In Vitro Study of Butyric Acid Deodorization Potential by Indigenously Constructed Bacterial Consortia and Pure Cultures from Pit Latrine Fecal Sludge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Cultures
2.2. Chemicals and Media
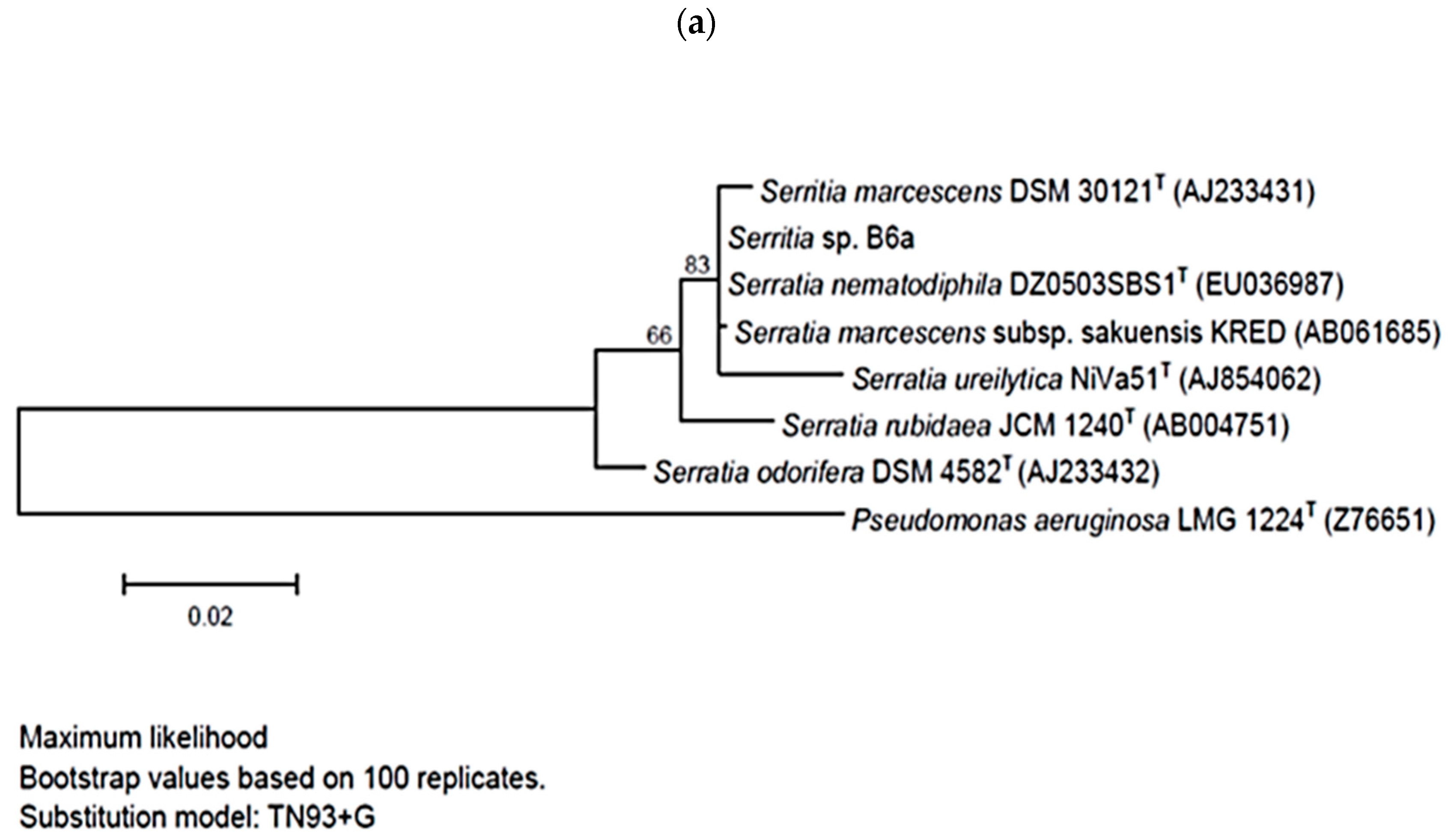
2.3. Phylogenetic Tree Construction
2.4. Bacterial Consortia Development
2.5. Butyric acid Degradation by Pure cultures and Bacterial Consortia
2.6. Effect of Environmental Parameters on Bacterial Growth and Butyric Acid Degradation
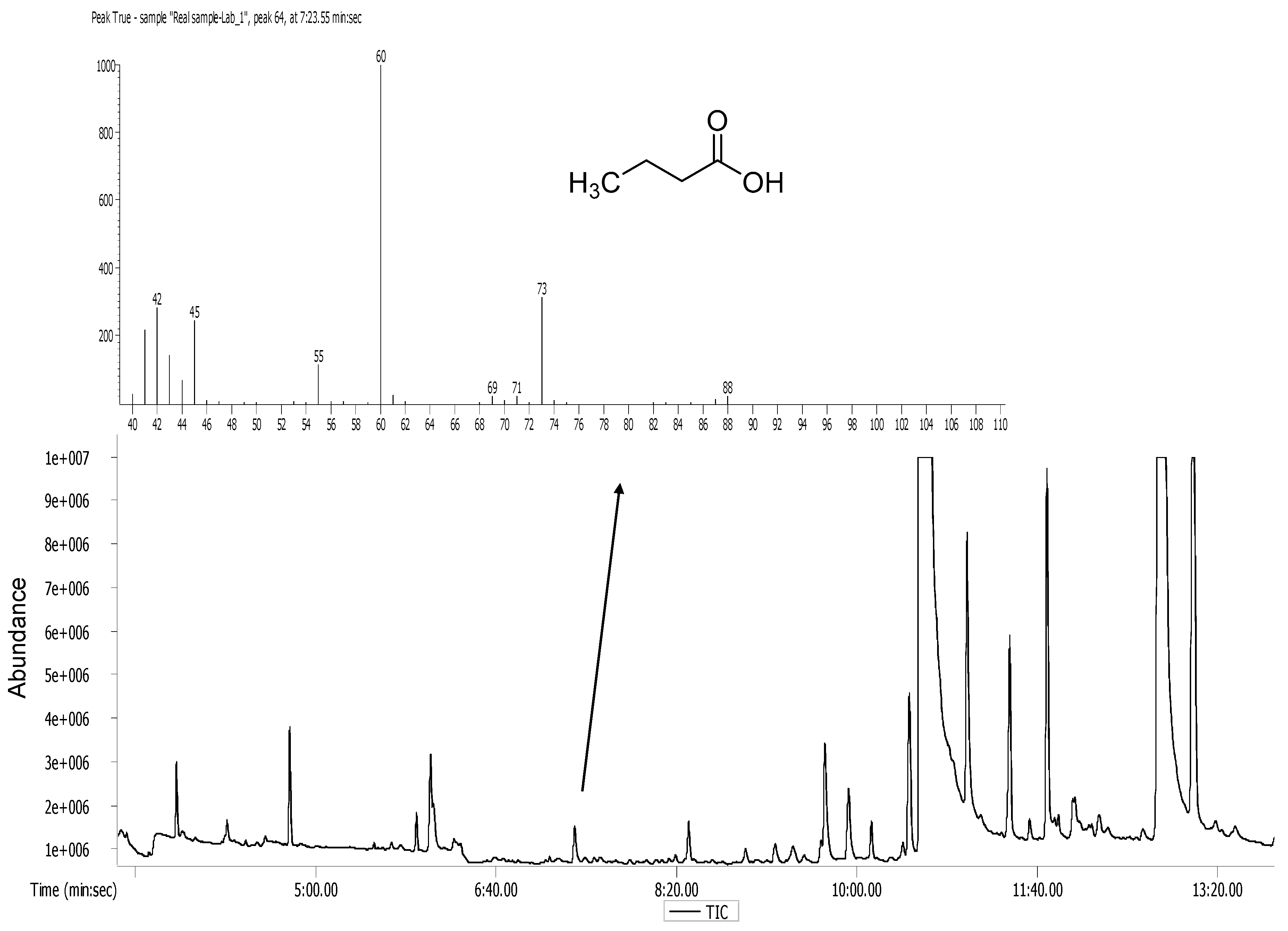
2.7. Analytical Methods
3. Results and Discussion
3.1. Selection of the Bacterial Strains
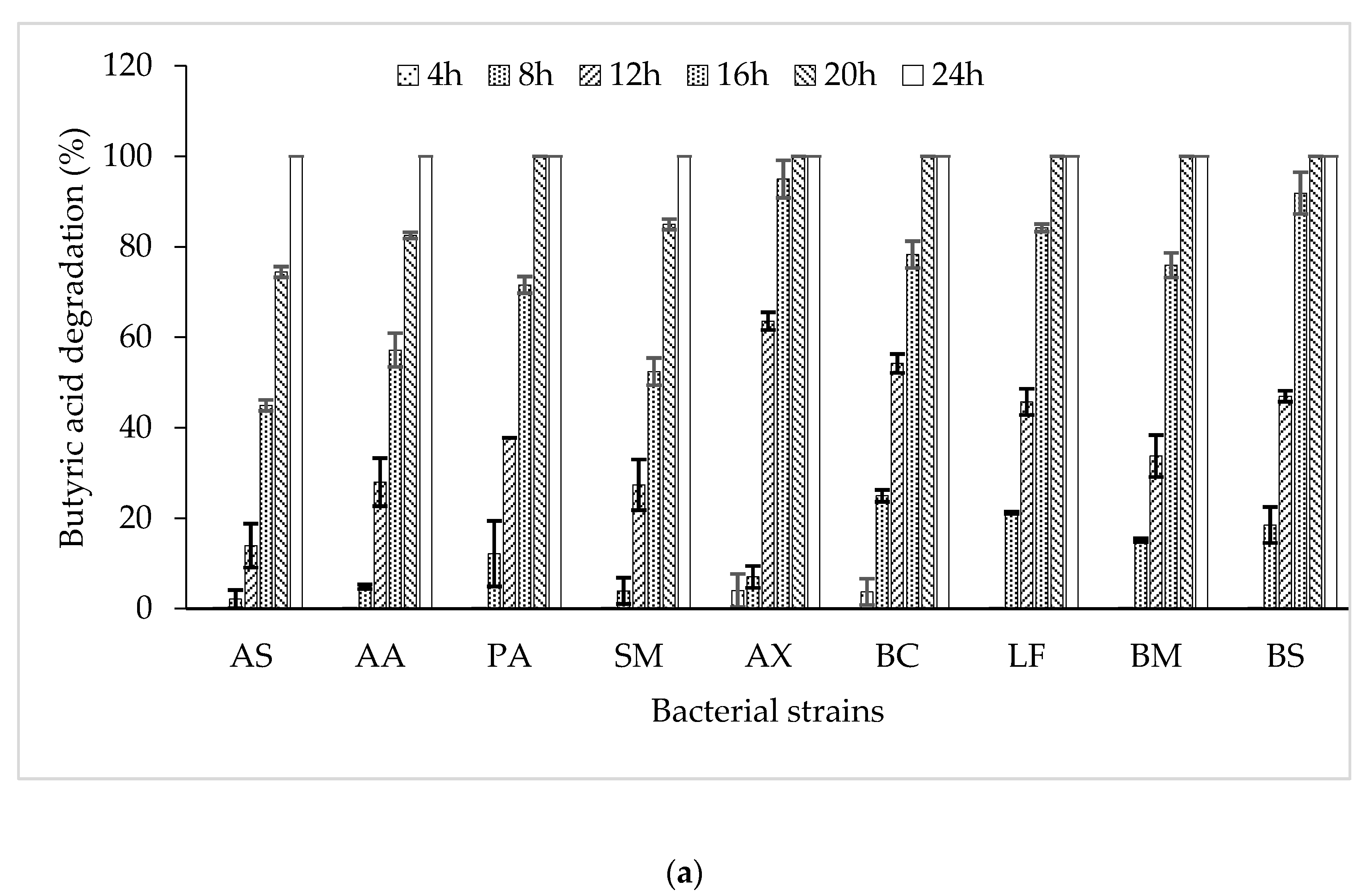
3.2. Butyric Acid Degradation by Pure Bacterial Strains
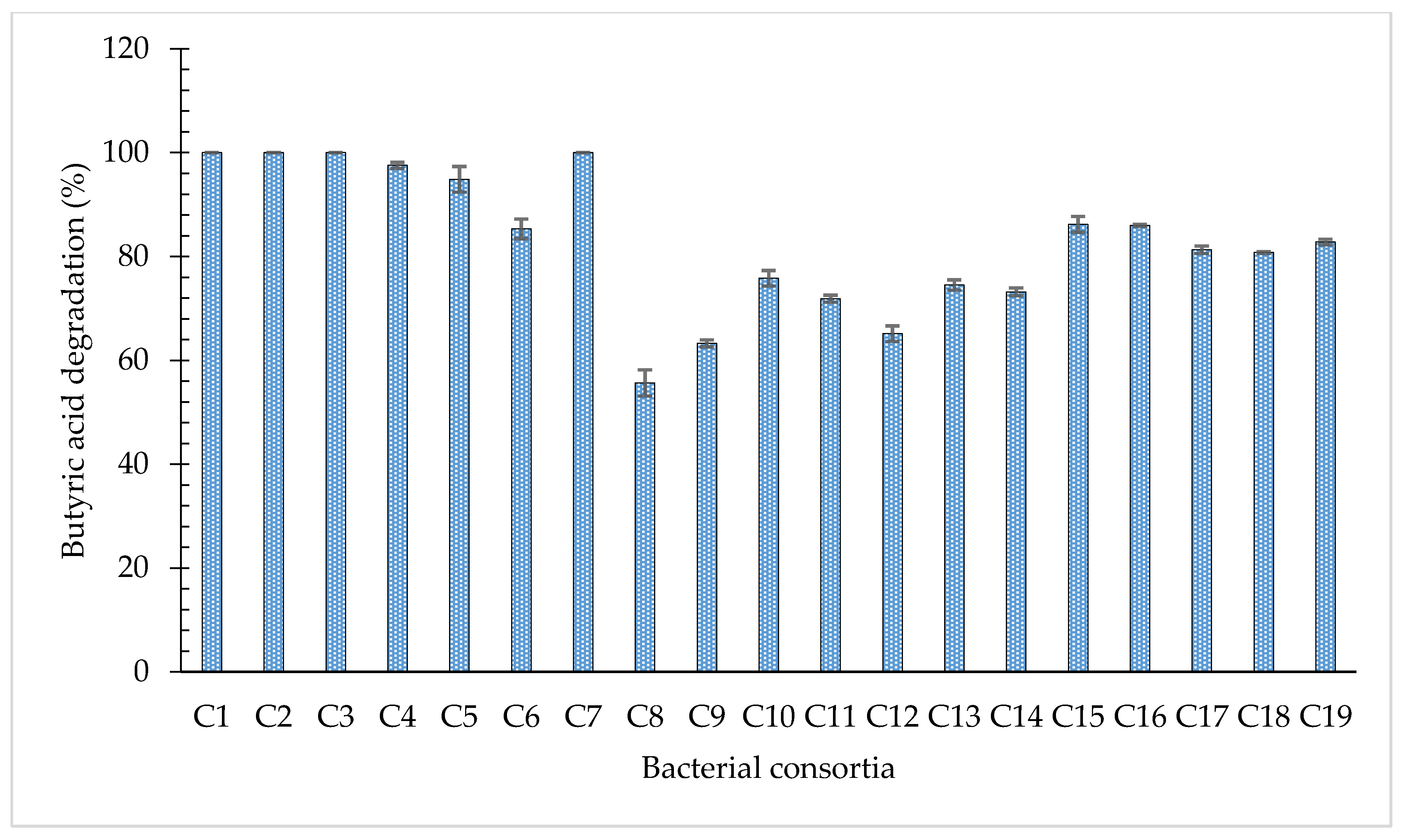
3.3. Degradation of Butyric Acid by Bacterial Consortia
- (i)
- the metabolic and physiological inadequacies of one bacterial strain in the consortium are compensated for by the presence of other bacterial strains in the consortium with the appropriate complementary physiology, which are able to provide the appropriate metabolic benefit to all bacterial strains involved [39];
- (ii)
- associated metabolism, wherein one bacterial strain in the consortium take up the intermediates of the metabolic pathway released during the degradation of butyric acid, which may be toxic and which may hinder the metabolic activities, thus, appearing to protect the other constituents of the bacterial consortium from toxicity that would otherwise accrue from the accumulation of the metabolites [40].
3.4. Environmental Factors Affecting the Growth and Butyric Acid Biodegradation by Bacterial Consortium, C3
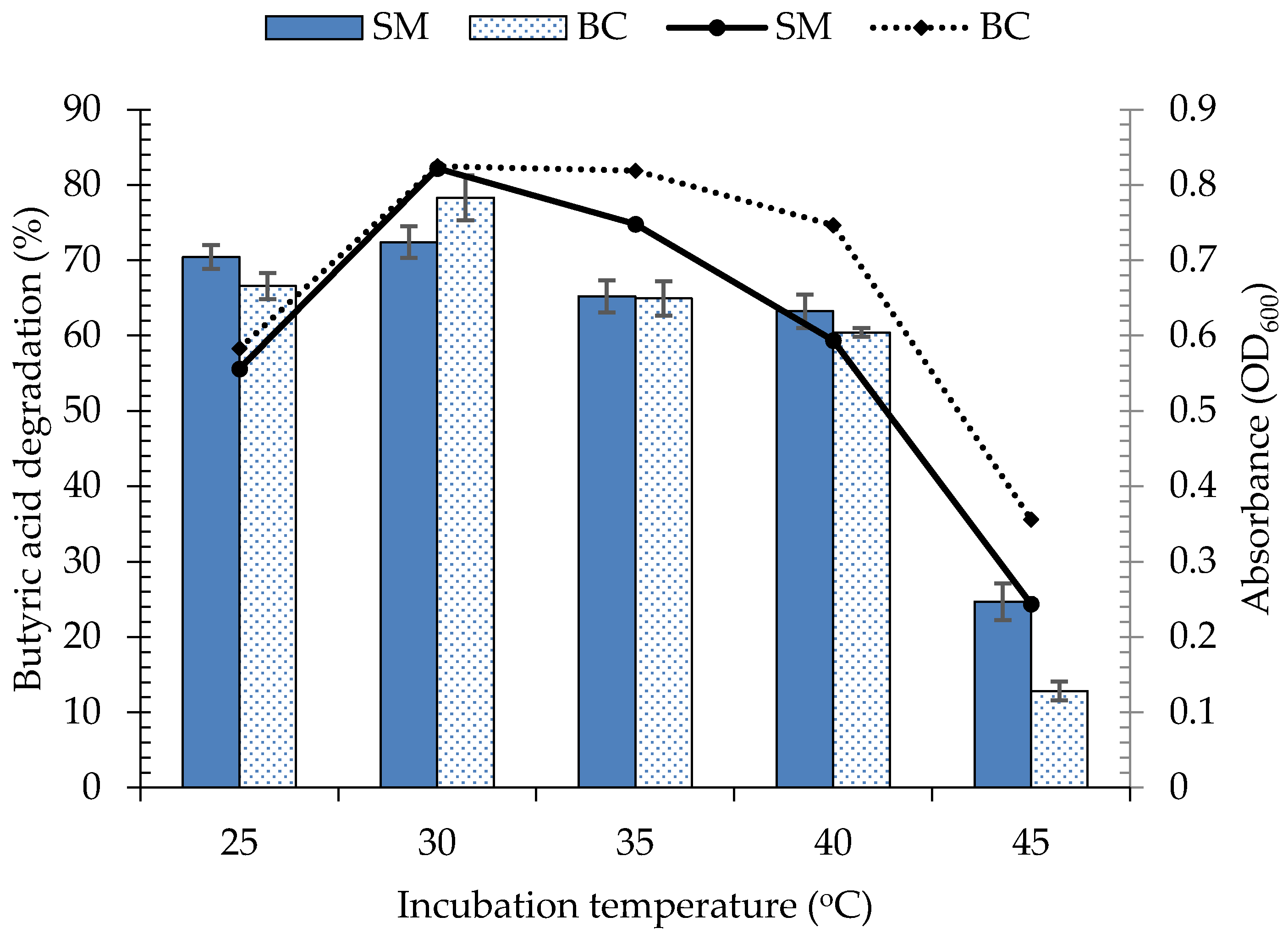
3.4.1. Effect of Incubation Temperature
3.4.2. Effect of Initial pH of the Medium
3.4.3. Effect of Initial Inoculum Size
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO; UNICEF. Progress on drinking water, sanitation and hygiene. 2017 Update and SDG Baselines Geneva. Available online: https://www.unicef.org/publications/index_96611.html (accessed on 14 March 2020).
- Evans, B.; Van Der Voorden, C.; Peal, A. Public Funding for Sanitation-The Many Faces of Sanitation Subsidies; Water Supply & Sanitation Collaborative Council: Geneva, Switzerland, 2009; p. 44. [Google Scholar]
- Bartram, J.; Cairncross, S. Hygiene, sanitationand water: Forgotten foundations of health. PLoS Med. 2010, 7, e1000367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guiteras, R.; Levinsohn, J.; Mobarak, A.M. Encouraging sanitation investment in the developing world: A cluster-randomized trial. Science 2015, 348, 903–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mascarini-Serra, L. Prevention of soil-transmitted helminthic infections. J. Global Infect. Dis. 2011, 3, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Stocks, M.E.; Ogden, S.; Haddad, D.; Addiss, D.G.; McGuire, C.; Freeman, M.C. Effect of water, sanitationand hygiene on the prevention of trachoma: A systematic review and meta-analysis. PLoS Med. 2014, 11, e1001605. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.A.; Bartram, J.; Elimelech, M. Increasing functional sustainability of water and sanitation supplies in rural Sub-Saharan Africa. Environ. Eng. Sci. 2014, 26, 5. [Google Scholar] [CrossRef]
- Thye, Y.P.; Templeton, M.R.; Ali, M. A critical review of technologies for pit latrine emptying in developing countries. Crit. Rev. Environ. Sci. Technol. 2011, 41, 1793–1819. [Google Scholar] [CrossRef]
- Graham, J.P.; Polizzotto, M.L. Pit latrines and their impacts on groundwater quality: A systematic review. Environ. Health Perspect. 2013, 121. [Google Scholar] [CrossRef] [Green Version]
- Rheinländer, T.; Keraita, B.; Konradsen, F.; Samuelsen, H.; Dalsgaard, A. Smell: An overlooked factor in sanitation promotion. Waterlines 2013, 32, 106–112. [Google Scholar] [CrossRef]
- Tsinda, A.; Abbott, P.; Pedley, S.; Charles, K.; Adogo, J.; Okurut, P.; Chenoweth, J. Challenges to achieving sustainable sanitation in informal settlements of Kigali, Rwanda. Int. J. Environ. Res. Public Health 2013, 10, 6939–6954. [Google Scholar] [CrossRef]
- Obeng, P.A.; Keraita, B.; Oduro-Kwarteng, S.; Brenghøj, H.; Abaidoo, R.C.; Awuah, E.; Konradsen, F. Usage and barriers to use of latrines in a Ghanaian peri-urban community. Environ. Process 2015, 2, 261–274. [Google Scholar] [CrossRef] [Green Version]
- Chappuis, C.J.-F.; Niclass, Y.; Cayeux, I.; Starkenmann, C. Sensory survey of key compounds of toilet malodour in Switzerland, India and Africa. Flavour Fragr. J. 2016, 31, 95–100. [Google Scholar] [CrossRef]
- Sheridan, B.A.; Curran, T.P.; Dodd, V.A. Biofiltration of n-butyric acid for the control of odour. Bioresour. Technol. 2003, 89, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Chaggu, E.J. Sustainable Environmental Protection Using Modified Pit Latrines. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2004. [Google Scholar]
- Buckley, C.A.; Foxon, K.M.; Brouckert, C.J.; Rodda, N.; Nwaneri, C.; Balboni, E.; Couderc, A.; Magagna, D. Scientific Support for the Design and Operation of Ventilated Improved Pit Latrines (VIPs) and the Efficacy of Pit Latrine Additives; WRC Report No. TT 357/08; Water Research Commission: Pretoria, South Africa, 2008. [Google Scholar]
- Lin, J.; Aoll, J.; Niclass, Y.; Velazco, M.I.; Wunsche, L.; Pika, J.; Starkenmann, C. Qualitative and quantitative analysis of volatile constituents from latrines. Environ. Sci. Technol. 2013, 47, 7876–7882. [Google Scholar] [CrossRef] [PubMed]
- Mainville, D.M. Biofiltration of Odours: Laboratory Studies Using Butyric Acid. Master’s. Thesis, University of Guelph, Guelph, ON, Canada, 1996. [Google Scholar]
- Brikké, F.; Bredero, M. Linking Technology Choice with Operation and Maintenance in the Context of Community Water Supply and Sanitation: A Reference Document for Planners and Project Staff; WHO: Geneva, Switzerland; IRC Water and Sanitation Centre: The Hague, The Netherlands, 2003. [Google Scholar]
- Chappuis, C.J.-F.; Huber, R.; Niclass, Y.; Starkenmann, C. Simulating latrines conditions to assess perfume performance against malodour. Flavour. Fragr. J. 2018, 33. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.G.; Lantz, S.E.; Blattman, B.O.; Chapman, P.J. Bench-scale evaluation of alternative biological treatment processes for the remediation of pentachlorophenol and creosote-contaminated materials solid phase bioremediation. Environ. Sci. Technol. 1991, 25, 1045–1055. [Google Scholar] [CrossRef]
- Nicolai, R.E.; Janni, K.A. Biofilter media mixture ratio of wood chips and compost treating swine odors. Water Sci. Technol. 2001, 44, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Chandran, P. Microbial degradation of petroleum hydrocarbon contaminants: An overview. Biotechnol. Res. Int. 2011, 2011, 941810. [Google Scholar] [CrossRef] [Green Version]
- Bourque, D.; Bisaillon, J.-G.; Beaudet, R.; Sylvestre, M.; Ishaque, M.; Morin, A. Microbiological degradation of malodorous substances of swine waste under aerobic conditions. Appl. Environ. Microbiol. 1997, 53, 137–141. [Google Scholar] [CrossRef] [Green Version]
- Yun, S.-I.; Ohta, Y. Some physiological properties of microorganisms capable of deodorizing farm animal feces. Bioresour. Technol. 1997, 60, 21–26. [Google Scholar] [CrossRef]
- Chin, S.; Ishmael, N.S.; Abudullar, A.A.; Yahya, A.R.M. Aerobic degradation of volatile fatty acids by bacterial strain isolated from rivers and cow farm in Malaysia. J. Bioremediat. Biodegrad. 2010, 1, 100–111. [Google Scholar] [CrossRef]
- Njalam’mano, J.B.J.; Chirwa, E.M.N. Indigenous butyric acid-degrading bacteria as surrogate pit latrine odour control: Isolation, biodegradability performance and growth kinetics. Ann. Microbiol. 2019, 69, 107–122. [Google Scholar] [CrossRef]
- Mukherjee, A.K.; Bordoloi, N.K. Bioremediation and reclamation of soil contaminated with petroleum oil hydrocarbons by exogenously seeded bacterial consortium: A pilot-scale study. Environ. Sci. Pollut. Res. 2011, 18, 471–478. [Google Scholar] [CrossRef]
- Roslev, P.; Madsen, P.L.; Thyme, J.B.; Henriksen, K. Degradation of phthalate and di-(2-ethylhexyl) phthalate by indigenous and inoculated microorganisms in sludge-amended soil. Appl. Environ. Microbiol. 1998, 64, 4711–4719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, K.; Strecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katdare, V.V.; Patil, Y.A. Quantitative Techniques, 2nd ed.; Prashant Publications: Jalgaon, India, 2014. [Google Scholar]
- Rao, S.M.; Malini, R.; Priscilla, A.; Lydia, A. Estimate of N2O release from pit-toilets. Environ. Earth Sci. 2015, 74, 2157. [Google Scholar] [CrossRef]
- Déportes, I.; Benoit-Guyod, J.L.; Zmirou, D.; Bouvier, M.C. Microbial disinfection of municipal solid wastes (MSW) composting. J. Appl. Microbiol. 1998, 85, 238–245. [Google Scholar] [CrossRef]
- Carrington, E.G. Evaluation of Sludge Treatments for Pathogen Reduction. European Commission. DG Environment Final Report; Office for official publications of the European Communities: Luxembourg, 2001; p. 44. [Google Scholar]
- Wu, M.; Chen, L.; Tian, Y.; Dick, W.A. Degradation of polycyclic aromatic hydrocarbons by microbial consortia enriched from three soils using two different culture media. Environ. Pollut. 2013, 178, 152–158. [Google Scholar] [CrossRef]
- Sarkar, P.; Rai, A.R.; Ghosh, S. Degradation of aromatic petroleum hydrocarbons (BTEX) by a solvent tolerant bacterial consortium. J. Urban Environ. Eng. 2013, 7, 274–279. [Google Scholar] [CrossRef] [Green Version]
- Brenner, K.; You, L.; Arnold, F.H. Engineering microbial consortia: A new frontier in synthetic biology. Trends Biotechnol. 2008, 26, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.-J.; Wang, S.Y. Synergistic growth in bacteria depends on substrate complexity. J. Microbiol. 2016, 54, 23–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dejonghe, W.; Berterloot, E.; Goris, J.; Boon, N.; Crul, K.; Höfte, M.; De Vos, P.; Verstraete, W.; Top, E.M. Synergistic degradation of linuron by a bacterial consortium and isolation of a single linuron-degrading Variovorax strain. Appl. Environ. Microbiol. 2003, 69, 1532–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghazali, F.M.; Rahman, R.N.Z.A.; Salleh, A.B.; Basri, M. Biodegradation of hydrocarbons in soil by microbial consortium. Int. Biodeter. Biodegr. 2004, 54, 61–67. [Google Scholar] [CrossRef]
- Isaac, P.; Martínez, F.L.; Bourguignon, N.; Sánchez, L.A.; Ferrero, M.A. Improve PAHs removal performance by a defined bacterial consortium indigenous Pseudomonas and Actinobacteria from Patagonia, Argentina. Int. Biodeter. Biodegr. 2015, 101, 21–31. [Google Scholar] [CrossRef]
- Sathishkumar, M.; Binupriya, A.R.; Balk, S.H.; Yun, S.E. Biodegradation of crude oil by individual bacterial strains and a mixed bacterial consortium isolated from hydrocarbon-contaminated areas. Clean—Soil Air Water 2008, 36, 92–96. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Chang, J.S.; Govindwar, S.P. Decolourization and biodegradation of reactive dyes and dye wastewater by a developed bacterial consortium. Biodegradation 2010, 2, 999–1015. [Google Scholar] [CrossRef]
- Tzintzun-Camacho, O.; Loera, O.; Ramírez-Saad, H.C.; Gutiérrez-Rojas, M. Comparison of mechanisms of hexadecane uptake among pure and mixed cultures derived from a bacterial consortium. Int. Biodeter. Biodegr. 2012, 70, 1–7. [Google Scholar] [CrossRef]
- Kristiansen, A.; Lindholst, S.; Feilberg, A.; Neilsen, P.H.; Neufeld, J.D.; Neilsen, J.L. Butyric acid-and dimethyl disulfide-assimilating microorganisms in a biofilter treating air emissions from a livestock facility. Appl. Environ. Microbiol. 2011, 11, 8595–8604. [Google Scholar] [CrossRef] [Green Version]
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, T.B. Bacterial competition: Surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 2010, 8, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Foster, K.R.; Bell, T. Competition, not cooperation, dominates interactions among culturable microbial species. Curr. Biol. 2012, 22, 1845–1850. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Philip, L. Enrichment and isolation of a mixed bacterial culture for complete mineralization of endosulfan. J. Environ. Sci. Health B 2006, 41, 81–96. [Google Scholar] [CrossRef]
- Guo, C.L.; Zhou, H.W.; Wong, Y.S.; Tam, N.F. Isolation of PAH-degrading bacteria from mangrove sediments and their biodegradation potential. Marine Poll. Bull. 2005, 51, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, S.; Imai, K.; Yoshikawa, S.; Kawada, K.; Uchibor, T. Surface activities, biodegradability and antimicrobial properties of n-alkyl glucosides, mannosides and galactosides. J. Am. Oil Chem. Soc. 1989, 67, 996–1001. [Google Scholar] [CrossRef]
- Schulte, P.M. The effects of temperature on aerobic metabolism: Towards a mechanistic understanding of the responses of ectotherms to a changing environment. J. Exp. Biol. 2015, 218, 1856–1866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torondel, B. Sanitation Ventures Literature Review: Onsite Sanitation Waste Characteristics; London School of Hygiene and Tropical Medicine: London, UK, 2010; p. 17. [Google Scholar]
- Kaleli, H.A.; Islam, M.R. Effect of temperature on the growth of wastewater bacteria. Toxicol. Environ. Chem. 1997, 59, 111–123. [Google Scholar] [CrossRef]
- Ghosal, D.; Ghosh, S.; Dutta, T.K.; Ahn, Y. Current state of knowledge in microbial degradation of polycyclic aromatic hydrocarbons (PAHs): A review. Front. Microbial. 2016, 7, 1369. [Google Scholar] [CrossRef] [Green Version]
- Sumitha, D. Histamine and Histamine Producing Microorganisms from Fermented Foods and Fish. Ph.D. Thesis, Department of Food Science, Pondicherry University, Puducherry, India, 2014. [Google Scholar]
- Sherpa, A.M.; Byamukama, D.; Roshan, R.S.; Haberl, R.; March, R.L.; Farnleitner, A.H. Use of feacal pollution indicators to estimate pathogen die off. J. Water Health. 2009, 7, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Nabateesa, S.; Zziwa, A.; Kabenge, I.; Kambugu, R.; Wanyama, T.; Komakech, A.J. Occurrence and survival of pathogens at different sludge depths in unlined pit latrines in Kampala slums. Water SA 2017, 43, 638–645. [Google Scholar] [CrossRef] [Green Version]
- Nwaneri, C.F.; Foxon, K.M.; Bakare, B.F.; Buckley, C.A. Biological degradation processes within a pit latrine. In Proceedings of the WISA 2008 Conference, Sun City, South Africa, 19–22 May 2008. [Google Scholar]
- Rose, C.; Parker, A.; Jefferson, B.; Cartmell, E. The Characterization of feces and urine: A review of the literature to inform advanced treatment technology. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1827–1879. [Google Scholar] [CrossRef] [Green Version]
- Zuma, L.; Velkushanova, K.; Buckley, C. Chemical and thermal properties of VIP latrine sludge. Water SA 2015, 41, 534–540. [Google Scholar] [CrossRef] [Green Version]
- Ratze, C.; Gore, J. Modifying and reacting to the environmental pH can drive bacterial interactions. PLoS Biol. 2018, 16, e2004248. [Google Scholar] [CrossRef] [Green Version]
- Surhio, M.A.; Talpur, F.N.; Nizamani, S.M.; Amin, F.; Bong, C.W.; Lee, C.W.; Ashraf, M.A.; Shah, M.R. Complete degradation of dimethyl phthalate by biochemical cooperation of the Bacillus thuringiensis strain isolated from cotton field soil. RSC Adv. 2014, 4, 55960. [Google Scholar] [CrossRef]
- Jensen, P.D.; Hardin, M.T.; Clarke, W.P. Effect of biomass concentration and inoculum sources on the rate of anaerobic cellulose solubilisation. Bioresour. Technol. 2014, 100, 5219–5225. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, B.R.; Imam, S.H.; Greene, R.V. Accelerated biodegradation of high and low concentrations of p-nitrophenol (PNP) by bacterial inoculation in industrial wastewater: The role of inoculum size on acclimation period. Curr. Microbiol. 1996, 33, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Bidlan, R.; Manonmani, H.K. Aerobic degradation of dichlorodiphenyltrichloroethane (DDT) by S. marcescens DT-1P. Process Biochem. 2002, 38, 49–56. [Google Scholar] [CrossRef]




| Bacterial Strains Group | Computed Bacterial Consortia Using Combinations | Consortium Designation |
|---|---|---|
| Top Two Best Bacterial Strains in Category 1 and Best Bacterial Strain in Category 2 [AX, SM, BC] | [AX, SM] [AX, BC] [SM, BC] | C1 C2 C3 |
| Top Two Best Bacterial Strains in Category 1 and Top Two Best Bacterial Strains in Category 2 [AX, SM, BC, AA] | [AX, SM, BC] [AX, SM, AA] [AX, BC, AA] [SM, BC, AA] | C4 C5 C6 C7 |
| All Three Bacterial Strains in Category 1 and Top Two Best Bacterial Strains in Category 2 [AX, SM, BC, AA, PA] | [AX, SM, BC, AA] [AX, SM, BC, PA] [AX, SM, AA, PA] [AX, BC, AA, PA] [SM, BC, AA, PA] | C8 C9 C10 C11 C12 |
| All Three Bacterial Strains in Category 1 and all Three Bacterial Strains in Category 2 [AX, SM, BC, AA, PA, AS] | [AX, SM, BC, AA, PA] [AX, SM, BC, AA, AS] [AX, SM, BC, PA, AS] [AX, SM, BC, PA, AS] [AX, BC, AA, PA, AS] [SM, BC, AA, PA, AS] | C13 C14 C15 C16 C17 C18 |
| All Six Bacterial strains | [AX, SM, BC, AA, PA, AS] | C19 |
| Degradation Efficiencies of Individual Bacterial Strains (%) | Mean Efficiency (%) | Consortium | Degradation Efficiencies of Consortia (%) | |||||
|---|---|---|---|---|---|---|---|---|
| AX | SM | BC | AA | PA | AS | |||
| 94.97 | 52.41 | - | - | - | - | 73.69 | C1 | 100 |
| 94.97 | - | 78.29 | - | - | - | 86.63 | C2 | 100 |
| - | 52.41 | 78.29 | - | - | - | 65.35 | C3 | 100 |
| 94.97 | 52.41 | 78.29 | - | - | - | 75.22 | C4 | 97.56 |
| 94.97 | 52.41 | - | 57.16 | - | - | 68.18 | C5 | 94.86 |
| 94.97 | - | 78.29 | 57.16 | - | - | 76.81 | C6 | 85.33 |
| - | 52.41 | 78.29 | 57.16 | - | - | 62.62 | C7 | 99.89 |
| 94.97 | 52.41 | 78.29 | 57.16 | - | - | 70.71 | C8 | 55.66 |
| 94.97 | 52.41 | 78.29 | - | 71.57 | - | 74.31 | C9 | 63.28 |
| 94.97 | 52.41 | - | 57.16 | 71.57 | - | 69.03 | C10 | 75.82 |
| 94.97 | - | 78.29 | 57.16 | 71.57 | - | 76.24 | C11 | 71.89 |
| - | 52.41 | 78.29 | 57.16 | 71.57 | - | 64.85 | C12 | 65.15 |
| 94.97 | 52.41 | 78.29 | 57.16 | 71.57 | - | 70.88 | C13 | 74.53 |
| 94.97 | 52.41 | 78.29 | 57.16 | - | 44.94 | 65.55 | C14 | 73.21 |
| 94.97 | 52.41 | 78.29 | - | 71.57 | 44.94 | 68.44 | C15 | 86.21 |
| - | 52.41 | 78.29 | 57.16 | 71.57 | 44.94 | 60.87 | C16 | 86.01 |
| 94.97 | - | 78.29 | 57.16 | 71.57 | 44.94 | 69.39 | C17 | 81.31 |
| 94.97 | 52.41 | - | 57.16 | 71.57 | 44.94 | 64.21 | C18 | 80.79 |
| 94.97 | 52.41 | 78.29 | 57.16 | 71.57 | 44.94 | 67.39 | C19 | 82.82 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Njalam’mano, J.B.J.; Chirwa, E.M.N.; Seabi, R.L. In Vitro Study of Butyric Acid Deodorization Potential by Indigenously Constructed Bacterial Consortia and Pure Cultures from Pit Latrine Fecal Sludge. Sustainability 2020, 12, 5156. https://doi.org/10.3390/su12125156
Njalam’mano JBJ, Chirwa EMN, Seabi RL. In Vitro Study of Butyric Acid Deodorization Potential by Indigenously Constructed Bacterial Consortia and Pure Cultures from Pit Latrine Fecal Sludge. Sustainability. 2020; 12(12):5156. https://doi.org/10.3390/su12125156
Chicago/Turabian StyleNjalam’mano, John Bright Joseph, Evans Martin Nkhalambayausi Chirwa, and Refilwe Lesego Seabi. 2020. "In Vitro Study of Butyric Acid Deodorization Potential by Indigenously Constructed Bacterial Consortia and Pure Cultures from Pit Latrine Fecal Sludge" Sustainability 12, no. 12: 5156. https://doi.org/10.3390/su12125156
APA StyleNjalam’mano, J. B. J., Chirwa, E. M. N., & Seabi, R. L. (2020). In Vitro Study of Butyric Acid Deodorization Potential by Indigenously Constructed Bacterial Consortia and Pure Cultures from Pit Latrine Fecal Sludge. Sustainability, 12(12), 5156. https://doi.org/10.3390/su12125156






