach to discriminate Rosmarinus officinalis L. plants with antioxidant activity, based on HPTLC fingerprint and targeted phenolic analysis combined with PCA. Ind. Crop. Prod. 2016, 94, 665–672. [Google Scholar] [CrossRef]
- Melito, S.; Petretto, G.L.; Chahine, S.; Pintore, G.; Chessa, M. Seasonal Variation of Essential Oil in Rosmarinus officinalis Leaves in Sardinia. Nat. Prod. Com. 2019, 14, 7. [Google Scholar] [CrossRef]
- Khorshidi, J.; Mohammadi, R.; Fakhr, M.T.; Nourbakhsh, H. Influence of Drying Methods, Extraction Time, and Organ Type on Essential Oil Content of Rosemary (Rosmarinus officinalis L.). Nat. Sci. 2009, 7, 42–44. [Google Scholar]
- Moreno, S.; Scheyer, T.; Romano, C.S.; Vojnov, A. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic. Res. 2006, 40, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, N.; Zu, Y.; Fu, Y. Antioxidative activity of Rosmarinus officinalis L. essential oil compared to its main components. Food Chem. 2008, 108, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Rozman, T.; Jersek, B. Antimicrobial activity of rosemary extracts (Rosmarinus officinalis L.) against different species of Listeria. Acta Agric. Slov. 2004, 93, 51–58. [Google Scholar]
- Moghtader, M.; Afzali, D. Study of the antibacterial properties of the essential oil of Rosemary. Am. Eur. J. Agric. Environ. Sci. 2009, 5, 393–397. [Google Scholar]
- Papachristos, D.P.; Stampoulos, D.C. Fumigant toxicity of three essential oils on the eggs of Acanthoscelide sobtectus (Say) (Coleoptera: Bruchidae). J. Stored Prod. Res. 2004, 40, 517–525. [Google Scholar] [CrossRef]
- Cuman, R.K. Anti-Inflammatory and Antinociceptive Effects of Rosmarinus officinalis L. Essential Oil in Experimental Animal Models. J. Med. Food. 2008, 11, 741–746. [Google Scholar]
- Takaki, I.; Bersani-Amado, L.E.; Vendruscolo, A.; Sartoretto, S.M.; Diniz, S.P.; Bersani-Amado, C.A.; Tunc, I.; Berger, B.M.; Erler, F.; Dagli, F. Ovicidal activity of essential oils from plants against two stored-product insects. J. Stored Prod. Res. 2000, 36, 161–168. [Google Scholar]
- Ozcan, M.M.; Chalchat, J. Chemical composition and antifungal activity of rosemary (Rosmarinus officinalis L) oil from Turkey. Int. J. Food Sci. Nutr. 2008, 59, 691–698. [Google Scholar] [CrossRef]
- Pozzatti, P.; Alves Scheid, L.; Borba Spader, T.; Linde Atayde, M.; Morais Santurio, J.; Hartz Alves, S. In vitro activity of essential oils extracted from plants used as spices against fluconazole-resistant and fluconazole-susceptible Candida spp. Can. J. Microbiol. 2008, 54, 950–956. [Google Scholar] [CrossRef]
- Isman, M.B. Plant essential oils for pest and disease management. Crop. Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Jones, B.; Nachtscheim, C.J. Split-Plot Designs: What, Why, and How. J. Quality Technol. 2009, 41, 340–361. [Google Scholar] [CrossRef]
- Petretto, G.L.; Fancello, F.; Zara, S.; Foddai, M.; Mangia, N.P.; Sanna, M.L.; Omer, E.A.; Menghini, L.; Chessa, M.; Pintore, G. Antimicrobial Activity against Beneficial Microorganisms and Chemical Composition of Essential Oil of Mentha suaveolens ssp. insularis Grown in Sardinia. J. Food Sci. 2014, 79, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Mannu, A.; Melito, S.; Petretto, G.L.; Manconi, P.; Pintore, G.M.; Chessa, M. Geographical variation of the chemical composition in essential oils extracted from Sardinian Salvia verbenaca. Nat. Prod. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Phene, C.J.; De Tar, W.R.; Clark, D.A. Real time irrigation scheduling of cotton with an automated pan evaporation system. Appl. Eng. Agric. 1992, 8, 787–793. [Google Scholar] [CrossRef]
- Bidondo, D.; Andreau, R.; Martinez, S.; Garbi, M.; Chale, W.; Cremaschi, G. XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): Comparison of the effect of surface and subsurface drip irrigation on water use growth and production of a greenhouse tomato crop. In Proceedings of the International Symposium on Greenhouse 2010 and Soilless Cultivationm, Lisbon, Portugal, 22 August 2010; pp. 309–313. [Google Scholar]
- Mannu, A.; Vlahopoulou, G.; Sireus, V.; Petretto, G.L.; Mulas, G.; Garroni, S. Bentonite as a Refining Agent in Waste Cooking Oils Recycling: Flash Point, Density and Color Evaluation. Nat. Prod. Commun. 2018, 13, 613–616. [Google Scholar] [CrossRef]
- Varma, R.S. Clay and clay-supported reagents in inorganic synthesis. Tetrahedron 2002, 58, 1235–1255. [Google Scholar] [CrossRef]
- Satje, A.; Nelson, P. Bentonite treatments can improve the nutrient and water holding capacity of sugarcane soils in the wet tropics. Proc. Aust Soc. Sugarcane Technol. 2009, 31, 166–176. [Google Scholar]
Sample Availability: Samples of the compounds are not available. |
| Environmental Data | Soil Properties | |||
|---|---|---|---|---|
| Latitude (N) | 30.397098 | O.M.% | 1.38 | |
| Longitude (E) | 31.551662 | CaCO3% | 4 | |
| Elevation (m) | 13 | pH (1:2:5) | 8.28 | |
| Distance (km) a | 80 | EC (dS/m) | 2.72 | |
| Direction | North-East | Available macronutrients (mg/100 g soil) | Ca++ | 369 |
| Max. temperature b | 27.4 | Mg++ | 100 | |
| Min. temperature b | 16.3 | Na+ | 231 | |
| T b | 11.1 | P | 1.82 | |
| Relative humidity (%) b | 56.7 | K | 27 | |
| Average temperature b (C) | 23.6 | Available micronutrients (ppm) | Fe | 10.2 |
| RH (%) | 61 | Mn | 13.4 | |
| WS (Km/day) | 144.7 | Zn | 2.6 | |
| PSSH (hr) | 9.3 | Cu | 0.3 | |
| Coarse sand (%) | 76.8 | |||
| Silt (%) | 8 | |||
| Clay (%) | 15.2 | |||
| Texture | Sand loamy | |||
| Treatment | Fresh Weight (g/Plant) | ||||||
|---|---|---|---|---|---|---|---|
| First Season | Second Season | ||||||
| 1st Cut | 2nd Cut | 1st Cut | 2nd Cut | 1st Cut | 2nd Cut | ||
| Mean (±) | Mean (±) | Mean (±) | Mean (±) | Mean (±) | Mean (±) | ||
| Surface | C | 35.89 (1.65) | 6.98 (0.80) | 42.87 (0.60) | 46.93 (2.43) | 9.67 (0.81) | 56.61 (1.15) |
| H | 36.33 (2.67) | 10.12 (1.76) | 46.46 (0.64) | 48.48 (1.41) | 12.02 (0.67) | 60.49 (0.52) | |
| BIO | 35.67 (2.00) | 9.40 (1.06) | 45.07 (0.67) | 46.45 (2.98) | 14.04 (1.70) | 60.49 (0.91) | |
| H + B10 | 38.55 (2.04) | 8.62 (0.57) | 47.17 (1.03) | 51.39 (1.43) | 10.15 (0.98) | 61.54 (0.32) | |
| Surface irrigation | 36.61 (2.09) | 8.78 (1.05) | 45.39 (0.74) | 25.89 (0.22) | 13.79 (0.37) | 59.78 (0.10) | |
| Subsurface | C | 38.78 (1.71) | 10.52 (0.84) | 49.30 (0.62) | 50.41 (3.35) | 13.29 (1.26) | 63.70 (1.48) |
| H | 41.22 (1.50) | 12.95 (1.30) | 54.18 (0.14) | 54.40 (3.12) | 17.49 (1.14) | 71.89 (1.40) | |
| BIO | 39.33 (2.33) | 15.92 (1.75) | 55.25 (0.41) | 53.66 (3.01) | 19.41 (1.38) | 73.07 (1.15) | |
| H + B10 | 42.33 (1.86) | 11.84 (1.72) | 54.17 (0.10) | 53.51 (3.66) | 17.76 (1.42) | 71.27 (1.58) | |
| Subsurface | 40.42 (1.85) | 12.81 (1.40) | 53.22 (0.32) | 28.58 (0.77) | 17.43 (0.32) | 69.98 (0.32) | |
| Soil conditioners | C | 37.33 (1.68) | 8.75 (0.82) | 46.09 (0.61) | 26.40 (0.15) | 13.92 (0.33) | 60.15 (0.13) |
| H | 38.78 (2.08) | 11.54 (1.53) | 50.32 (0.39) | 27.42 (0.80) | 16.19 (0.29) | 66.19 (0.36) | |
| BIO | 37.50 (2.17) | 12.66 (1.40) | 50.16 (0.54) | 26.52 (0.61) | 16.72 (0.05) | 66.78 (0.50) | |
| H + B10 | 40.44 (1.95) | 10.23 (1.15) | 50.67 (0.57) | 28.60 (0.41) | 15.61 (0.11) | 66.41 (0.21) | |
| LSD 5% irrigation | ns | 1.69 | 2.08 | ns | 2.83 | 2.46 | |
| LSD 5% soil conditioners | ns | 0.2 | 2.94 | ns | ns | 3.48 | |
| LSD 5% Interaction | ns | ns | ns | ns | ns | ns | |
| Treatment | Dry Weight (g/Plant) | ||||||
|---|---|---|---|---|---|---|---|
| First Season | Second Season | ||||||
| 1st Cut | 2nd Cut | Total | 1st Cut | 2nd Cut | Total | ||
| Mean (±) | Mean (±) | Mean (±) | Mean (±) | Mean (±) | Mean (±) | ||
| Surface | C | 8.97 (0.84) | 3.16 (0.57) | 12.14 0.20) | 11.73 (0.99) | 3.53 (0.55) | 15.26 (0.32) |
| H | 9.08 (0.67) | 4.81 (0.57) | 13.89 (0.06) | 12.12 (1.29) | 4.67 (1.19) | 16.79 (0.07) | |
| BIO | 8.84 (0.88) | 4.59 (0.68) | 13.42 (0.14) | 11.62 (1.25) | 5.50 (1.46) | 17.12 (0.15) | |
| H + B10 | 9.64 (1.50) | 4.24 (0.30) | 13.88 (0.64) | 12.84 (1.60) | 4.51 (0.47) | 17.35 (0.80) | |
| Surface irrigation | 9.13 (0.97) | 4.20 (0.60) | 13.33 (0.26) | 12.08 (1.28) | 4.55 (0.92) | 16.63 (0.33) | |
| Subsurface | C | 9.69 (1.58) | 4.93 (0.33) | 14.62 (0.88) | 12.60 (1.84) | 6.23 (0.32) | 18.83 (1.07) |
| H | 10.31 (1.27) | 6.89 (0.88) | 17.20 (0.28) | 13.60 (1.61) | 10.21 (0.68) | 23.81 (0.66) | |
| BIO | 9.83 (0.92) | 8.49 (1.09) | 18.32 (0.12) | 13.42 (1.03) | 14.20 (0.86) | 27.62 (0.12) | |
| H + B10 | 10.58 (0.85) | 6.65 (0.83) | 17.23 (0.01) | 13.38 (1.66) | 11.63 (1.24) | 25.01 (0.30) | |
| Subsurface | 10.10 (1.15) | 6.74 (0.78) | 16.84 (0.26) | 13.25 (1.54) | 10.57 (0.77) | 23.82 (0.54) | |
| Soil conditioners | C | 9.33 (1.21) | 4.05 (0.45) | 13.38 (0.54) | 12.17 (1.42) | 4.88 (0.43) | 17.05 (0.69) |
| H | 9.70 (0.97) | 5.85 (0.73) | 15.54 (0.17) | 12.86 (1.45) | 7.44 (0.94) | 20.30 (0.36) | |
| BIO | 9.34 (0.90) | 6.54 (0.88) | 15.87 (0.01) | 12.52 (1.14) | 9.85 (1.16) | 22.37 (0.14) | |
| H + B10 | 10.11 (1.18) | 5.45 (0.71) | 15.56 (0.33) | 13.11 (1.63) | 8.07 (0.86) | 21.18 (0.55) | |
| LSD 5% irrigation | ns | 1.26 | 1.25 | ns | 1.26 | 1.41 | |
| LSD 5% soil conditioners | ns | ns | 1.77 | ns | 1.79 | 1.99 | |
| LSD 5% Interaction | ns | ns | ns | ns | 1.60 | 2.82 | |
| Treatment | Fresh Yield (kg/Fad.) | ||||||
|---|---|---|---|---|---|---|---|
| First Season | Second Season | ||||||
| 1st Cut | 2nd Cut | Total | 1st Cut | 2nd Cut | Total | ||
| Mean (±) | Mean (±) | Mean (±) | Mean (±) | Mean (±) | Mean (±) | ||
| Surface | C | 825.5 (37.8) | 160.6 (18.3) | 986.1 (13.8) | 1079.4 (55.8) | 222.5 (18.5) | 1301.9 (26.3) |
| H | 835.7 (61.3) | 232.8 (40.4) | 1068.5 (14.8) | 1115.0 (32.4) | 276.3 (15.4) | 1391.3 (12.0) | |
| BIO | 820.4 (46.0) | 216.2 (24.3) | 1036.6 (15.3) | 1068.5 (68.6) | 323.0 (39.0) | 1391.3 (20.9) | |
| H + B10 | 886.7 (46.8) | 198.1 (13.2) | 1084.9 (23.8) | 1181.9 (32.8) | 233.5 (22.4) | 1415.5 (7.3) | |
| Surface irrigation | 842.1 (48.0) | 201.9 (24.0) | 1044.0 (16.9) | 1111.2 (47.4) | 263.8 (23.8) | 1375.0 (16.7) | |
| Subsurface | C | 891.9 (39.4) | 241.9 (19.3) | 1133.8 (14.2) | 1159.4 (77.0) | 305.6 (29.0) | 1465.0 (33.9) |
| H | 948.1 (34.6) | 297.9 (29.9) | 1246.1 (3.3) | 1251.2 (71.8) | 402.3 (26.1) | 1653.6 (32.3) | |
| BIO | 904.7 (53.7) | 366.0 (40.2) | 1270.7 (9.5) | 1234.2 (69.3) | 446.4 (31.8) | 1680.7 (26.5) | |
| H + B10 | 973.7 (42.7) | 272.3 (39.5) | 1245.9 (2.3) | 1230.8 (84.2) | 408.5 (32.7) | 1639.3 (36.4) | |
| Subsurface | 929.6 (42.6) | 294.5 (32.2) | 1224.1 (7.3) | 1218.9 (75.6) | 390.7 (29.9) | 1609.6 (32.3) | |
| Soil conditioners | C | 858.7 (38.6) | 201.3 (18.8) | 1060.0 (14.0) | 1119.4 (66.4) | 264.0 (23.8) | 1383.5 (30.1) |
| H | 891.9 (47.9) | 265.4 (35.2) | 1157.3 (9.0) | 1183.1 (52.1) | 339.3 (20.6) | 1522.4 (22.2) | |
| BIO | 862.5 (49.8) | 291.1 (32.2) | 1153.7 (12.4) | 1151.3 (68.9) | 384.7 (35.4) | 1536.0 (23.7) | |
| H + B10 | 930.2 (44.8) | 235.2 (26.4) | 1165.4 (13.0) | 1206.4 (58.5) | 321.0 (27.6) | 1527.4 (21.9) | |
| LSD 5% irrigation | ns | 39.0 | 47.8 | ns | 65.1 | 56.7 | |
| LSD 5% soil conditioners | ns | 55.1 | 67.7 | ns | ns | 80.2 | |
| LSD 5% Interaction | ns | ns | ns | ns | ns | ns | |
| Treatment | Fresh Yield (kg/Fad.) | ||||||
|---|---|---|---|---|---|---|---|
| First Season | First Season | ||||||
| 1st Cut | 1st Cut | 1st Cut | 1st Cut | 1st Cut | 1st Cut | ||
| Mean (±) | Mean (±) | Mean (±) | Mean (±) | Mean (±) | Mean (±) | ||
| Surface | C | 206.4 (19.4) | 72.7 (13.0) | 279.1 (4.5) | 269.9 (22.9) | 81.09 (12.6) | 350.98(7.2) |
| H | 208.9 (15.3) | 110.6 (13.2) | 319.5 (1.5) | 278.7 (29.6) | 107.44 (27.4) | 386.2 (1.5) | |
| BIO | 203.2 (20.2) | 105.5 (15.6) | 308.7 (3.2) | 267.2 (28.6) | 126.60 (33.5) | 393.8 (3.4) | |
| H + B10 | 221.6 (34.6) | 97.6 (13.7) | 319.2 (14.7) | 295.4 (36.8) | 103.70 (10.8) | 399.1 (18.4) | |
| Surface irrigation | 210.0 (22.4) | 96.6 (13.9) | 306.7 (6.0) | 277.8 (29.5) | 104.71 (21.1) | 382.5 (5.9) | |
| Subsurface | C | 222.9 (36.3) | 113.3 (7.6) | 336.3 (20.3) | 289.83 (42.2) | 143.4 (7.3) | 433.2 (24.7) |
| H | 237.0 (29.3) | 158.4 (20.3) | 395.5 (6.3) | 312.84 (37.1) | 234.8 (15.6) | 547.7 (15.2) | |
| BIO | 226.2 (21.0) | 195.3 (25.0) | 421.4 (2.8) | 308.57 (23.8) | 326.7 (19.7) | 635.2 (2.9) | |
| H + B10 | 243.4 (19.5) | 15.9 (19.1) | 396.3 (0.2) | 307.70 (38.2) | 267.6 (28.6) | 575.3 (6.8) | |
| Subsurface | 232.4 (26.5) | 155.0 (18.0) | 387.4 (6.0) | 304.73 (35.3) | 243.1 (17.8) | 547.8 (12.4) | |
| Soil conditioners | C | 214.7 (27.8) | 93.0 (10.3) | 307.7 (12.4) | 279.9 (32.6) | 112.2 (10.0) | 392.1 (16.0) |
| H | 223.0 (22.3) | 134.5 (16.7) | 357.5 (3.9) | 295.8 (33.3) | 171.1 (21.5) | 466.9 (8.4) | |
| BIO | 214.7 (20.6) | 150.4 (20.3) | 365.1 (0.2) | 287.9 (26.2) | 226.6 (26.6) | 514.5 (0.3) | |
| H + B10 | 232.5 (27.0) | 125.2 (16.4) | 357.8 (7.5) | 301.6 (37.5) | 185.6 (19.7) | 487.2 (12.6) | |
| LSD 5% irrigation | ns | 29.07 | 28.8 | ns | 29.2 | 32.5 | |
| LSD 5% soil conditioners | ns | ns | 40.8 | ns | 41.3 | 46.0 | |
| LSD 5% Interaction | ns | ns | ns | ns | 37.0 | 65.0 | |
| Treatment | EO% | ||||||
|---|---|---|---|---|---|---|---|
| First Season | First Season | ||||||
| 1st Cut | 1st Cut | 1st Cut | 1st Cut | 1st Cut | 1st Cut | ||
| Mean (±) | Mean (±) | Mean (±) | Mean (±) | Mean (±) | Mean (±) | ||
| Surface | C | 3.63 (0.06) | 3.75 (0.13) | 7.38 (0.05) | 3.63 (0.03) | 3.73 (0.09) | 7.36 (0.04) |
| H | 3.75 (0.13) | 3.85 (0.03) | 7.60 (0.07) | 3.72 (0.11) | 3.81 (0.02) | 7.53 (0.06) | |
| BIO | 3.92 (0.03) | 3.94 (0.01) | 7.86 (0.01) | 3.90 (0.03) | 3.93 (0.04) | 7.83 (0.01) | |
| H + B10 | 3.70 (0.04) | 3.83 (0.10) | 7.53 (0.22) | 3.75 (0.24) | 3.87 (0.10) | 7.62 (0.10) | |
| Surface irrigation | 3.75 (0.15) | 3.84 (0.07) | 7.59 (0.06) | 3.75 (0.10) | 3.83 (0.06) | 7.59 (0.03) | |
| Subsurface | C | 3.80 (0.10) | 3.89 (0.03) | 7.69 (0.05) | 3.83 (0.04) | 3.82 (0.03) | 7.65 (0.01) |
| H | 4.23 (0.06) | 4.42 (0.07) | 8.66 (0.01) | 4.18 (0.06) | 4.26 (0.04) | 8.44 (0.02) | |
| BIO | 4.10 (0.10) | 4.32 (0.03) | 8.42 (0.05) | 4.21 (0.02) | 4.30 (0.03) | 8.51 (0.01) | |
| H + B10 | 4.03 (0.15) | 4.23 (0.10) | 8.26 (0.04) | 4.04 (0.21) | 4.21 (0.04) | 8.25 (0.13) | |
| Subsurface | 4.04 (0.10) | 4.22 (0.06) | 8.26 (0.03) | 4.06 (0.08) | 4.15 (0.03) | 8.21 (0.04) | |
| Soil conditioners | C | 3.72 (0.08) | 3.82 (0.08) | 7.54 (0.00) | 3.73 (0.03) | 3.78 (0.06) | 7.50 (0.02) |
| H | 3.99 (0.10) | 4.14 (0.05) | 8.13 (0.03) | 3.95 (0.09) | 4.03 (0.03) | 7.98 (0.04) | |
| BIO | 4.01 (0.06) | 4.13 (0.02) | 8.14 (0.03) | 4.06 (0.02) | 4.12 (0.03) | 8.17 (0.01) | |
| H + B10 | 3.87 (0.28) | 4.03 (0.10) | 7.90 (0.13) | 3.89 (0.23) | 4.04 (0.07) | 7.94 (0.11) | |
| LSD 5% irrigation | 0.14 | 0.06 | 0.19 | 0.11 | 0.05 | 0.13 | |
| LSD 5% soil conditioners | 0.20 | 0.09 | 0.26 | 0.15 | 0.07 | 0.18 | |
| LSD 5% Interaction | ns | 0.29 | ns | ns | 0.09 | 0.25 | |
| Treatment | EO (mL/plant) | ||||||
|---|---|---|---|---|---|---|---|
| First Season | First Season | ||||||
| 1st Cut | 1st Cut | 1st Cut | 1st Cut | 1st Cut | 1st Cut | ||
| Mean (±) | Mean (±) | Mean (±) | Mean (±) | Mean (±) | Mean (±) | ||
| Surface | C | 0.33 (0.03) | 0.12 (0.03) | 0.44 (0.00) | 0.43 (0.03) | 0.13 (0.02) | 0.56 (0.01) |
| H | 0.34 (0.03) | 0.19 (0.02) | 0.53 (0.01) | 0.45 (0.05) | 0.18 (0.05) | 0.63 (0.01) | |
| BIO | 0.35 (0.08) | 0.18 (0.03) | 0.53 (0.03) | 0.45 (0.09) | 0.22 (0.06) | 0.67 (0.02) | |
| H + B10 | 0.36 (0.09) | 0.16 (0.03) | 0.52 (0.05) | 0.48 (0.09) | 0.17 (0.02) | 0.66 (0.05) | |
| Surface irrigation | 0.34 (0.06) | 0.16 (0.03) | 0.51 (0.02) | 0.45 (0.07) | 0.18 (0.04) | 0.63 (0.02) | |
| Subsurface | C | 0.37 (0.06) | 0.19 (0.01) | 0.56 (0.04) | 0.48 (0.09) | 0.24 (0.01) | 0.72 (0.06) |
| H | 0.44 (0.12) | 0.30 (0.04) | 0.74 (0.06) | 0.57 (0.14) | 0.43 (0.10) | 1.00 (0.03) | |
| BIO | 0.40 (0.10) | 0.37 (0.05) | 0.77 (0.03) | 0.57 (0.13) | 0.61 (0.04) | 1.18 (0.06) | |
| H + B10 | 0.43 (0.04) | 0.28 (0.03) | 0.71 (0.01) | 0.54 (0.09) | 0.49 (0.11) | 1.03 (0.01) | |
| Subsurface | 0.41 (0.08) | 0.29 (0.03) | 0.69 (0.03) | 0.54 (0.11) | 0.44 (0.06) | 0.98 (0.03) | |
| Soil conditioners | C | 0.35 (0.04) | 0.16 (0.02) | 0.50 (0.02) | 0.45 (0.06) | 0.19 (0.02) | 0.64 (0.03) |
| H | 0.39 (0.07) | 0.24 (0.03) | 0.63 (0.03) | 0.51 (0.10) | 0.31 (0.07) | 0.82 (0.02) | |
| BIO | 0.37 (0.09) | 0.27 (0.04) | 0.65 (0.03) | 0.51 (0.11) | 0.41 (0.05) | 0.92 (0.04) | |
| H + B10 | 0.39 (0.07) | 0.22 (0.03) | 0.62 (0.03) | 0.51 (0.09) | 0.33 (0.07) | 0.85 (0.02) | |
| LSD 5% irrigation | ns | 0.05 | 0.07 | 0.08 | 0.05 | 0.09 | |
| LSD 5% soil conditioners | ns | 0.07 | 0.1 | ns | 0.07 | 0.12 | |
| LSD 5% Interaction | ns | ns | ns | ns | 0.10 | ns | |
| Treatment | EO (L/Fad.) | ||||||
|---|---|---|---|---|---|---|---|
| First Season | First Season | ||||||
| 1st Cut | 1st Cut | 1st Cut | 1st Cut | 1st Cut | 1st Cut | ||
| Mean (±) | Mean (±) | Mean (±) | Mean (±) | Mean (±) | Mean (±) | ||
| Surface | C | 7.5 (0.6) | 2.7 (0.6) | 10.2 (0.1) | 9.8 (0.8) | 3.0 (0.5) | 12.8 (0.2) |
| H | 7.8 (0.7) | 4.3 (0.5) | 12.1 (0.1) | 10.4 (1.2) | 4.1 (1.0) | 14.5 (0.1) | |
| BIO | 8.0 (1.7) | 4.2 (0.6) | 12.1 (0.8) | 10.4 (2.0) | 5.0 (1.3) | 15.4 (0.4) | |
| H + B10 | 8.3 (2.2) | 3.7 (0.6) | 12.0 (1.1) | 11.1 (2.1) | 4.0 (0.5) | 15.2 (1.1) | |
| Surface irrigation | 7.9 (1.3) | 3.7 (0.6) | 11.6 (0.5) | 10.4 (1.5) | 4.0 (0.9) | 14.5 (0.5) | |
| Subsurface | C | 8.4 (1.5) | 4.4 (0.3) | 12.8 (0.8) | 11.1 (2.2) | 5.5 (0.3) | 16.6 (1.3) |
| H | 10.0 (2.7) | 7.0 (0.9) | 17.0 (1.3) | 13.0 (3.2) | 10.0 (2.2) | 23.1 (0.7) | |
| BIO | 9.2 (2.2) | 8.4 (1.1) | 17.7 (0.7) | 13.0 (3.0) | 14.1 (0.9) | 27.1 (1.4) | |
| H + B10 | 9.8 (0.9) | 6.5 (0.7) | 16.3 (0.1) | 12.5 (2.0) | 11.3 (2.5) | 23.7 (0.3) | |
| Subsurface | 9.4 (1.8) | 6.6 (0.7) | 16.0 (0.7) | 12.4 (2.6) | 10.2 (1.5) | 22.6 (0.9) | |
| Soil conditioners | C | 8.0 (1.0) | 3.6 (0.4) | 11.5 (0.4) | 10.4 (1.5) | 4.3 (0.4) | 14.7 (0.7) |
| H | 8.9 (1.7) | 5.6 (0.7) | 14.6 (0.7) | 11.7 (2.2) | 7.0 (1.6) | 18.8 (0.4) | |
| BIO | 8.6 (2.0) | 6.3 (0.9) | 14.9 (0.8) | 11.7 (2.5) | 9.5 (1.1) | 21.2 (0.9) | |
| H + B10 | 9.1 (1.5) | 5.1 (0.7) | 14.2 (0.6) | 11.8 (2.1) | 7.6 (1.5) | 19.4 (0.4) | |
| LSD 5% irrigation | ns | 1.2 | 1.6 | 1.9 | 1.2 | 2.0 | |
| LSD 5% soil conditioners | ns | 1.7 | 2.3 | ns | 1.7 | 2.8 | |
| LSD 5% Interaction | ns | ns | ns | ns | 2.4 | ns | |
| Name | KI | Surface | Subsurface | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C | HZ | BENT | HZ + BENT | C | HZ | BENT | HZ + BENT | ||
| Tricyclene | 900 | 0.2 | 0.2 | 0.2 | 0.2 | 0.1 | 0.2 | 0.2 | 0.1 |
| α-Pinene | 909 | 19.3 | 16.7 | 16.8 | 21.1 | 14.5 | 18.8 | 20.6 | 15.4 |
| Camphene | 927 | 4.8 | 4.7 | 4.6 | 4.8 | 4.4 | 4.8 | 4.7 | 4.2 |
| thuja 2,4-Diene | 930 | 0.4 | 0.5 | 0.5 | 0.4 | 0.5 | 0.5 | 0.4 | 0.4 |
| β-Pinene | 956 | 0.5 | 0.4 | 0.4 | 0.6 | 0.4 | 0.4 | 0.5 | 0.3 |
| β-Myrcene | 957 | 0.7 | 0.5 | 0.5 | 0.8 | 0.5 | 0.5 | 0.6 | 0.4 |
| Phellandrene | 984 | 0.3 | 0.2 | 0.2 | 0.3 | 0.2 | 0.2 | 0.3 | 0.2 |
| δ-2-Carene | 1005 | 0.7 | 0.6 | 0.6 | 0.7 | 0.6 | 0.6 | 0.7 | 0.5 |
| p-cymene | 1026 | 1.1 | 0.9 | 0.9 | 1.2 | 1.0 | 0.9 | 1.0 | 0.8 |
| Eucalyptol | 1036 | 14.5 | 14.5 | 14.4 | 15.5 | 14.4 | 14.1 | 13.7 | 13.1 |
| γ-Terpinene | 1062 | 1.0 | 0.8 | 0.74 | 1.0 | 0.8 | 0.7 | 0.9 | 0.5 |
| Terpinolene | 1065 | 1.4 | 1.1 | 1.0 | 1.3 | 1.1 | 1.0 | 1.2 | 0.9 |
| Linalool | 1102 | 1.0 | 1.0 | 1.0 | 1.1 | 1.0 | 0.9 | 1.1 | 0.9 |
| α-Campholenal | 1106 | 0.5 | 0.4 | 0.5 | 0.5 | 0.5 | 0.4 | 0.4 | 0.3 |
| Camphor | 1145 | 40.0 | 44.1 | 44.3 | 40.7 | 44.0 | 41.9 | 38.6 | 44.8 |
| Pinocarvone | 1161 | 0.2 | 0.1 | 0.1 | 0.3 | 0.3 | 0.3 | 0.3 | 0.1 |
| Terpinen-4-ol | 1163 | 0.9 | 0.9 | 0.9 | 0.7 | 1.0 | 0.9 | 0.9 | 2.3 |
| Thymol | 1165 | 0.1 | 0.1 | 0.2 | 0.9 | 0.8 | 0.8 | 0.9 | 0.1 |
| α-Terpineol | 1180 | 2.5 | 2.2 | 2.3 | 2.1 | 2.5 | 2.2 | 2.4 | 3.3 |
| Verbenone | 1223 | 7.8 | 6.9 | 7.1 | 5.2 | 9.1 | 7.7 | 7.9 | 6.3 |
| Bornyl acetate | 1261 | 0.4 | 0.4 | 0.3 | 0.1 | 0.3 | 0.4 | 0.4 | 0.2 |
| Caryophyllene | 1400 | 0.2 | 0.2 | 0.3 | - | 0.3 | 0.2 | 0.2 | 0.2 |
| α-Humulene | 1406 | 0.4 | 0.3 | 0.4 | - | 0.4 | 0.4 | 0.4 | 0.1 |
| Bisabolene, trans- | 1479 | 0.1 | 0.2 | 0.0 | - | 0.1 | 0.1 | - | 0.1 |
| Monoterpene hydrocarbons | 30.4 | 26.7 | 26.4 | 32.3 | 24.1 | 28.6 | 31.2 | 23.7 | |
| Oxygenated hydrocarbons | 68.0 | 70.8 | 71.0 | 67.2 | 74.0 | 69.6 | 66.6 | 71.5 | |
| Sesquiterpene hydrocarbons | 0.7 | 0.8 | 0.7 | 0.0 | 0.8 | 0.7 | 0.7 | 0.3 | |
| Total of identified compounds | 99.1 | 98.2 | 98.2 | 99.5 | 98.9 | 98.9 | 98.4 | 95.6 | |
| Unidentified compounds | 0.9 | 1.8 | 1.8 | 0.4 | 1.1 | 1.1 | 1.6 | 4.4 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omer, E.; Hendawy, S.; ElGendy, A.N.; Mannu, A.; Petretto, G.L.; Pintore, G. Effect of Irrigation Systems and Soil Conditioners on the Growth and Essential Oil Composition of Rosmarinus officinalis L. Cultivated in Egypt. Sustainability 2020, 12, 6611. https://doi.org/10.3390/su12166611
Omer E, Hendawy S, ElGendy AN, Mannu A, Petretto GL, Pintore G. Effect of Irrigation Systems and Soil Conditioners on the Growth and Essential Oil Composition of Rosmarinus officinalis L. Cultivated in Egypt. Sustainability. 2020; 12(16):6611. https://doi.org/10.3390/su12166611
Chicago/Turabian StyleOmer, Elsayed, Saber Hendawy, Abdel Nasser ElGendy, Alberto Mannu, Giacomo L. Petretto, and Giorgio Pintore. 2020. "Effect of Irrigation Systems and Soil Conditioners on the Growth and Essential Oil Composition of Rosmarinus officinalis L. Cultivated in Egypt" Sustainability 12, no. 16: 6611. https://doi.org/10.3390/su12166611
APA StyleOmer, E., Hendawy, S., ElGendy, A. N., Mannu, A., Petretto, G. L., & Pintore, G. (2020). Effect of Irrigation Systems and Soil Conditioners on the Growth and Essential Oil Composition of Rosmarinus officinalis L. Cultivated in Egypt. Sustainability, 12(16), 6611. https://doi.org/10.3390/su12166611







