Impacts of Moringa oleifera Foliage Substituted for Concentrate Feed on Growth, Nutrient Digestibility, Hematological Attributes, and Blood Minerals of Growing Goats under Abu Dhabi Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Moringa Preparation and Chemical Composition
2.2. Animal, Design, and Alimentation
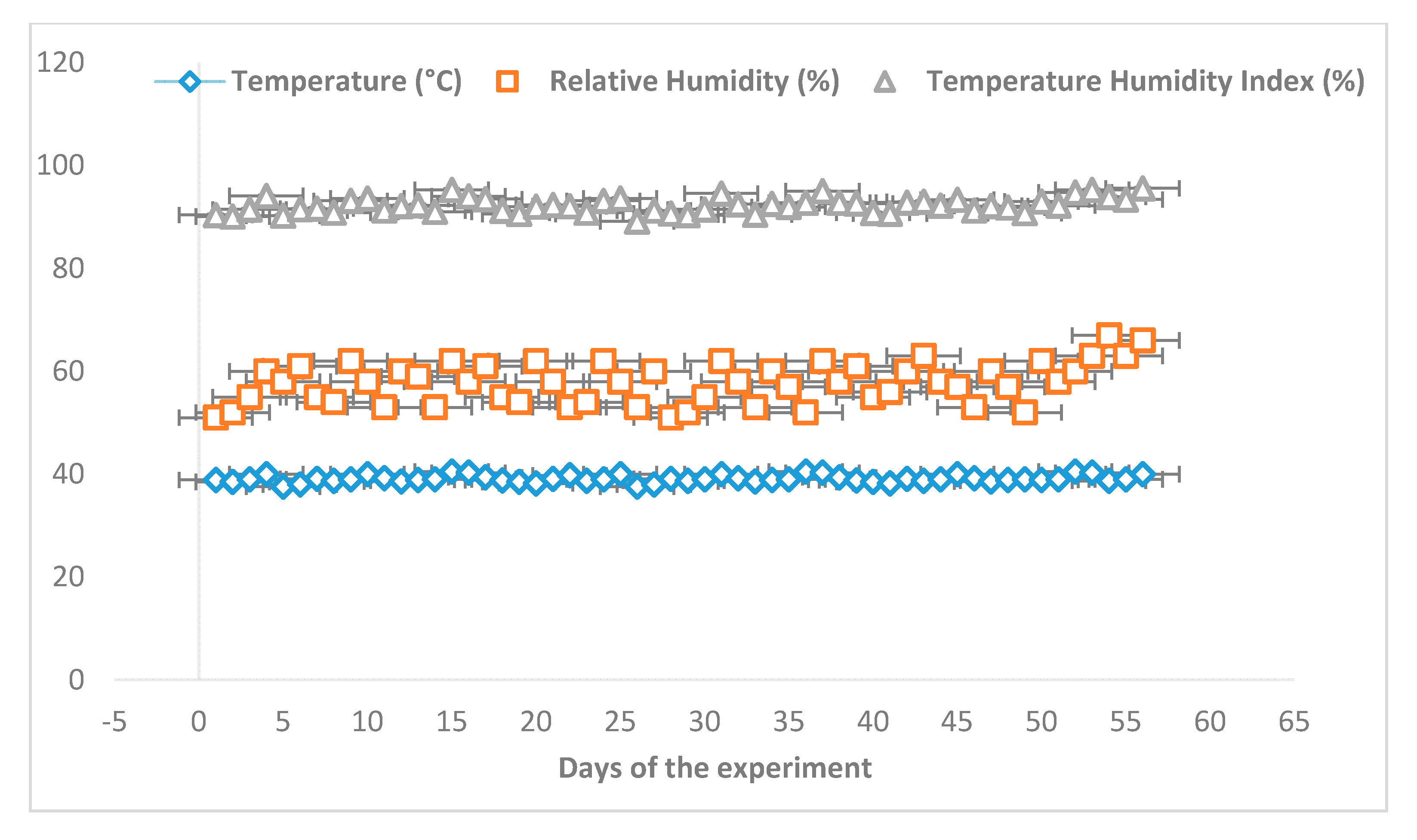
2.3. Temperature Humidity Index (THI)
2.4. Growth Performance and Digestibility
2.5. Hematological and Serum Biochemical Analysis
2.6. Statistical Analysis
3. Results
3.1. Temperature Humidity Index (THI)
3.2. Growth Performance
3.3. Hematological Analysis
3.4. Serum Metabolites
3.5. Minerals
4. Discussions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abdelnour, S.A.; Abd El-Hack, M.E.; Khafaga, A.F.; Arif, M.; Taha, A.E.; Noreldin, A.E. Stress biomarkers and proteomics alteration to thermal stress in ruminants: A review. J. Therm. Biol. 2019, 79, 120–134. [Google Scholar] [CrossRef]
- FAO. World Livestock 2011—Livestock in Food Security; FAO: Rome, Italy, 2011. [Google Scholar]
- FAO. The State of Food and Agriculture Climate Change, Agriculture and Food Security 2016, Livestock in food Security; FAO: Rome, Italy, 2016. [Google Scholar]
- Al-Samawi, K.A.; Al-Hassan, M.J.; Swelum, A.A. Thermoregulation of female Aardi goats exposed to environmental heat stress in Saudi Arabia. Indian J. Anim. Res. 2014, 48, 344–34911. [Google Scholar] [CrossRef]
- Ashour, E.A.; El-Kholy, M.S.; Alagawany, M.; Abd El-Hack, M.E.; Mohamed, E.; Mohamed, L.A.; Taha, A.E.; El Sheikh, A.I.; Laudadio, V.; Tufarelli, V. Effect of dietary supplementation with Moringa oleifera leaves and/or seeds powder on production, egg characteristics, hatchability and blood chemistry of laying Japanese quails. Sustainability 2020, 12, 2463. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Alagawany, M.; Elrys, A.S.; Desoky, E.M.; Tolba, H.M.N.; Elnahal, A.S.M.; Elnesr, S.S.; Swelum, A.A. Effect of forage Moringa oleifera L. (moringa) on animal health and nutrition and its beneficial applications in soil, plants and water purification. Agriculture 2018, 8, 145. [Google Scholar] [CrossRef]
- Nouman, W.; Basra, S.M.A.; Siddiqui, M.T.; Yasmeen, A.; Gull, T.; Alcayde, M.A.C. Potential of Moringa oleifera L. as livestock fodder crop: A review. Turk. J. Agric. For. 2014, 38, 1–14. [Google Scholar] [CrossRef]
- Mendieta-Araica, B.; Spörndly, R.; Reyes-Sánchez, N.; Spörndly, E. Moringa (Moringa oleifera) leaf meal as a source of protein in locally produced concentrates for dairy cows fed low protein diets in tropical areas. Livest. Sci. 2011, 137, 10–17. [Google Scholar] [CrossRef]
- Soliva, C.R.; Kreuzer, M.; Foidl, N.; Foidl, G.; Machmüller, A.; Hess, H.D. Feeding value of whole and extracted Moringa oleifera leaves for ruminants and their effects on ruminal fermentation in vitro. Anim. Feed Sci. Technol. 2005, 118, 47–62. [Google Scholar] [CrossRef]
- Moyo, B.; Masika, P.J.; Muchenje, V. Effect of supplementing crossbred Xhosa lop-eared goat castrates with Moringa oleifera leaves on growth performance, carcass and non-carcass characteristics. Trop. Anim. Health Prod. 2012, 44, 801–809. [Google Scholar] [CrossRef]
- Aregheore, E.M. Intake and digestibility of Moringa oleifera–batiki grass mixtures by growing goats. Small Rumin. Res. 2002, 46, 23–28. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Abdelnour, S.A.; Taha, A.E.; Khafaga, A.F.; Arif, M.; Ayasan, T.; Swelum, A.A.; Abukhalil, M.H.; Alkahtani, S.; Aleya, L.; et al. Herbs as thermoregulatory agents in poultry: An overview. Sci. Total Environ. 2020, 703, 134399. [Google Scholar] [CrossRef]
- Kholif, A.E.; Gouda, G.A.; Morsy, T.A.; Salem, A.Z.M.; Lopez, S.; Kholif, A.M. Moringa oleifera leaf meal as a protein source in lactating goat’s diets: Feed intake, digestibility, ruminal fermentation, milk yield and composition, and its fatty acids profile. Small Rumin. Res. 2015, 129, 129–137. [Google Scholar] [CrossRef]
- Kholif, A.E.; Morsy, T.A.; Gouda, G.A.; Anele, U.Y.; Galyean, M.L. Effect of feeding diets with processed Moringa oleifera meal as protein source in lactating Anglo-Nubian goats. Anim. Feed Sci. Technol. 2016, 217, 45–55. [Google Scholar] [CrossRef]
- Abd Mahfuz, S.; Piao, X.S. Application of Moringa (Moringa oleifera) as natural feed supplement in poultry diets. Animals 2019, 9, 431. [Google Scholar] [CrossRef]
- Becker, K. Studies on utilization of Moringa oleifera leaves as animal feed. Inst. Anim. Prod. Trop. Subtrop. 1995, 480, 15. [Google Scholar]
- Al-Juhaimi, F.Y.; Alsawmahi, O.N.; Abdoun, K.A.; Ghafoor, K.; Babiker, E.E. Antioxidant potential of Moringa leaves for improvement of milk and serum quality of Aardi goats. South Afr. J. Bot. 2019. [Google Scholar] [CrossRef]
- Kholif, A.E.; Gouda, G.A.; Olafadehan, O.A.; Abdo, M.M. Effects of replacement of Moringa oleifera for berseem clover in the diets of Nubian goats on feed utilisation, and milk yield, composition and fatty acid profile. Animal 2018, 12, 964–972. [Google Scholar] [CrossRef]
- National Research Council; Committee on the Nutrient Requirements of Small Ruminants; Board on Agriculture; Division on Earth; Life Studies. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World camelids; China Legal Publishing House: Beijing, China, 2007. [Google Scholar]
- LPHSI. Livestock and Poultry Heat Stress Indices: Agriculture Engineering Guide; Clemson 744 University: Clemson, SC, USA, 1990. [Google Scholar]
- Sultana, N.; Alimon, A.; Huque, K.; Baba, M.; Hossain, J. Evaluation of Moringa Foliage (Moringa oleifera) as Goat Feed. Iran. J. App. Anim. Sci. 2015, 5, 865–871. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis: Changes in Official Methods of Analysis Made at the Annual Meeting; Supplement; Association of Official Analytical Chemists: Washington, DC, USA, 1990; Volume 15. [Google Scholar]
- Su, B.; Chen, X. Current Status and Potential of Moringa oleifera Leaf as an Alternative Protein Source for Animal Feeds. Front. Vet. Sci. 2020, 7, 53. [Google Scholar] [CrossRef]
- Sultana, N.; Alimon, A.; Huque, K.; Sazili, A.; Yaakub, H.; Hossain, J.; Baba, M. The feeding value of Moringa (Moringa oleifera) foliage as replacement to conventional concentrate diet in Bengal goats. Adv. Anim. Vet. Sci. 2015, 3, 164–173. [Google Scholar] [CrossRef]
- Ewuola, E.O.; Sokunbi, O.A.; Sanni, K.M.; Oyedemi, O.M.; Lawal, T.T. Haematological and serum biochemical responses of rabbit does to crude Moringa oleifera leaf extract at gestation and lactation. Trop. Anim. Health Prod. 2015, 47, 637–642. [Google Scholar] [CrossRef]
- Hashem, N.M.; Soltan, Y.A.; El-Desoky, N.I.; Morsy, A.S.; Sallam, S.M.A. Effects of Moringa oleifera extracts and monensin on performance of growing rabbits. Livest. Sci. 2019, 228, 136–143. [Google Scholar] [CrossRef]
- Gupta, A.; Gautam, M.K.; Singh, R.K.; Kumar, M.V.; Rao, C.; Goel, R.K.; Anupurba, S. Immunomodulatory effect of Moringa oleifera Lam. extract on cyclophosphamide induced toxicity in mice. Indian J. Exp. Biol. 2010, 48, 1157–1160. [Google Scholar] [PubMed]
- Yusuf, A.O.; Mlambo, V.; Iposu, S.O. A nutritional and economic evaluation of Moringa oleifera leaf meal as a dietary supplement in West African Dwarf goats. S. Afr. J. Anim. Sci. 2018, 48, 81–87. [Google Scholar] [CrossRef]
- Villarruel-López, A.; López-de la Mora, D.A.; Vázquez-Paulino, O.D.; Puebla-Mora, A.G.; Torres-Vitela, M.R.; Guerrero-Quiroz, L.A.; Nuño, K. Effect of Moringa oleifera consumption on diabetic rats. BMC Complement. Altern. Med. 2018, 18, 127. [Google Scholar] [CrossRef]
- Vargas-Sánchez, K.; Garay-Jaramillo, E.; González-Reyes, R.E. Effects of Moringa oleifera on glycaemia and insulin levels: A review of animal and human studies. Nutrients 2019, 11, 2907. [Google Scholar] [CrossRef]
- Aju, B.Y.; Rajalakshmi, R.; Mini, S. Protective role of Moringa oleifera leaf extract on cardiac antioxidant status and lipid peroxidation in streptozotocin induced diabetic rats. Heliyon 2019, 5, e02935. [Google Scholar] [CrossRef]
- Meel, P.; Gurjar, M.L.; Nagda, R.K.; Sharma, M.C.; Gautam, L. Effect of Moringa oleifera leaves feeding on hemato-biochemical profile of Sirohi goat kids. J. Entomol. Zool. Studies. J. Entomol. Zool. Stud. 2018, 6, 41–48. [Google Scholar]
- Ndukaku, O.Y.; Emmanuel, E.U.; Mercy, E.A.; Caroline, N.O. Evaluation of the serum liver enzymes markers, lipid profile and kidney function parameters in typhoid patients. IJTDH 2015, 8, 79–89. [Google Scholar] [CrossRef]
- Yousef, F.M.A.; Khattab, H.A.H.; Sindi, H.A.A. Effectiveness of Moringa oleifera L. Leaves Extract Against Methotrexate-induced Acute Hepatotoxicity in Male Rats. Int. J. Pharm. 2018, 14, 1029–1037. [Google Scholar]
- Toledo, J.B.; Furlan, A.C.; Pozza, P.C.; Carraro, J.M.; Gabriel, F.S.L.; Gallego, A.G. Reduction of the crude protein content of diets supplemented with essential amino acids for piglets weighing 15 to 30 kilograms. Rev. Bras. Zootec. 2014, 43, 301–309. [Google Scholar] [CrossRef][Green Version]
- Arigony, A.L.; de Oliveira, I.M.; Machado, M.; Bordin, D.L.; Bergter, L.; Prá, D.; Henriques, J.A. The influence of micronutrients in cell culture: A reflection on viability and genomic stability. Biomed Res Int. 2013, 2013, 597282. [Google Scholar] [CrossRef] [PubMed]
- El-Desoky, N.I.; Hashem, N.M.; Elkomy, A.; Abo-elezz, Z.R. Physiological response and semen quality of rabbit bucks supplemented with Moringa leaves ethanolic extract during summer season. Animal 2017, 11, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Bin-Jumah, M.; Abd El-Hack, M.E.; Abdelnour, S.A.; Hendy, Y.A.; Ghanem, H.A.; Alsafy, S.A.; Khafaga, A.F.; Noreldin, A.E.; Shaheen, H.; Samak, D.; et al. Potential use of chromium to combat thermal stress in animals: A review. Sci. Total Environ. 2020, 707, 135996. [Google Scholar] [CrossRef] [PubMed]
- Sejian, V.; Singh, A.K.; Sahoo, A.; Naqvi, S.M.K. Effect of mineral mixture and antioxidant supplementation on growth, reproductive performance and adaptive capability of Malpura ewes subjected to heat stress. J. Anim. Physiol. Anim. Nutr. 2014, 98, 72–83. [Google Scholar] [CrossRef]
| Feed Type | Concentrate Feed | Alfalfa Hay | Moringa |
| Dry matter (%) | 93.03 | 93.21 | 24.36 |
| ADF (%) | 30.28 | 29.49 | 28.86 |
| Crude protein (%) | 9.44 | 14.19 | 18.38 |
| P (%) | 0.53 | 0.32 | 0.33 |
| K (%) | 0.74 | 2.34 | 1.63 |
| Ca (%) | 0.29 | 0.48 | 2.2 |
| Mg (%) | 0.29 | 0.48 | 0.27 |
| S (%) | 0.84 | 0.47 | 1.36 |
| Na (%) | 0.04 | 0.16 | 0.07 |
| Fe (ppm) | 838 | 549 | 331 |
| Cu (ppm) | 10.6 | 12.8 | 9.7 |
| Mn (ppm) | 70.57 | 33.84 | 30.38 |
| Zn (ppm) | 55.18 | 31.12 | 26.1 |
| Cr (ppm) | 5.61 | 4.76 | 3.47 |
| Se (ppm) | 0.23 | 0.12 | ND |
| Items | Diets | ||||
| MF0 | MF25 | MF50 | MF75 | MF100 | |
| Concentrate Feed | 1700 g | 1300 g | 850 g | 500 g | --- |
| Alfalfa Hay | 1200 g | 1200 g | 1200 g | 1200 g | 1200 g |
| Moringa Oleifera | ---- | 400 g | 850 g | 1200 g | 1700 g |
| Items | Diets | Pooled SEM | p-Value | ||||
| MF0 | MF25 | MF50 | MF75 | MF100 | |||
| Growth performance | |||||||
| IBW (kg) | 15.83 | 15.75 | 15.77 | 15.73 | 15.78 | 0.147 | >0.999 |
| FBW (kg) | 20.06 a | 19.81 a | 18.63 ab | 17.25 b | 17.05 b | 0.334 | 0.001 |
| ADG (kg) | 0.083 a | 0.080 a | 0.056 b | 0.030 c | 0.025 c | 0.005 | <0.001 |
| Digestibility of crude protein and acid detergent fiber (% on DM basis) | |||||||
| ADF (% of DM) | 52.30 b | 57.25 a | 57.07 a | 58.09 a | 55.24 ab | 0.62 | 0.010 |
| CP (%) | 9.13 ab | 9.43 a | 8.98 abc | 7.61 bc | 7.25 c | 0.28 | 0.043 |
| Items | Diets | Pooled SEM | p Value | ||||
| MF0 | MF25 | MF50 | MF75 | MF100 | |||
| HTC (%) | 30.31 | 32.43 | 32.42 | 33.01 | 31.14 | 0.51 | 0.447 |
| Hb (g/dL) | 14.43 ab | 15.34 a | 14.15 b | 14.45 ab | 13.38 b | 0.19 | 0.009 |
| RBCs (106/µL) | 12.62 | 12.44 | 12.72 | 13.25 | 11.61 | 0.19 | 0.117 |
| MCV (fL) | 24.31 | 25.43 | 25.58 | 25.12 | 24.53 | 0.32 | 0.529 |
| MCH (pg) | 11.43 | 11.15 | 11.37 | 11.40 | 11.22 | 0.07 | 0.682 |
| MCHC (g/dL) | 47.72 | 44.77 | 44.75 | 45.72 | 45.33 | 0.56 | 0.456 |
| WBCs (103/µL) | 12.92 | 13.37 | 15.74 | 14.66 | 14.68 | 0.36 | 0.990 |
| NEU (%) | 61.32 | 73.25 | 63.38 | 69.05 | 66.02 | 1.75 | 0.218 |
| LYPH (%) | 30.21 | 21.91 | 27.43 | 24.72 | 27.42 | 1.37 | 0.414 |
| MONO (%) | 4.71 | 2.35 | 3.47 | 3.45 | 3.23 | 0.32 | 0.249 |
| ESOIN (%) | 2.78 | 2.83 | 3.28 | 1.88 | 2.13 | 0.19 | 0.146 |
| BASO (%) | 1.03 | 0.83 | 0.87 | 0.88 | 0.72 | 0.50 | 0.410 |
| Items | Diets | Pooled SEM | p Value | ||||
| MF0 | MF25 | MF50 | MF75 | MF100 | |||
| Glucose (mg/dL) | 40.93 b | 42.21 b | 43.32 b | 48.67 a | 50.24 a | 0.971 | <0.001 |
| Bulirubin (µmol/L) | 22.80 | 24.23 | 25.02 | 25.04 | 24.91 | 0.374 | 0.288 |
| Crea (mg/dL) | 0.69 a | 0.66 a | 0.59 b | 0.597 b | 0.6 b | 0.010 | <0.001 |
| TP (g/dL) | 6.25 b | 6.50 b | 7.06 a | 6.96 a | 7.01 a | 0.072 | <0.001 |
| ALB (g/dL) | 3.45 c | 3.483 c | 3.6b c | 3.8 a | 3.7 ab | 0.041 | <0.001 |
| BUN (mg/dL) | 54.95 a | 53.92 a | 49.86 b | 49.85 b | 50.33 b | 0.484 | <0.001 |
| LDL (mg/dL) | 487.66 a | 397.54 b | 464.21 a | 360.16 b | 387.83 b | 10.48 | <0.001 |
| CK (IU/L) | 295.21 | 318.83 | 351.72 | 360.01 | 337.30 | 12.97 | 0.545 |
| ALT (IU/L) | 14.21 ab | 16.05 a | 13.23 b | 14.84 ab | 16.11 a | 0.351 | 0.032 |
| AST (IU/L) | 89.56 | 82.63 | 79.76 | 79.73 | 73.88 | 1.97 | 0.149 |
| ALP (IU/L) | 206.66 a | 196.83 a | 158.16 b | 134.16 b | 151.66 b | 6.93 | <0.001 |
| GGT (IU/L) | 73.66 | 74.16 | 68.00 | 77.83 | 73.83 | 1.927 | 0.642 |
| Items µg/dL | Diets | Pooled SEM | p-Value | ||||
| MF0 | MF25 | MF50 | MF75 | MF100 | |||
| Ca | 10.68 | 11.05 | 10.73 | 11.02 | 10.85 | 0.085 | 0.24 |
| P | 8.45 a | 8.51 a | 8.73 a | 7.43 b | 7.11 b | 0.19 | 0.01 |
| Na | 149.32 | 151.53 | 153.21 | 150.83 | 149.3 | 0.50 | 0.09 |
| Fe | 145.23 | 143.41 | 142.51 | 153.38 | 154.5 | 4.53 | 0.88 |
| K | 5.87 | 5.67 | 5.85 | 5.65 | 5.34 | 0.067 | 0.08 |
| Cu | 83.51 | 85.66 | 78.02 | 77.53 | 78.33 | 2.05 | 0.65 |
| Zn | 68.67 ab | 66.83 ab | 70.50 a | 58.33 bc | 53.33 c | 2.01 | 0.02 |
| Cl | 106.01 b | 107.26 ab | 108.7 a | 105.43 b | 105.33 b | 0.42 | 0.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaher, H.A.; Alawaash, S.A.; Tolba, A.M.; Swelum, A.A.; Abd El-Hack, M.E.; Taha, A.E.; Abdelnour, S.A. Impacts of Moringa oleifera Foliage Substituted for Concentrate Feed on Growth, Nutrient Digestibility, Hematological Attributes, and Blood Minerals of Growing Goats under Abu Dhabi Conditions. Sustainability 2020, 12, 6096. https://doi.org/10.3390/su12156096
Zaher HA, Alawaash SA, Tolba AM, Swelum AA, Abd El-Hack ME, Taha AE, Abdelnour SA. Impacts of Moringa oleifera Foliage Substituted for Concentrate Feed on Growth, Nutrient Digestibility, Hematological Attributes, and Blood Minerals of Growing Goats under Abu Dhabi Conditions. Sustainability. 2020; 12(15):6096. https://doi.org/10.3390/su12156096
Chicago/Turabian StyleZaher, Hany A., Saeed A. Alawaash, Amir M. Tolba, Ayman A. Swelum, Mohamed E. Abd El-Hack, Ayman E. Taha, and Sameh A. Abdelnour. 2020. "Impacts of Moringa oleifera Foliage Substituted for Concentrate Feed on Growth, Nutrient Digestibility, Hematological Attributes, and Blood Minerals of Growing Goats under Abu Dhabi Conditions" Sustainability 12, no. 15: 6096. https://doi.org/10.3390/su12156096
APA StyleZaher, H. A., Alawaash, S. A., Tolba, A. M., Swelum, A. A., Abd El-Hack, M. E., Taha, A. E., & Abdelnour, S. A. (2020). Impacts of Moringa oleifera Foliage Substituted for Concentrate Feed on Growth, Nutrient Digestibility, Hematological Attributes, and Blood Minerals of Growing Goats under Abu Dhabi Conditions. Sustainability, 12(15), 6096. https://doi.org/10.3390/su12156096









