1. Introduction
Poultry slaughterhouse wastewater originates from meat production processes containing high concentrations of organic substances. The produced wastewater is highly polluted and requires treatment before discharging to the environment [
1]. The quantity of wastewater discharged from a poultry plant operations is estimated to be from 20 to 40 L per bird, with 25 L being a typical value [
2]. Excessive usage of water in a poultry farm leads to the release of huge amounts of wastewater characterized by high concentrations of pollutants [
3,
4]. The wastewater generated from a poultry slaughterhouse contains high organic content (chemical oxygen demand (COD), biochemical oxygen demand (BOD)) due to protein and fats, fibers, pathogens, veterinary pharmaceuticals, total nitrogen (TN), total phosphorus (TP), and total suspended solids (TSS) [
5]. Depending on the desired degree of treatment, poultry wastewater is treated by using physical, chemical, and biological treatment systems. Each treatment technology possesses advantages and disadvantages. The advantage of biological treatment systems (anaerobic and aerobic) is the great adaptability of microorganisms to a wide variety of wastewater composition. However, the biological treatment systems are slow treatment processes requiring a large physical area and generating large amounts of sludge [
6]. Electrochemical processes offer an alternative as they are robust, require little space, are easy to operate, and they are flexible under fluctuating wastewater composition [
7]. However, the variability of electrode materials and design, as well as wastewater characteristics, makes it challenging to predict the behavior of electrochemical wastewater treatment systems [
8].
In recent years, there has been a growing interest in electrochemical (EC) technologies for wastewater treatment [
9,
10]. An electrochemical system consists of at least two electrodes, an anode and a cathode, and an intermediate space filled with electrolyte [
11,
12]. Either a voltage source (electrolysis cell) or an electrical load (galvanic element) is essential for the electrical circuit, which is closed through electrical wires. Furthermore, in most cases, either a membrane or diaphragm (acting as a separator) is used to separate the reactor into an anode and cathode compartment [
7]. The electrodes can be arranged in a monopolar or bipolar mode. Several materials including aluminum, graphite, and iron in a plate or packed form of scraps, such as steel turnings and millings, have been used [
13]. When the system is connected to a power source, the oxidation process occurs in the anode, making it electrochemically corroded, while passivation occurs in the cathode where, during the process, amorphous M(OH)
3, also known as sweep flocs, are formed. The formed amorphous M(OH)
3 is useful for the removal of colloidal particles through a rapid adsorption of soluble organic compounds [
14]. In addition, through polymerization, the formed flocs can be removed by sedimentation and floatation [
15]. Despite the general applicability of the electrochemical technologies for treating wastewater of different kinds, they have also been increasingly used for the treatment of poultry wastewater [
16,
17,
18] under different electrode combinations, such as using aluminum electrodes [
19] as well as iron electrodes [
20]. Moreover, the performance of electrodes in a wastewater treatment system can be highly affected by the choice of electrode materials [
21], combination [
22], as well as the characteristics of the wastewater to be treated. The characteristics of a poultry slaughterhouse wastewater are source-dependent even within the same facility. Understanding the response of wastewater when subjected to a treatment system is a key aspect of designing efficient processes. Although various studies [
23,
24,
25] have shown the general performance of the electrochemical technologies for the treatment of poultry slaughterhouse wastewater, the response of a poultry slaughterhouse wastewater treatment system when subjected to different combinations of electrode materials has not been comprehensively studied. Therefore, a comparison of different electrode combinations for a wastewater treatment system is of great importance before adopting a certain combination. The present study provides a comprehensive comparative analysis in terms of performance for three types of electrode combinations for poultry slaughterhouse wastewater with the help of a water quality index (WQI).
Understanding all alternative wastewater treatment scenarios and the achieved water quality requires technical knowledge, which makes the need for simple and easy ways to present the results attractive and even necessary. The typical manner of presenting water quality reports may be too technical, detailed, and hard to understand, especially for readers who are not from the water quality field. Moreover, this kind of analysis produces some doubtful results; for example, presenting lab analysis results with many parameters and different concentrations with different units cannot be easily understandable by non-experts [
26]. The use of the WQI is one of the handy ways to present information related to water quality with an easy-to-understand approach [
27,
28]. The WQI approach for water quality analysis has been widely used in various studies [
29,
30,
31]. A WQI provides a summary of a technical and large amount of data from water quality analyses by replacing them with simplified terms such as extremely bad, bad, good, and excellent which are easier for non-experts to understand [
32]. In 1965, Horton defined the WQI with a status classification from poor to the ideal, where the range was from 0 to 100 [
33]. The definition was based on eight indicators or parameters of water quality, which were given a weight following their influence on the quality of water. The National Sanitation Foundation (NSF-WQI) of the United States and the Canadian Council of Ministers of the Environment (CCME-WQI) of Canada are members of the organizations that have been working on water quality indices for many years. There have been many research studies since then that have utilized and developed the idea further [
34,
35,
36]. Despite many approaches and methodologies for water quality evaluation, there are still some challenges in the field, such as the selection of the appropriate water quality parameters for a particular requirement [
37].
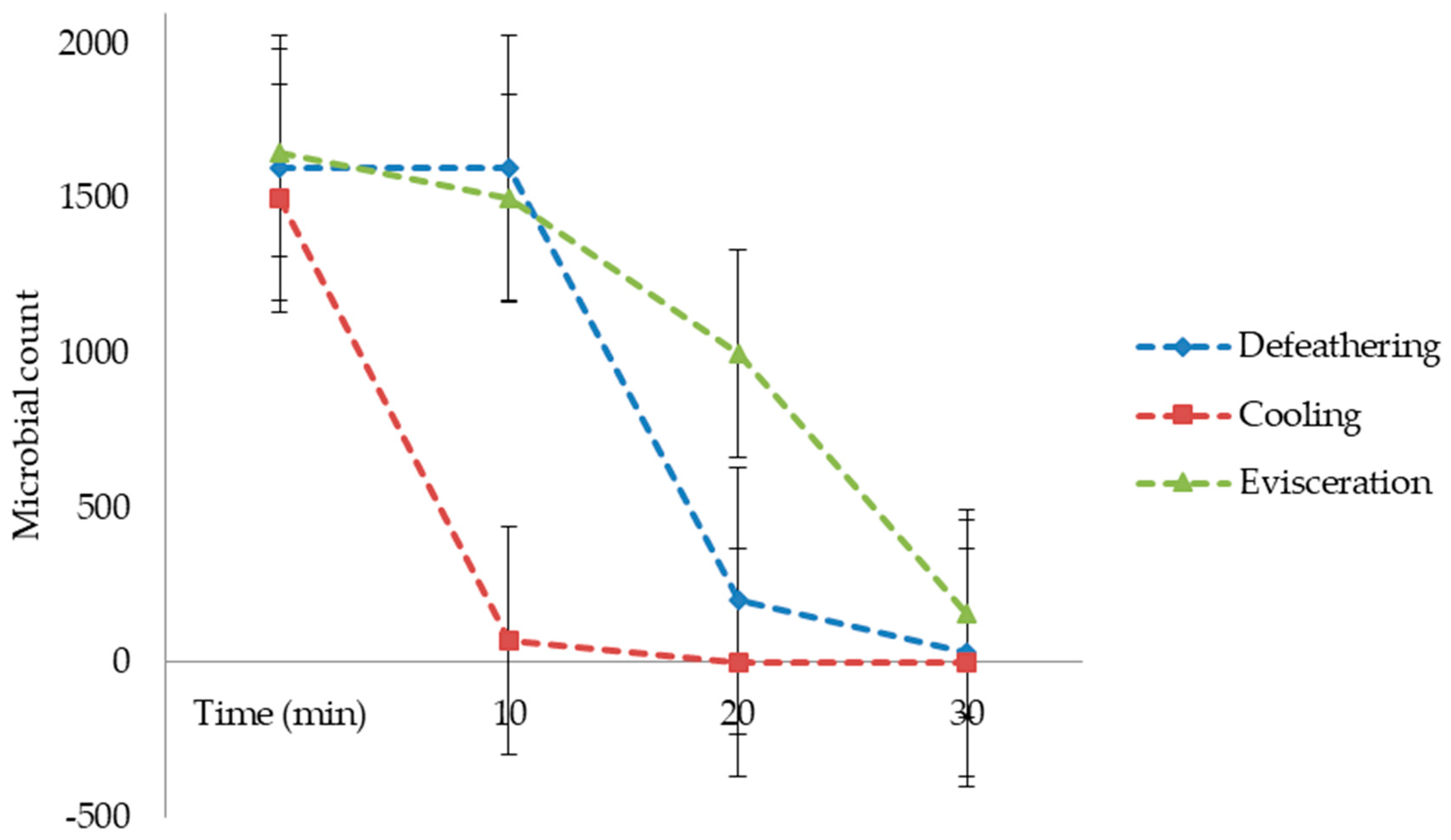
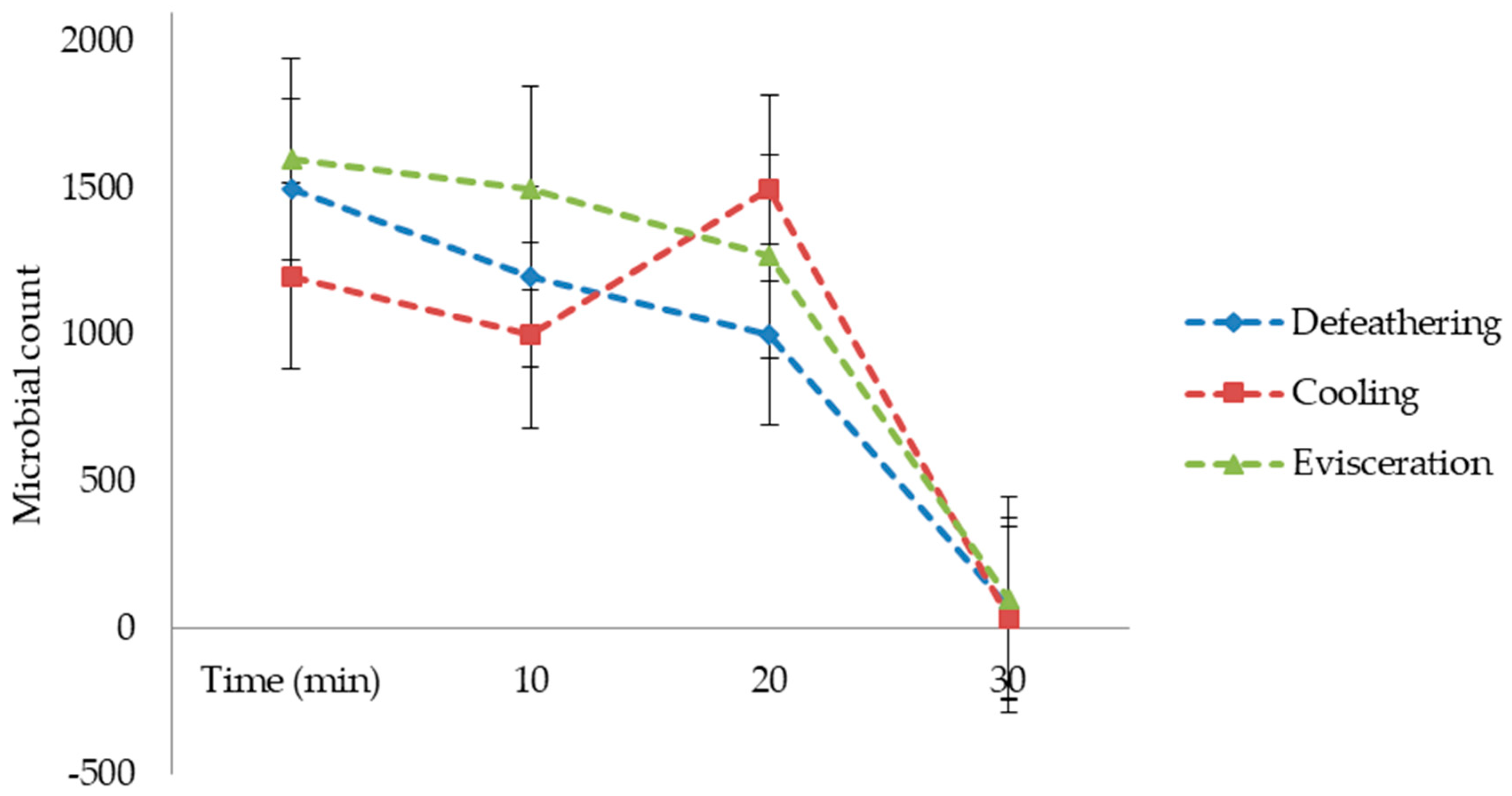
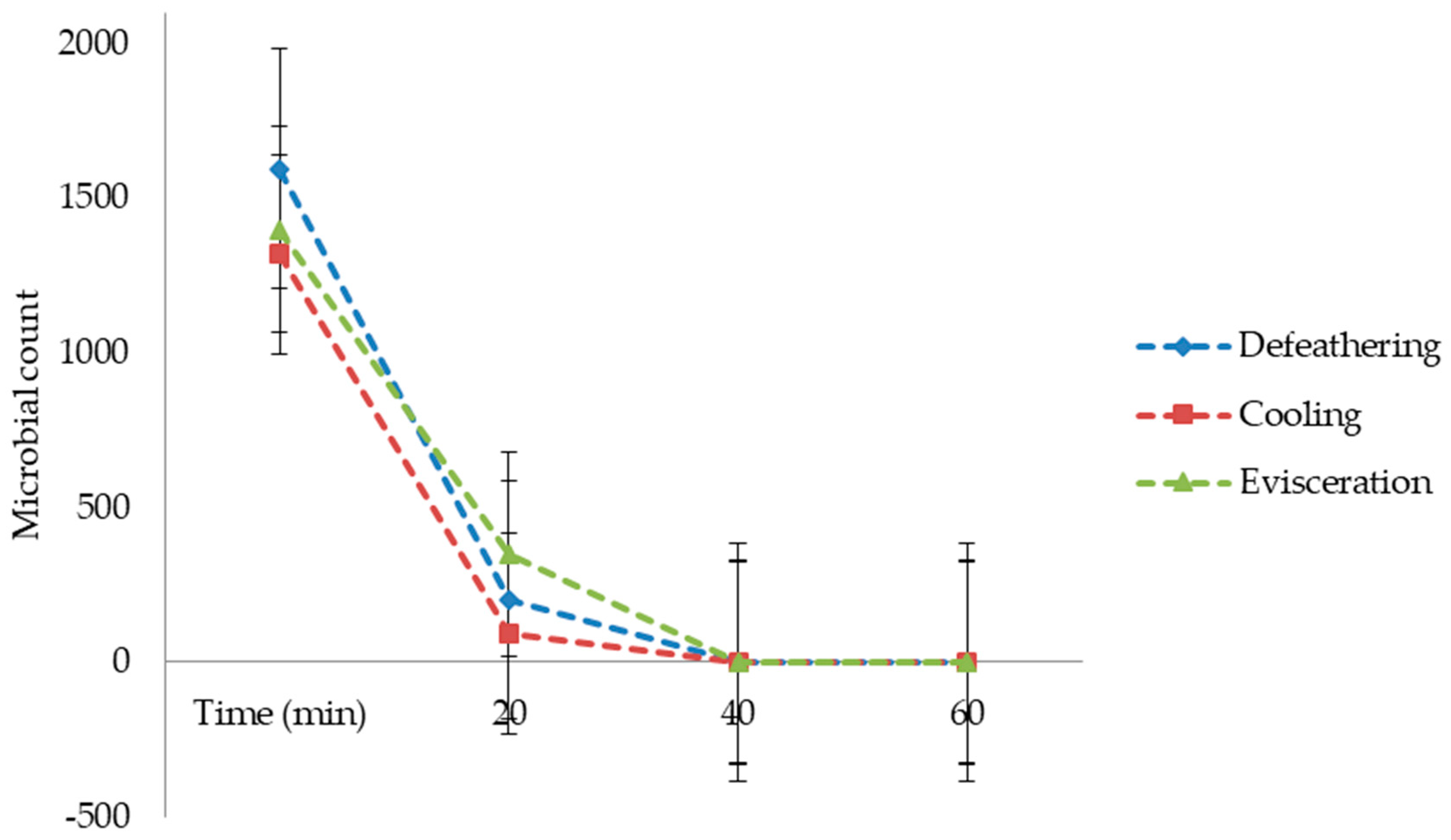
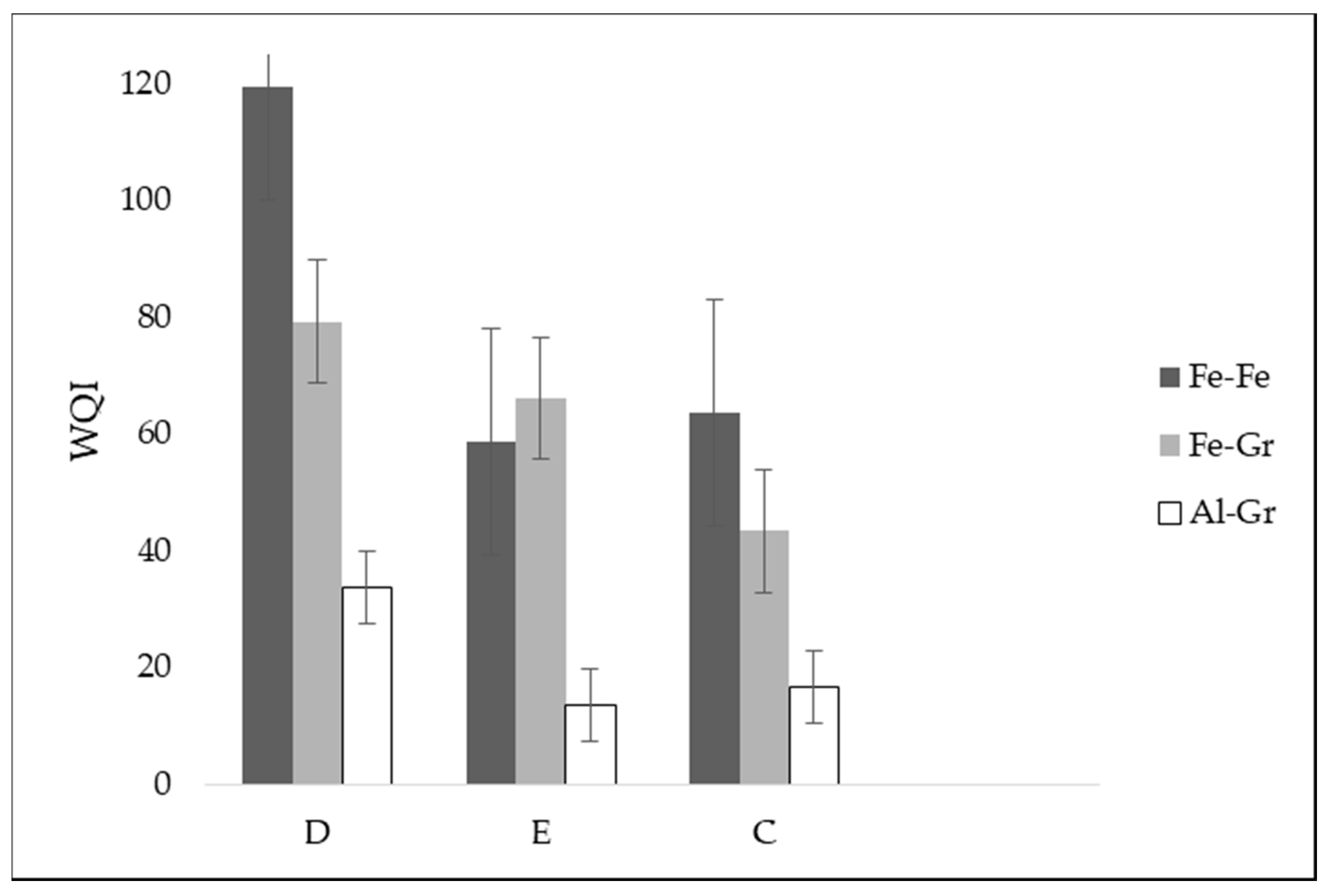
In this study, wastewater samples collected from the Izhevski poultry slaughterhouse industry in Izhevskoye village, Kazakhstan, were used. Three wastewater samples coming from different poultry slaughterhouse production processes were used, namely defeathering, evisceration, and cooling. The samples were transported to the Water and Environmental Management laboratory at the L.N. Gumilyov Eurasian National University for analysis and then treated in a lab-scale electrochemical process by using three different electrode combinations: iron to iron (Fe-Fe), iron to graphite (Fe-Gr), and aluminum to graphite (Al-Gr). This study aimed to utilize the potential of the WQI as a tool for assessing the performance of different electrode combinations for purifying wastewater from a poultry slaughterhouse. Based on the developed WQIs, the quality of the treated water obtained from each of the three electrode combinations was then classified from the “excellent” status to the “unsuitable for drinking” status, with drinking water as a reference. The selection of drinking-water quality standards as references was based on the fact that the studied treatment methods were investigated for their potential to produce high-quality water for the cooling section of the slaughterhouse.
4. Conclusions
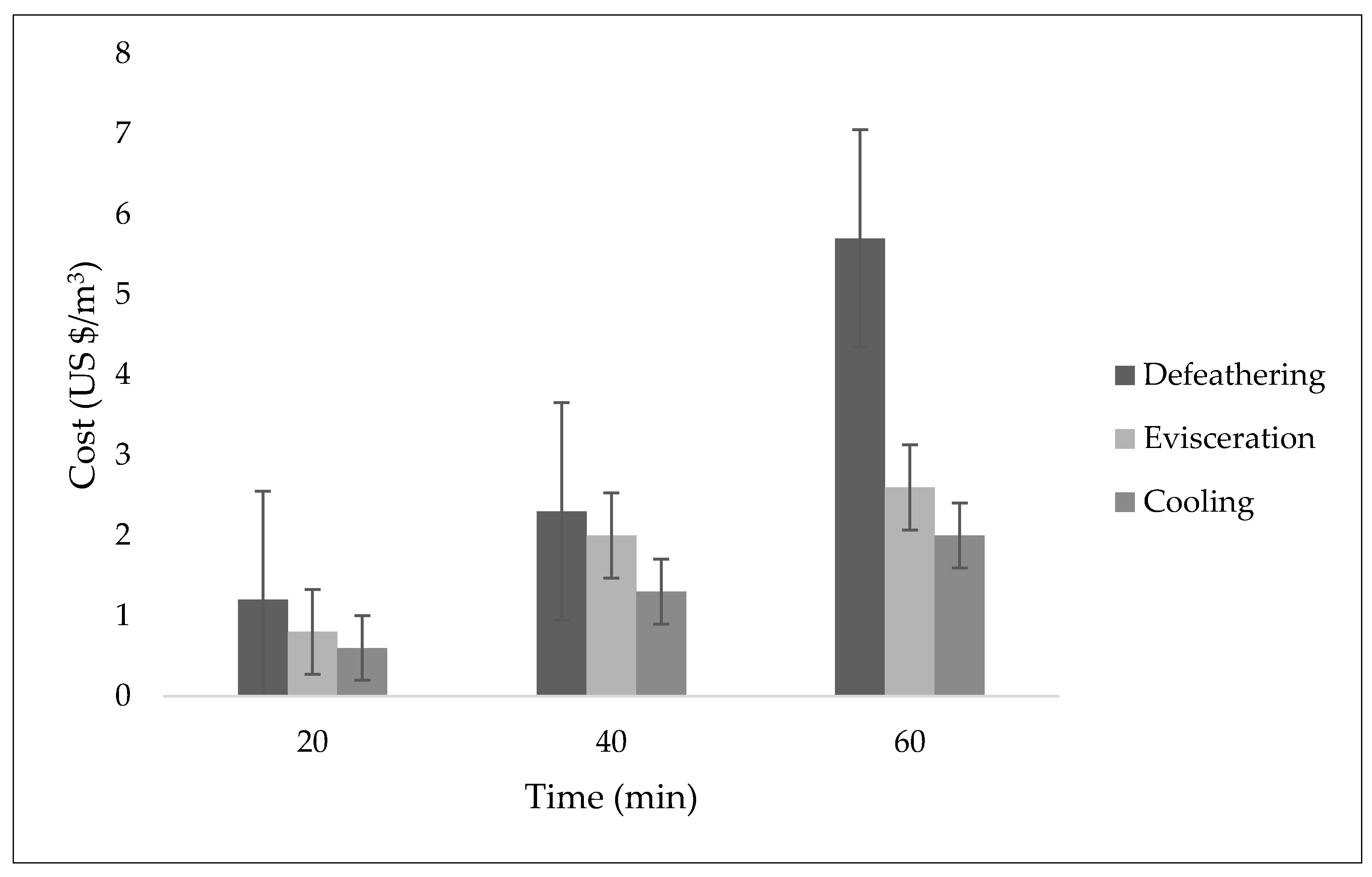
The potential influence of electrode combination on the treatability of a poultry slaughterhouse wastewater was studied. In this study, the efficiency of three different electrode combinations (Fe-Fe, Fe-Gr, and Al-Gr) for electrochemical treatment technology was evaluated with drinking water quality standards as references. The samples were analyzed before and after the purification to determine the treatment efficiency of the investigated combinations of electrodes. Water quality classification for the samples treated using Fe-Fe, Fe-Gr, and Al-Gr was successfully achieved using WQIs. From this study, the Al-Gr electrode combination showed an impressive treatment performance, achieving an “excellent” status for all of the three studied sources of wastewater. From the developed WQIs, this study was able to reveal that the wastewater from the evisceration process in the poultry slaughterhouse had higher quality after the purification process compared to that of defeathering and cooling processes. To improve the performance of Fe-Fe electrodes and the defeathering process combination, it would be ideal to integrate the combination with a post-treatment unit, especially for the removal of turbidity and COD. In general, seven out of nine WQIs developed in this study indicated an impressive performance of the electrochemical electrodes studied. In general, the use of WQI provides a relatively easy-to-understand approach for presenting information related to water quality to experts and non-experts. The findings of this study show how a change in electrode combination may influence the treatment performance of an electrochemical plant. The results reveal further the possibility of unexpected behavior when applying a certain type of electrode combination. From the developed WQIs, it is also evident that depending on the sources of wastewater, the treatment efficiency of a wastewater treatment system may be affected. Moreover, the Al-Gr electrode combination was also found to be the most cost-effective option among the studied electrode combinations.