Synergies of Cutting Air Pollutants and CO2 Emissions by the End-of-Pipe Treatment Facilities in a Typical Chinese Integrated Steel Plant
Abstract
:1. Introduction
2. Materials and Methods
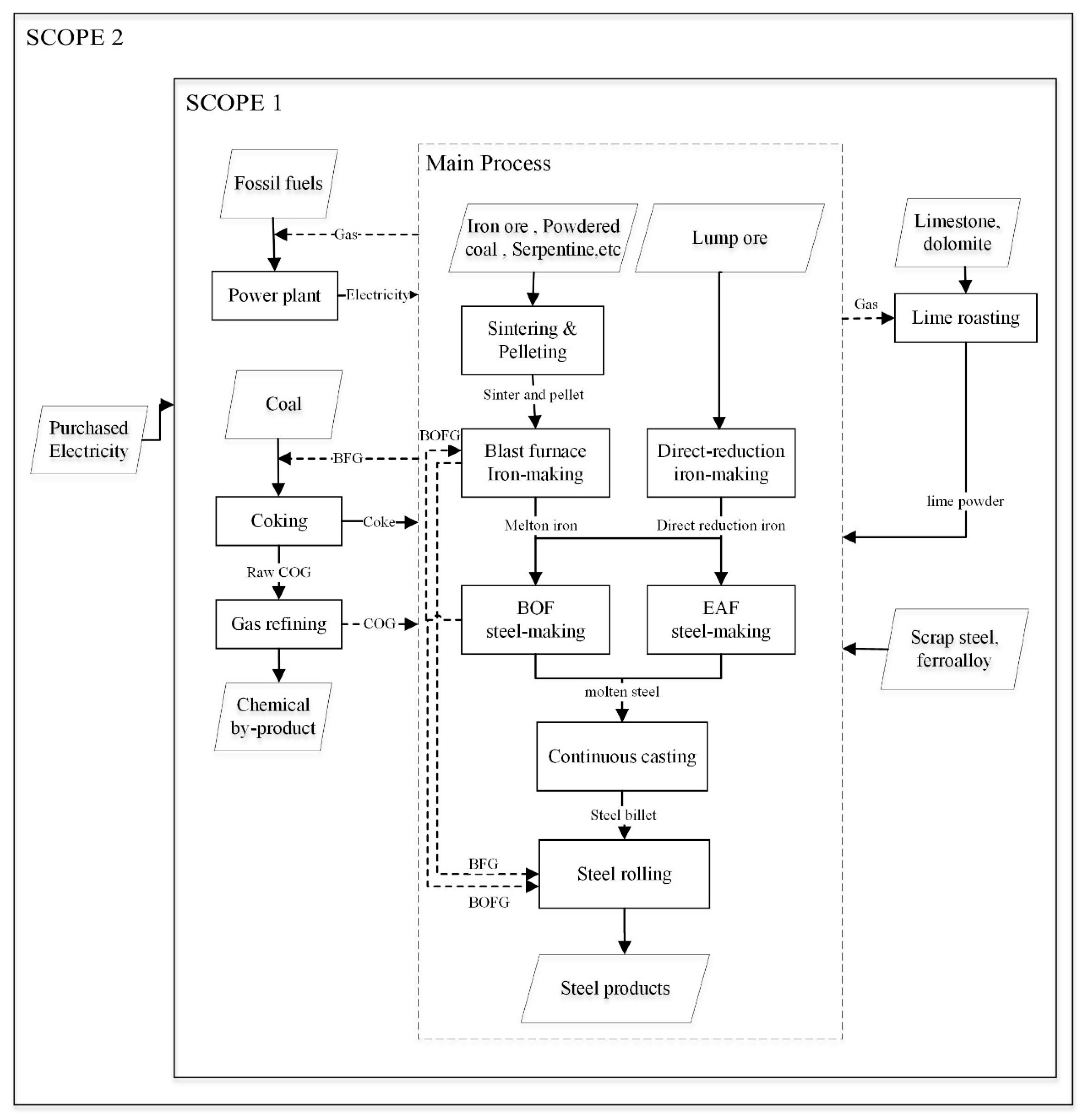
2.1. Research Boundary
2.2. Emissions Calculation
2.2.1. Calculation of Air Pollutant Emissions
2.2.2. Calculation of CO2 Emissions
2.3. Synergies Assessment
2.3.1. Calculation of Synergistic Emissions
2.3.2. Pollutant Reduction Cross-Elasticity
3. Results
3.1. Overview of the Target Plant
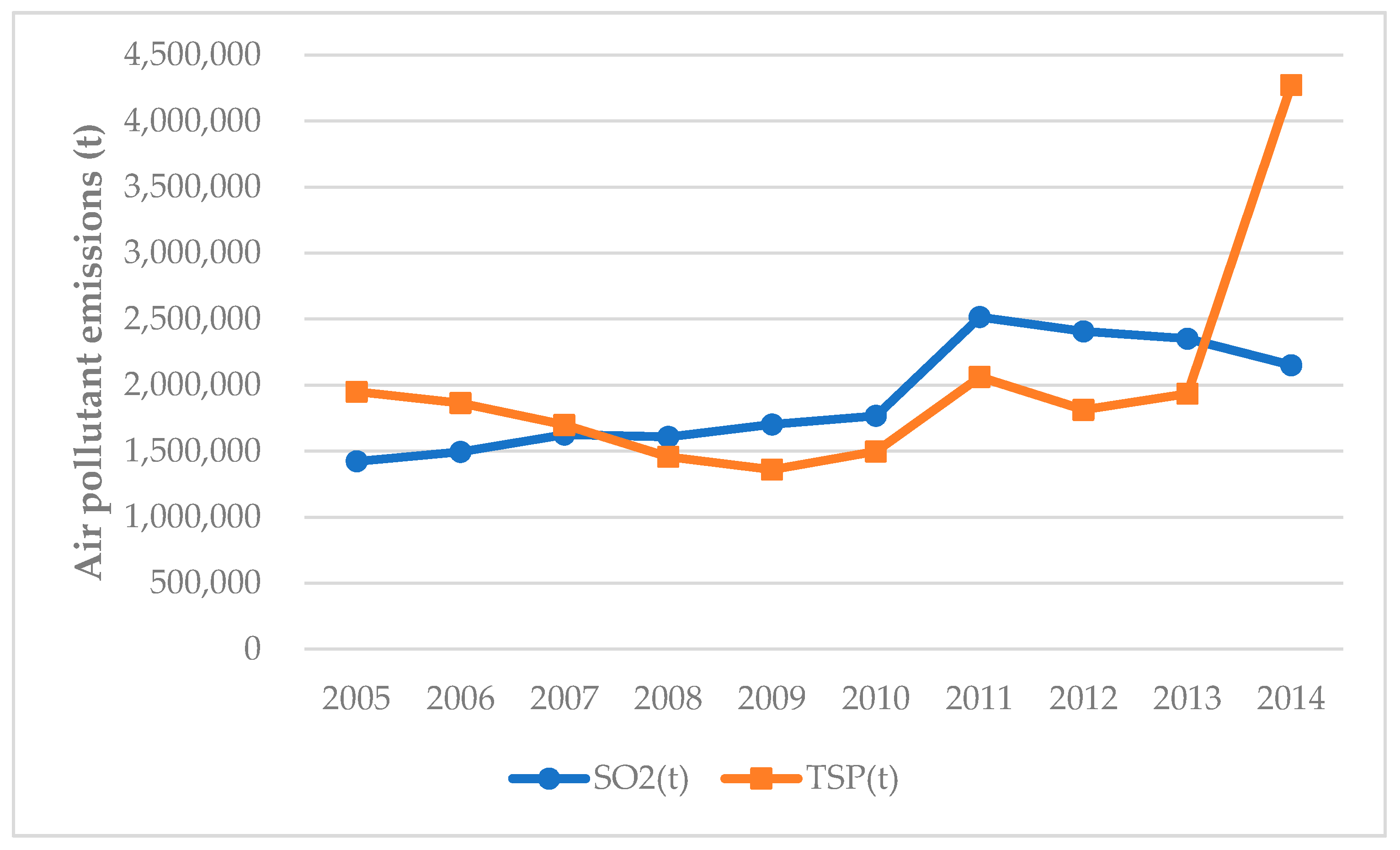
3.2. Production, Reduction, and Discharge of Air Pollutants
3.3. CO2 Emissions Analysis
3.3.1. CO2 Emissions
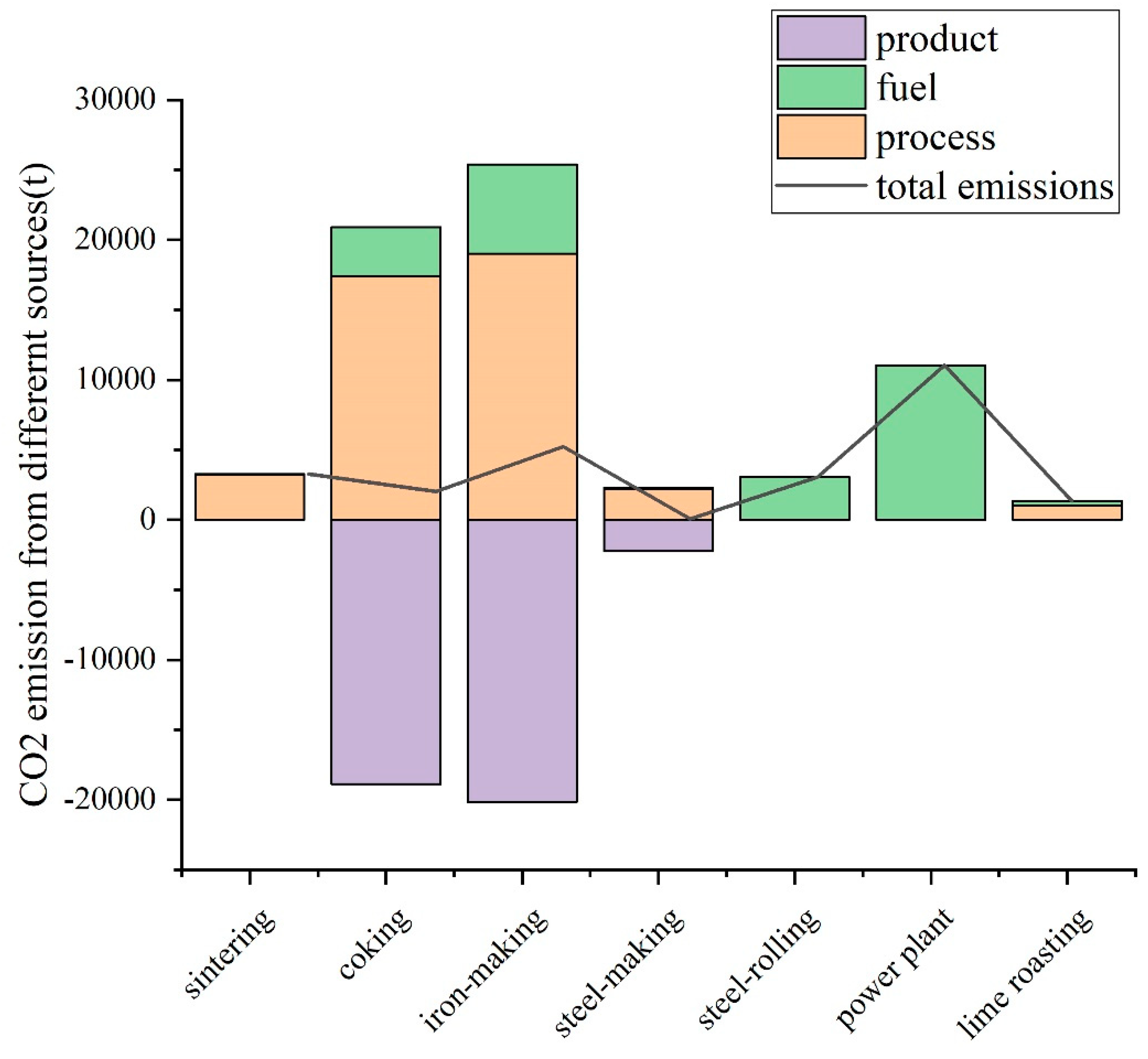
3.3.2. CO2 Emission Sources
3.4. Synergies of End-of-Pipe Treatment Equipment
4. Discussion
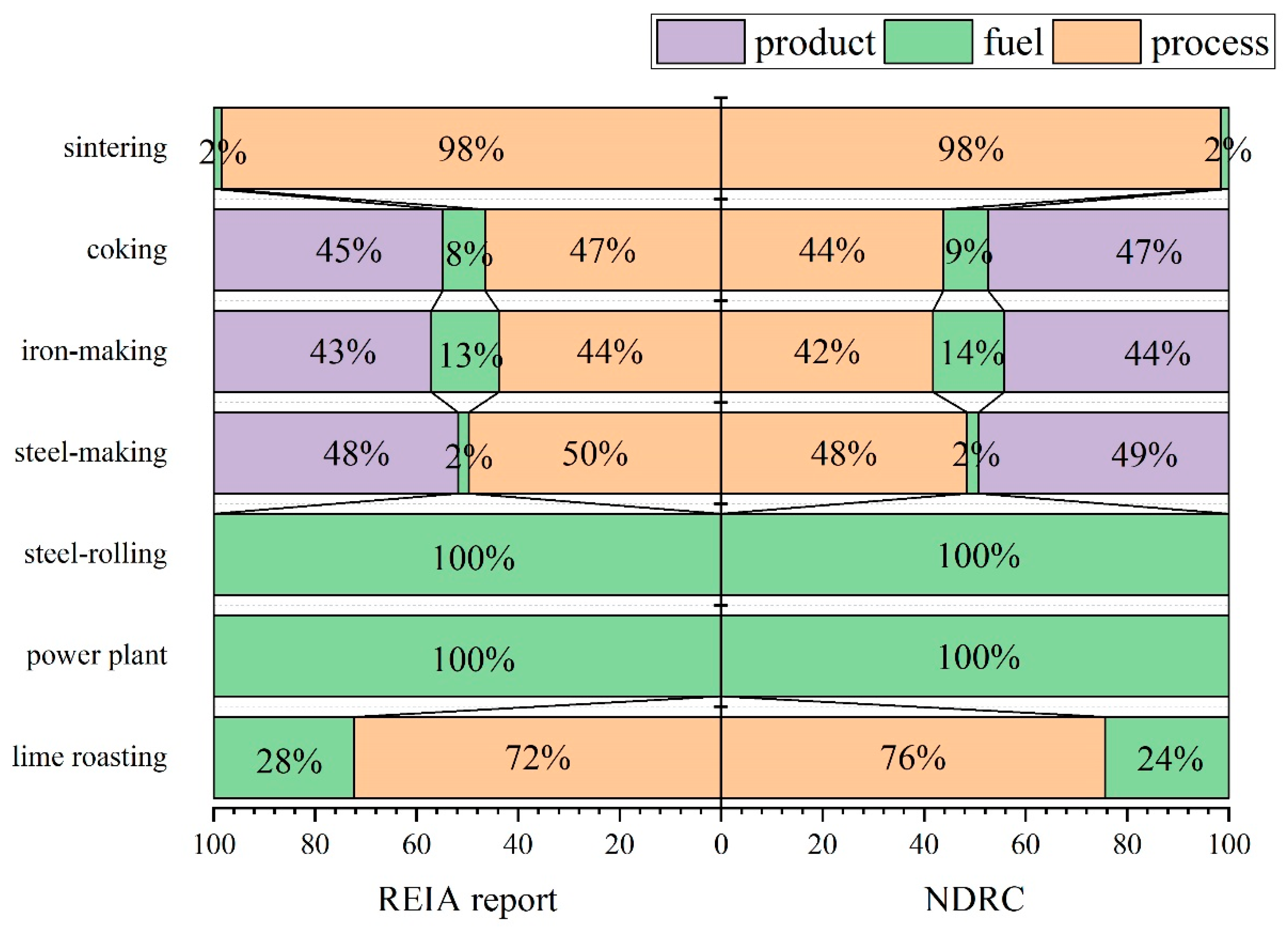
4.1. Applicability of CO2 Emission Calculation Methods
4.2. Power Consumption Coefficient
4.3. Denitration Facilities
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| GHG | greenhouse gas | CE | electric power consumption coefficient |
| TSP | total suspended particulate | Els | cross-elasticity |
| PM | particulate matter | wh | annual working hours |
| ISI | iron and steel industry | a | air pollutant |
| FGD | flue gas desulfurization | i | department |
| ESP | electrostatic precipitator | j | raw material type |
| LTDR | Lurgi-Thyssen dust removal | k | fuel type |
| EF | emission factor | m | main product type |
| LCA | life cycle analysis | n | by-product type |
| BOF | basic oxygen furnace | pg | regional power grid |
| EAF | electric arc furnace | α | carbon content |
| COG | coke oven gas | η | removal rate of pollutant |
| BFG | blast furnace gas | El | the electric power purchased from the power grid |
| BOFG | basic oxygen furnace gas | F | input of fuel |
| P | production | M | input of raw material |
| E | emission | MP | output of main products |
| E’ | emission related to the power consumption of the end-of-pipe treatment facilities | BP | output of by-product |
| R | reduction | CC | carbon content |
| IPCC | Intergovernmental Panel on Climate Change | OF | carbon oxidation rate |
| NDRC | Greenhouse Gas Emission Accounting Method and Reporting Guidelines for Chinese Iron and Steel Enterprises (on trial) | LCV | low calorific value |
| REIA report | Retrospective Environmental Impact Assessment of the Enterprise |
Appendix B
| Department | Sintering | Coking | Iron-Making | Steel-Making (EAF) |
|---|---|---|---|---|
| (t·CO2/t·product) | 0.21 | 0.51 | 1.43 | 0.18 |
| Department | Steel-Making (BOF) | Steel-Rolling | Power Plant | Lime Roasting |
| (t·CO2/t·product) | 0.15 | * | * | 0.75 |
Appendix C
| NDRC | REIA Report | |||||||
|---|---|---|---|---|---|---|---|---|
| Type | LCV (GJ/t or GJ/104 Nm3) | CCCV (t·C/TJ) | OF (%) | Type | LCV (GJ/t or GJ/104 Nm3) | CCCV (t·C/TJ) | CCM (t·C/t) | |
| Nongaseous fuel (t·C/t·fuel) | washed coal | 20.304 | 27.49 | 0.94 | washed coal | / | / | 0.7865 |
| lignite | 14.08 | 28 | 0.96 | steam coal | / | / | 0.6701 | |
| Fuel oil | 41.816 | 21.1 | 0.98 | Fuel oil | 41.816 | 21.1 | / | |
| diesel oil | 42.652 | 20.2 | 0.98 | diesel oil | 42.652 | 20.2 | / | |
| Gaseous fuel (t·C/104 Nm3) | COG | 173.54 | 12.1 | 0.99 | COG | / | / | 2.277 |
| BFG | 33 | 70.8 | 0.99 | BFG | / | / | 2.43 | |
| BOFG | 84 | 49.6 | 0.99 | BOFG | / | / | 4.118 | |
| natural gas | 389.31 | 15.3 | 0.99 | natural gas | 389.31 | 15.3 | / | |
| other gas | 52.27 | 12.2 | 0.99 | city gas | 167.26 | 12.1 | / | |
| producer gas | 167.26 | 12.1 | / | |||||
| NDRC | REIA Report | ||
|---|---|---|---|
| Type | Carbon Content Per Unit Mass (t·C/t) | Type | Carbon Content Per Unit Mass (t·C/t) |
| coke | 0.839 | coke | 0.879 |
| washed coal | 0.669 | washed coal | 0.787 |
| other coal products | 0.587 | soft asphalt | 0.885 |
| crude benzene | 0.949 | crude benzene | 0.922 |
| coal tar | 0.736 | coal tar | 0.924 |
| anthracite | 0.558 | injection coal | 0.788 |
| BF slag | / | BF slag | 0.006 |
| pig-iron | 0.041 | pig-iron | 0.047 |
| crude steel | 0.00248 | crude steel | 0.007 |
| scrap | 0.003 | ||
| steel slag | 0.011 | ||
| dolomite | 0.12 | dolomite | 0.13 |
| limestone | 0.128 | limestone | 0.119 |
| EAF carbon electrode | 0.999 | EAF carbon electrode | / |
Appendix D
| Project | Power Proportion (%) | Related CO2 Emissions (×106 t) |
|---|---|---|
| Power plant | 87.01 | 14.15 |
| CDQ | 1.94 | 0.00 |
| TRT | 5.07 | 0.00 |
| Purchased from the power grid | 4.02 | 1.31 |
| Transferred to the power grid | 8.02 | 0.00 |
| Loss | 0.02 | 0.00 |
| Total | 100 | 15.46 |
References
- WSA. Steel Statistical Yearbook 2018; WSA: Brussels, Belgium, 2018. [Google Scholar]
- NBS. China Statistics Yearbook on Environment; China Statistics Press: Beijing, China, 2006. Available online: http://www.stats.gov.cn/ztjc/ztsj/hjtjzl/2006/ (accessed on 21 June 2020).
- MEE. China’s 2018 Annual Report on Policies and Actions to Address Climate Change; MEE: Beijing, China, 2018. [Google Scholar]
- Zeng, S.; Lan, Y.; Huang, J. Mitigation paths for Chinese iron and steel industry to tackle global climate change. Int. J. Greenh. Gas. Control 2009, 3, 675–682. [Google Scholar] [CrossRef]
- Liu, Z.; Mao, X.; Jiang, H. Key Directions and Contents of Ecological Environment Protection During the 14th Five-Year Plan Period. Chin. J. Environ. Manag. 2019, 3, 40–45. [Google Scholar]
- Gao, C.; Gao, W.; Song, K.; Na, H.; Tian, F.; Zhang, S. Spatial and temporal dynamics of air-pollutant emission inventory of steel industry in China: A bottom-up approach. Resour. Conserv. Recycl. 2019, 143, 184–200. [Google Scholar] [CrossRef]
- Zhao, C.L.; Wu, T.; Bo, X.; Su, Y. The status quo of sintering flue gas desulfurization of iron & steel industry and pollutants co-treatment suggestion. Environ. Eng. 2014, 10, 76–78. [Google Scholar]
- Wang, A.; Cai, J.-J.; Li, X.-P.; Wang, D.; Zhou, Q.-A. Affecting Factors and Improving Measures for Converter Gas Recovery. J. Iron Steel Res. Int. 2007, 14, 22–26. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Mitigation of Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Kuramochi, T. Assessment of midterm CO2 emissions reduction potential in the iron and steel industry: A case of Japan. J. Clean. Prod. 2016, 132, 81–97. [Google Scholar] [CrossRef]
- Xuan, Y.; Yue, Q. Scenario analysis on resource and environmental benefits of imported steel scrap for China’s steel industry. Resour. Conserv. Recycl. 2017, 120, 186–198. [Google Scholar] [CrossRef]
- Griffin, P.W.; Hammond, G.P. Industrial energy use and carbon emissions reduction in the iron and steel sector: A UK perspective. Appl. Energy 2019, 249, 109–125. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, L.; Zhang, Y.; Zhao, L.; Zheng, C.; Gao, X.; Cen, K. Cost and potential of energy conservation and collaborative pollutant reduction in the iron and steel industry in China. Appl. Energy 2016, 184, 171–183. [Google Scholar] [CrossRef]
- Mao, X.; Zeng, A.; Hu, T.; Zhou, J.; Xing, Y.; Liu, S. Co-control of Local Air Pollutants and CO2 in the Chinese Iron and Steel Industry. Environ. Sci. Technol. 2013, 47, 12002–12010. [Google Scholar] [CrossRef]
- Gu, A.; Teng, F.; Feng, X. Effects of pollution control measures on carbon emission reduction in China: Evidence from the 11th and 12th Five-Year Plans. Clim. Policy 2016, 18, 198–209. [Google Scholar] [CrossRef]
- Zhang, S.; Worrell, E.; Crijns-Graus, W.; Wagner, F.; Cofala, J. Co-benefits of energy efficiency improvement and air pollution abatement in the Chinese iron and steel industry. Energy 2014, 78, 333–345. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Zhang, W.; Xu, J. Energy and resource conservation and air pollution abatement in China’s iron and steel industry. Resour. Conserv. Recycl. 2019, 147, 67–84. [Google Scholar] [CrossRef]
- Li, H.; Tan, X.; Guo, J.; Zhu, K.; Huang, C. Study on an Implementation Scheme of Synergistic Emission Reduction of CO2 and Air Pollutants in China’s Steel Industry. Sustainabilty 2019, 11, 352. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Xing, J.; Zhou, Y.; Wang, S.; Zhao, B.; Zheng, H.; Zhao, X.; Chang, H.; Jang, C.; Zhu, Y.; et al. Estimation of abatement potentials and costs of air pollution emissions in China. J. Environ. Manag. 2020, 260, 110069. [Google Scholar] [CrossRef]
- Liu, Z.; Guan, D.; Wei, W.; Davis, S.J.; Ciais, P.; Li, X.; Peng, S.; Zhang, Q.; Hubacek, K.; Marland, G.; et al. Reduced carbon emission estimates from fossil fuel combustion and cement production in China. Nature 2015, 524, 335–338. [Google Scholar] [CrossRef] [Green Version]
- Shan, Y.; Liu, J.; Liu, Z.; Xu, X.; Shao, S.; Wang, P.; Guan, D. New provincial CO2 emission inventories in China based on apparent energy consumption data and updated emission factors. Appl. Energy 2016, 184, 742–750. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Wan, B.; Zhu, T.; Shao, M. CO2 emissions from China’s iron and steel industry. J. Clean. Prod. 2016, 139, 1504–1511. [Google Scholar] [CrossRef]
- Na, H.-M.; Gao, C.-K.; Tian, M.-Y.; Qi, Z.-Q.; Ye, Z. MFA-based analysis of CO2 emissions from typical industry in urban—As a case of steel industry. Ecol. Model. 2017, 365, 45–54. [Google Scholar] [CrossRef]
- Jing, R.; Cheng, J.C.; Gan, V.J.; Woon, K.S.; Lo, I.M. Comparison of greenhouse gas emission accounting methods for steel production in China. J. Clean. Prod. 2014, 83, 165–172. [Google Scholar] [CrossRef]
- Li, L.; Lei, Y.; Pan, D. Study of CO2 emissions in China’s iron and steel industry based on economic input–output life cycle assessment. Nat. Hazards 2015, 81, 957–970. [Google Scholar] [CrossRef] [Green Version]
- NBS. China Statistical Yearbook on Environment; China Statistics Press: Beijing, China, 2010. Available online: http://www.stats.gov.cn/ztjc/ztsj/hjtjzl/2010/ (accessed on 21 June 2020).
- IPCC. 2006 IPCC Guidelines for National Greenhouse Gas Inventories; IGES: Hayama, Japan, 2006; Available online: https://www.ipcc-nggip.iges.or.jp/public/2006gl/index.html (accessed on 21 June 2020).
- Gitarskiy, M.; Yu, A.; Izrael Institute of Global Climate and Ecology IPCC. 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories. Fundam. Available online: https://www.ipcc.ch/report/2019-refinement-to-the-2006-ipcc-guidelines-for-national-greenhouse-gas-inventories/ (accessed on 20 June 2020).
- NDRC. Greenhouse Gas Emission Accounting Method and Reporting Guidelines for Chinese Iron and Steel Enterprises (on Trial); NDRC: Beijing, China, 2013. Available online: http://www.gov.cn/zwgk/2013-11/04/content_2520743.htm (accessed on 21 June 2020).
- Bin, J.; Xiong, X. Discussion on Recovery Process and Implementation of Iron, Carbon and Zinc Resources in Blast Furnace Gas and Gas Ash. Iron Steel Scrap China 2006, 5, 30–32. [Google Scholar]
- MEE. The Production and Emission Accounting Coefficient Manual for the Second National Pollution Source Census (Trial Version); MEE: Beijing, China, 2019. [Google Scholar]
- Zhao, H.; Ma, W.; Dong, H.; Jiang, P. Analysis of Co-Effects on Air Pollutants and CO2 Emissions Generated by End-of-Pipe Measures of Pollution Control in China’s Coal-Fired Power Plants. Sustainability 2017, 9, 499. [Google Scholar] [CrossRef] [Green Version]
- Hou, Q. Study on Reforming of Dust Removal in High Ore Bunker in Lian Yuan Steel Sintering Plant. Master’s Thesis, Wuhan University of Technology, Wuhan, China, April 2006. [Google Scholar]
- Liu, W.; Zhang, R.; Guo, Y.; Hu, X.; Hou, F. Tianjin Steel Sintering Flue Gas Desulfurization Treatment Project Practice. J. EMCC 2014, 24, 3. [Google Scholar]
- Wu, Z. The Improvement and Application of Coking Production Systemin Chongqing Steel Group. Master’s Thesis, Chongqing University, Chongqing, China, November 2007. [Google Scholar]
- Gui, X. Design and Realization of Blast furnace Casthouse Bag Dust Removal Control System. Master’s Thesis, Northeastern University, Liaoning, China, June 2013. [Google Scholar]
- Gong, W. Analysis on the selection of new OG method and LT method for converter primary flue gas removal. Iron Steel Technol. 2009, 46–50. [Google Scholar]
- Liu, C. Power saving technology for dust removal system of converter steelmaking plant. World Metal Guide 2017, 5, B13. [Google Scholar]
- Wei, H. Research and Modification of Dust Removal Technology for 80 Tons Electric Furnace in Hangzhou Iron and Steel Co. Master’s Thesis, Jiangxi University of Science and Technology, Ganzhou, China, June 2011. [Google Scholar]
- Feng, Z. Upgrade Transform of Baosteel 2050 Hot Rolling Plant Dedust Automation System. Metall. Equip. 2016, 1, 52–56. [Google Scholar]
- Cheng, C. Practice of dust removal technology in lime production in Jiugang Sintering Plant. Gansu Metall 2004, 26, 68–70; discussion 73. [Google Scholar]
- Jia, X.; Xin, G.; Qian, Y.; Qian, Y. Sectoral co-control of air pollutants: Case of a chlor-alkali/polyvinyl chloride sector in China. J. Clean. Prod. 2016, 112, 1667–1675. [Google Scholar] [CrossRef]
- Zhao, X.; Bai, H.; Hao, J. A review on the optimal scheduling of byproduct gases in steel making industry. Energy Procedia 2017, 142, 2852–2857. [Google Scholar] [CrossRef]
- Maddaloni, A.; Porzio, G.F.; Nastasi, G.; Colla, V.; Branca, T.A. Multi-objective optimization applied to retrofit analysis: A case study for the iron and steel industry. Appl. Therm. Eng. 2015, 91, 638–646. [Google Scholar] [CrossRef]
- Li, X.; Xie, H.; Kou, W.; Wang, W.; Zhang, N. Discussion and case study on the CO2 emission calculation methods in iron and steel industry. J. Saf. Environ. 2016, 16, 320–324. [Google Scholar]
- Feng, C.; Gao, X.; Tang, Y.; Zhang, Y. Comparative life cycle environmental assessment of flue gas desulphurization technologies in China. J. Clean. Prod. 2014, 68, 81–92. [Google Scholar] [CrossRef]
- Xu, G.; Yang, Y.; Wang, N.; Yuan, X.; Li, J.; Song, X. Analysis on Energy Consumption and Optimal Operation of FGD System in Power Plant. In Proceedings of the 2010 Asia-Pacific Power and Energy Engineering Conference, Chengdu, China, 28–31 March 2010. [Google Scholar]
- Sakamoto, Y.; Tonooka, Y. Estimation of CO2 emission for each process in the Japanese steel industry: A process analysis. Int. J. Energy Res. 2000, 24, 625–632. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, X.; Hou, J. Development Report on Desulfurization and Denitration Industry Technology. China Environ. Prot. Ind. 2018, 9, 14–22. [Google Scholar]

| Department | Section | TSP | SO2 | ||
|---|---|---|---|---|---|
| End-of-Pipe Treatment Facilities | Power Consumption Coefficient (kWh/t) | End-of-Pipe Treatment Facilities | Power Consumption Coefficient (kWh/t) | ||
| Sintering | Sintering machine head | electrostatic precipitator(ESP) | 185.00 [33] | Limestone–gypsum wet Flue gas desulfurization (FGD) | 4795.20 [34] |
| Sintering machine tail and others | bag filter | 229.81 [33] | |||
| Coking | − | bag filter | 237.11 [35] | ||
| Iron-making | − | bag filter | 510.74 [36] | ||
| Steel-making | Primary flue Gas in BOF | Lurgi–Thyssen dust removal (LTDR) | 2042.46 [37] | ||
| Ordinary flue gas in BOF | bag filter | 273.41 [38] | |||
| EAF | bag filter | 1973.27 [39] | |||
| Steel-rolling | − | bag filter | 17.43 [40] | ||
| Lime roasting | − | bag filter | 146.16 [41] | ||
| Scope | Department | SO2 | TSP | ||||
|---|---|---|---|---|---|---|---|
| Production (t) | Reduction (t) | Discharge (t) | Production (t) | Reduction (t) | Discharge(t) | ||
| 1 | sintering | 10,732.76 | 9659.48 | 1073.28 | 247,334.85 | 246,827.89 | 506.97 |
| coking | 1161.55 | 0.00 | 1161.55 | 43,325.65 | 42,808.58 | 517.07 | |
| iron-making | 1017.16 | 0.00 | 1017.16 | 200,075.66 | 199,743.24 | 332.42 | |
| steel-making | 92.68 | 0.00 | 92.68 | 155,244.15 | 152,038.00 | 3206.15 | |
| steel-rolling | 1875.39 | 0.00 | 1875.39 | 4723.24 | 4250.12 | 473.12 | |
| power plant | 27,300.05 | 26,262.52 | 1037.52 | 319,682.40 | 318,552.77 | 1129.63 | |
| lime roasting | 106.78 | 0.00 | 106.78 | 1363.14 | 1349.51 | 13.63 | |
| subtotal | 42,286.37 | 35,922.00 | 6364.36 | 971,749.09 | 965,570.11 | 6178.99 | |
| 2 | purchased power | 4.64 | 0.00 | 4.64 | 1.11 | 0.00 | 1.11 |
| total | 42,291.01 | 35,922.00 | 6369.00 | 971,750.20 | 965,570.11 | 6180.10 | |
| Scope | Department | EF Method | Carbon Balance Method | |
|---|---|---|---|---|
| IPCC | NDRC | REIA Report | ||
| Scope 1 | sintering | 3700.51 | 3273.99 | 3463.07 |
| coking | 2664.71 | 2021.56 | 4305.37 | |
| iron-making | 20,779.16 | 5222.37 | 7125.31 | |
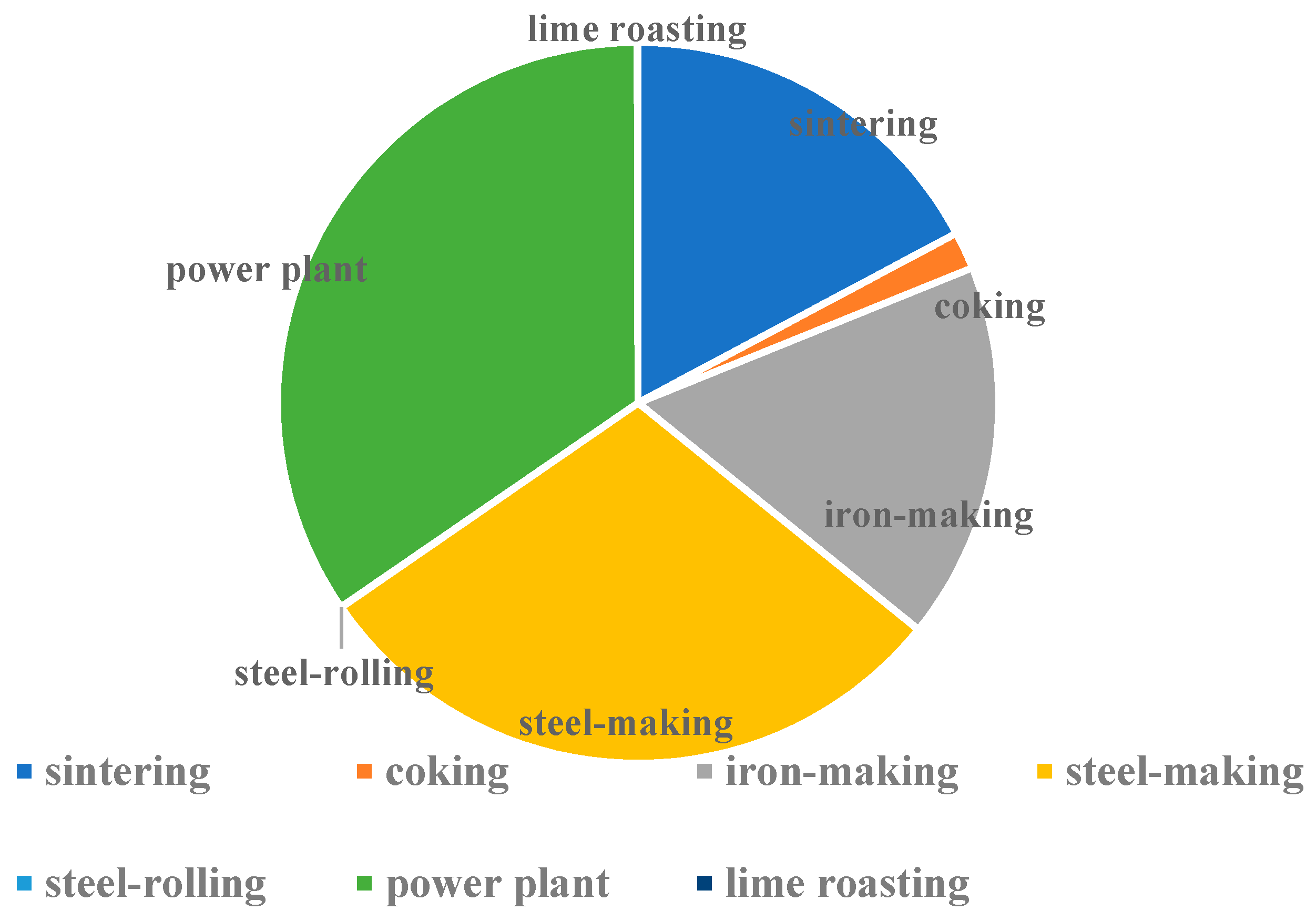
| steel-making | 2385.05 | 64.65 | 195.09 | |
| steel-rolling | 3053.71 | 3061.02 | 3226.65 | |
| power plant | 10,432.01 | 11,055.10 | 14,146.39 | |
| lime roasting | 943.24 | 1342.35 | 1399.49 | |
| subtotal | 43,958.38 | 26,041.04 | 33,861.38 | |
| Scope 2 | purchased power | 1308.52 | 1308.52 | 1308.52 |
| Total | 45,266.90 | 27,349.56 | 35,169.90 | |
| Department | Dust Removal | Desulfurization | ||
|---|---|---|---|---|
| Power Consumption (MWh) | Proportion of Power Consumption (%) | Power Consumption (MWh) | Proportion of Power Consumption (%) | |
| Sintering | 52,183.33 | 0.49 | 45,301.89 | 0.43 |
| Coking | 10,150.47 | 0.10 | / | / |
| Iron-making | 102,016.90 | 0.96 | / | / |
| Steel-making | 178,076.80 | 1.68 | / | / |
| Steel-rolling | 74.08 | 0.00 | / | / |
| Power plant | 41,400.00 | 0.39 | 150,058.25 | 1.42 |
| Lime roasting | 197.24 | 0.00 | / | / |
| Total | 384,098.82 | 3.63 | 195,360.14 | 1.85 |
| Local Air Pollutants | Department | End-of-Pipe Treatment Equipment | Reduction (kt/a) | Related Indirect CO2 Emission (kt/a) | Proportion of Related CO2 Emission (%) |
|---|---|---|---|---|---|
| TSP | Sintering | ESP + bag filter | 246.83 | 56.46 | 0.21 |
| Coking | bag filter | 42.81 | 10.98 | 0.04 | |
| Iron-making | bag filter | 199.74 | 110.39 | 0.40 | |
| Steel-making | LT process + bag filter | 152.04 | 192.69 | 0.70 | |
| Steel-rolling | bag filter | 4.25 | 0.08 | 0.00 | |
| Power plant | ESP | 318.45 | 44.80 | 0.16 | |
| Lime roasting | bag filter | 1.35 | 0.21 | 0.001 | |
| Subtotal | / | 965.46 | 415.61 | 1.52 | |
| SO2 | Sintering | limestone–gypsum wet FGD | 9.66 | 49.02 | 0.18 |
| Power plant | limestone–gypsum wet FGD | 26.26 | 162.37 | 0.59 | |
| Subtotal | / | 35.92 | 211.39 | 0.77 | |
| Total | / | / | / | 627.00 | 2.29 |
| Target Air Pollutant | Measure | Pollutant Reduction (t) | Related CO2 Emissions (t) | Els |
|---|---|---|---|---|
| TSP | ESP | 419,900.14 | 65,083.81 | −5.51 |
| Bag filter | 482,118.35 | 210,075.18 | −15.48 | |
| LTDR | 63,551.60 | 140,450.63 | −78.52 | |
| SO2 | Limestone–gypsum wet FGD | 35,922.00 | 237,188.83 | −10.21 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, H.; Jiang, P.; He, J.; Ma, W. Synergies of Cutting Air Pollutants and CO2 Emissions by the End-of-Pipe Treatment Facilities in a Typical Chinese Integrated Steel Plant. Sustainability 2020, 12, 5157. https://doi.org/10.3390/su12125157
Tang H, Jiang P, He J, Ma W. Synergies of Cutting Air Pollutants and CO2 Emissions by the End-of-Pipe Treatment Facilities in a Typical Chinese Integrated Steel Plant. Sustainability. 2020; 12(12):5157. https://doi.org/10.3390/su12125157
Chicago/Turabian StyleTang, Haoyue, Ping Jiang, Jia He, and Weichun Ma. 2020. "Synergies of Cutting Air Pollutants and CO2 Emissions by the End-of-Pipe Treatment Facilities in a Typical Chinese Integrated Steel Plant" Sustainability 12, no. 12: 5157. https://doi.org/10.3390/su12125157
APA StyleTang, H., Jiang, P., He, J., & Ma, W. (2020). Synergies of Cutting Air Pollutants and CO2 Emissions by the End-of-Pipe Treatment Facilities in a Typical Chinese Integrated Steel Plant. Sustainability, 12(12), 5157. https://doi.org/10.3390/su12125157




