Hydroglycerolic Solvent and Ultrasonication Pretreatment: A Green Blend for High-Efficiency Extraction of Salvia fruticosa Polyphenols
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material—Handling and Preparation
2.3. Ultrasonication Pretreatment
2.4. Batch Stirred-Tank Solid–Liquid Extraction
2.5. Extraction Kinetics and Temperature Effects
2.6. Determinations
2.7. Chromatographic Determinations
2.8. Statistical Analysis
3. Results and Discussion
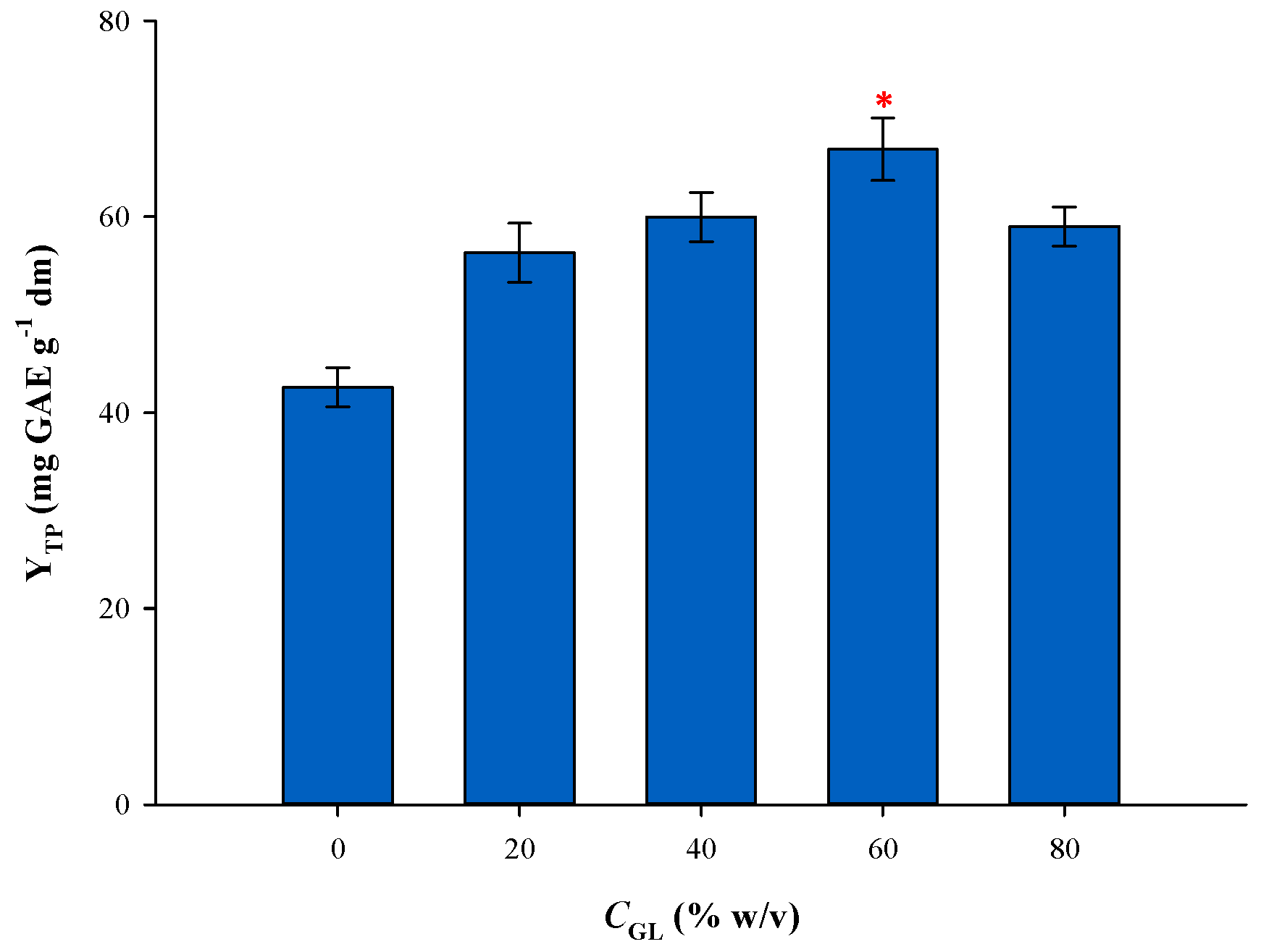
3.1. Effect of Solvent Composition
3.2. Effect of Ultrasonication Pretreatment
3.3. Extraction Kinetics and the Effect of Temperature
3.4. Antioxidant Properties and Polyphenolic Profile
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Anton, R.; Mathioudakis, B.; Pramono, S.; Sezik, E.; Sharma, S. Traditional use of botanicals and botanical preparations. Eur. Food Feed Law Rev. 2019, 14, 132–141. [Google Scholar]
- Colombo, F.; Restani, P.; Biella, S.; Di Lorenzo, C. Botanicals in functional foods and food supplements: Tradition, efficacy and regulatory aspects. Appl. Sci. 2020, 10, 2387. [Google Scholar] [CrossRef]
- Campa, M.; Baron, E. Anti-aging effects of select botanicals: Scientific evidence and current trends. Cosmetics 2018, 5, 54. [Google Scholar] [CrossRef]
- Xu, J.; Wei, K.; Zhang, G.; Lei, L.; Yang, D.; Wang, W.; Han, Q.; Xia, Y.; Bi, Y.; Yang, M. Ethnopharmacology, phytochemistry, and pharmacology of Chinese Salvia species: A review. J. Ethnopharmacol. 2018, 225, 18–30. [Google Scholar] [CrossRef]
- Hao, D.-C.; Ge, G.-B.; Xiao, P.-G. Anticancer drug targets of Salvia phytometabolites: Chemistry, biology and omics. Curr. Drug Targets 2018, 19, 1–20. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Polyphenolics of Salvia—A review. Phytochemistry 2002, 59, 117–140. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Gong, X.; Yang, M.; Zhang, C.; Li, M. Biosynthesis, chemistry, and pharmacology of polyphenols from Chinese Salvia species: A review. Molecules 2019, 24, 155. [Google Scholar] [CrossRef]
- Sarrou, E.; Martens, S.; Chatzopoulou, P. Metabolite profiling and antioxidative activity of sage (Salvia fruticosa Mill.) under the influence of genotype and harvesting period. Ind. Crops Prod. 2016, 94, 240–250. [Google Scholar] [CrossRef]
- Exarchou, V.; Kanetis, L.; Charalambous, Z.; Apers, S.; Pieters, L.; Gekas, V.; Goulas, V. HPLC-SPE-NMR characterization of major metabolites in Salvia fruticosa Mill. extract with antifungal potential: Relevance of carnosic acid, carnosol, and hispidulin. J. Agric. Food Chem. 2015, 63, 457–463. [Google Scholar] [CrossRef]
- Duletić-Laušević, S.; Aradski, A.A.; Šavikin, K.; Knežević, A.; Milutinović, M.; Stević, T.; Vukojević, J.; Marković, S.; Marin, P. Composition and biological activities of Libyan Salvia fruticosa Mill. and S. lanigera Poir. extracts. S. Afr. J. Bot. 2018, 117, 101–109. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. TrAC Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Vidović, S.; Redovniković, I.R.; Jokić, S. New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod. Process. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Li, Z.; Smith, K.H.; Stevens, G.W. The use of environmentally sustainable bio-derived solvents in solvent extraction applications—A review. Chin. J. Chem. Eng. 2016, 24, 215–220. [Google Scholar] [CrossRef]
- Díaz-Álvarez, A.E.; Francos, J.; Lastra-Barreira, B.; Crochet, P.; Cadierno, V. Glycerol and derived solvents: New sustainable reaction media for organic synthesis. Chem. Commun. 2011, 47, 6208–6227. [Google Scholar] [CrossRef]
- Wolfson, A.; Dlugy, C.; Shotland, Y. Glycerol as a green solvent for high product yields and selectivities. Environ. Chem. Lett. 2007, 5, 67–71. [Google Scholar] [CrossRef]
- Gu, Y.; Jérôme, F. Glycerol as a sustainable solvent for green chemistry. Green Chem. 2010, 12, 1127–1138. [Google Scholar] [CrossRef]
- Apostolakis, A.; Grigorakis, S.; Makris, D.P. Optimisation and comparative kinetics study of polyphenol extraction from olive leaves (Olea europaea) using heated water/glycerol mixtures. Sep. Purif. Technol. 2014, 128, 89–95. [Google Scholar] [CrossRef]
- Kyriakidou, K.; Mourtzinos, I.; Biliaderis, C.G.; Makris, D.P. Optimization of a green extraction/inclusion complex formation process to recover antioxidant polyphenols from oak acorn husks (Quercus robur) using aqueous 2-hydroxypropyl-β-cyclodextrin/glycerol mixtures. Environments 2016, 3, 3. [Google Scholar] [CrossRef]
- Makris, D.P. Kinetics of ultrasound-assisted flavonoid extraction from agri-food solid wastes using water/glycerol mixtures. Resources 2016, 5, 7. [Google Scholar] [CrossRef]
- Mourtzinos, I.; Anastasopoulou, E.; Petrou, A.; Grigorakis, S.; Makris, D.; Biliaderis, C.G. Optimization of a green extraction method for the recovery of polyphenols from olive leaf using cyclodextrins and glycerin as co-solvents. J. Food Sci. Technol. 2016, 53, 3939–3947. [Google Scholar] [CrossRef]
- Huang, H.; Belwal, T.; Jiang, L.; Hu, J.; Limwachiranon, J.; Li, L.; Ren, G.; Zhang, X.; Luo, Z. Valorization of lotus byproduct (Receptaculum Nelumbinis) under green extraction condition. Food Bioprod. Process. 2019, 115, 110–117. [Google Scholar] [CrossRef]
- Ciganović, P.; Jakimiuk, K.; Tomczyk, M.; Zovko Končić, M. Glycerolic licorice extracts as active cosmeceutical ingredients: Extraction optimization, chemical characterization, and biological activity. Antioxidants 2019, 8, 445. [Google Scholar] [CrossRef] [PubMed]
- El Kantar, S.; Rajha, H.N.; Boussetta, N.; Vorobiev, E.; Maroun, R.G.; Louka, N. Green extraction of polyphenols from grapefruit peels using high voltage electrical discharges, deep eutectic solvents and aqueous glycerol. Food Chem. 2019, 295, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Eyiz, V.; Tontul, I.; Turker, S. Optimization of green extraction of phytochemicals from red grape pomace by homogenizer assisted extraction. J. Food Meas. Charact. 2020, 14, 39–47. [Google Scholar] [CrossRef]
- Lantzouraki, D.Z.; Tsiaka, T.; Soteriou, N.; Asimomiti, G.; Spanidi, E.; Natskoulis, P.; Gardikis, K.; Sinanoglou, V.J.; Zoumpoulakis, P. Antioxidant profiles of Vitis vinifera L. and Salvia triloba L. leaves using high-energy extraction methodologies. J. AOAC Int. 2019. [Google Scholar] [CrossRef] [PubMed]
- Huamán-Castilla, N.L.; Mariotti-Celis, M.S.; Martínez-Cifuentes, M.; Pérez-Correa, J.R. Glycerol as alternative co-Solvent for water extraction of polyphenols from Carménère pomace: Hot pressurized liquid extraction and computational chemistry calculations. Biomolecules 2020, 10, 474. [Google Scholar] [CrossRef]
- Lakka, A.; Karageorgou, I.; Kaltsa, O.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D.P. Polyphenol extraction from Humulus lupulus (hop) using a neoteric glycerol/L-alanine deep eutectic solvent: Optimisation, kinetics and the effect of ultrasound-assisted pretreatment. AgriEngineering 2019, 1, 30. [Google Scholar] [CrossRef]
- Peleg, M.; Normand, M.D.; Corradini, M.G. The Arrhenius equation revisited. Crit. Rev. Food Sci. Nutr. 2012, 52, 830–851. [Google Scholar] [CrossRef]
- van Boekel, M.A. Kinetic modeling of food quality: A critical review. Compr. Rev. Food Sci. Food Saf. 2008, 7, 144–158. [Google Scholar] [CrossRef]
- Karakashov, B.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Optimisation of polyphenol extraction from Hypericum perforatum (St. John’s Wort) using aqueous glycerol and response surface methodology. J. Appl. Res. Med. Aromat. Plants 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Kaltsa, O.; Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D.P. A green extraction process for polyphenols from elderberry (Sambucus nigra) flowers using deep eutectic solvent and ultrasound-assisted pretreatment. Molecules 2020, 25, 921. [Google Scholar] [CrossRef] [PubMed]
- Michail, A.; Sigala, P.; Grigorakis, S.; Makris, D.P. Kinetics of ultrasound-assisted polyphenol extraction from spent filter coffee using aqueous glycerol. Chem. Eng. Commun. 2016, 203, 407–413. [Google Scholar] [CrossRef]
- Karakashov, B.; Grigorakis, S.; Loupassaki, S.; Mourtzinos, I.; Makris, D.P. Optimisation of organic solvent-free polyphenol extraction from Hypericum triquetrifolium Turra using Box–Behnken experimental design and kinetics. Int. J. Ind. Chem. 2015, 6, 85–92. [Google Scholar] [CrossRef]
- Blidi, S.; Bikaki, M.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. A comparative evaluation of bio-solvents for the efficient extraction of polyphenolic phytochemicals: Apple waste peels as a case study. Waste Biomass Valorization 2015, 6, 1125–1133. [Google Scholar] [CrossRef]
- Shehata, E.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Extraction optimisation using water/glycerol for the efficient recovery of polyphenolic antioxidants from two Artemisia species. Sep. Purif. Technol. 2015, 149, 462–469. [Google Scholar] [CrossRef]
- Katsampa, P.; Valsamedou, E.; Grigorakis, S.; Makris, D.P. A green ultrasound-assisted extraction process for the recovery of antioxidant polyphenols and pigments from onion solid wastes using Box–Behnken experimental design and kinetics. Ind. Crops Prod. 2015, 77, 535–543. [Google Scholar] [CrossRef]
- Trasanidou, D.; Apostolakis, A.; Makris, D.P. Development of a green process for the preparation of antioxidant and pigment-enriched extracts from winery solid wastes using response surface methodology and kinetics. Chem. Eng. Commun. 2016, 203, 1317–1325. [Google Scholar] [CrossRef]
- Lakka, A.; Grigorakis, S.; Kaltsa, O.; Karageorgou, I.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D.P. The effect of ultrasonication pretreatment on the production of polyphenol-enriched extracts from Moringa oleifera L. (drumstick tree) using a novel bio-based deep eutectic solvent. Appl. Sci. 2020, 10, 220. [Google Scholar] [CrossRef]
- Philippi, K.; Tsamandouras, N.; Grigorakis, S.; Makris, D.P. Ultrasound-assisted green extraction of eggplant peel (Solanum melongena) polyphenols using aqueous mixtures of glycerol and ethanol: Optimisation and kinetics. Environ. Process. 2016, 3, 369–386. [Google Scholar] [CrossRef]
- Chemat, F.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Nayak, A.; Bhushan, B.; Rosales, A.; Turienzo, L.R.; Cortina, J. Valorisation potential of Cabernet grape pomace for the recovery of polyphenols: Process intensification, optimisation and study of kinetics. Food Bioprod. Process. 2018, 109, 74–85. [Google Scholar] [CrossRef]
- Grigorakis, S.; Benchennouf, A.; Halahlah, A.; Makris, D.P. High-performance green extraction of polyphenolic antioxidants from Salvia fruticosa using cyclodextrins: Optimization, kinetics and composition. Appl. Sci. 2020, 10, 3447. [Google Scholar] [CrossRef]
- Boussetta, N.; Vorobiev, E.; Deloison, V.; Pochez, F.; Falcimaigne-Cordin, A.; Lanoisellé, J.-L. Valorisation of grape pomace by the extraction of phenolic antioxidants: Application of high voltage electrical discharges. Food Chem. 2011, 128, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.; Goulas, V.; Tsakona, S.; Manganaris, G.A.; Gekas, V. A knowledge base for the recovery of natural phenols with different solvents. Int. J. Food Prop. 2013, 16, 382–396. [Google Scholar] [CrossRef]
- Khiari, Z.; Makris, D.P.; Kefalas, P. An investigation on the recovery of antioxidant phenolics from onion solid wastes employing water/ethanol-based solvent systems. Food Bioprocess Technol. 2009, 2, 337. [Google Scholar] [CrossRef]
- Karageorgou, I.; Grigorakis, S.; Lalas, S.; Makris, D.P. Enhanced extraction of antioxidant polyphenols from Moringa oleifera Lam. leaves using a biomolecule-based low-transition temperature mixture. Eur. Food Res. Technol. 2017, 243, 1839–1848. [Google Scholar] [CrossRef]
- Shang, X.; Dou, Y.; Zhang, Y.; Tan, J.-N.; Liu, X.; Zhang, Z. Tailor-made natural deep eutectic solvents for green extraction of isoflavones from chickpea (Cicer arietinum L.) sprouts. Ind. Crops Prod. 2019, 140, 111724. [Google Scholar] [CrossRef]
- Mourtzinos, I.; Makris, D.P.; Yannakopoulou, K.; Kalogeropoulos, N.; Michali, I.; Karathanos, V.T. Thermal stability of anthocyanin extract of Hibiscus sabdariffa L. in the presence of β-cyclodextrin. J. Agric. Food Chem. 2008, 56, 10303–10310. [Google Scholar] [CrossRef]
- Atwi, M.; Weiss, E.-K.; Loupassaki, S.; Makris, D.P.; Ioannou, E.; Roussis, V.; Kefalas, P. Major antioxidant polyphenolic phytochemicals of three Salvia species endemic to the island of Crete. J. Herbs Spices Med. Plants 2016, 22, 27–34. [Google Scholar] [CrossRef]

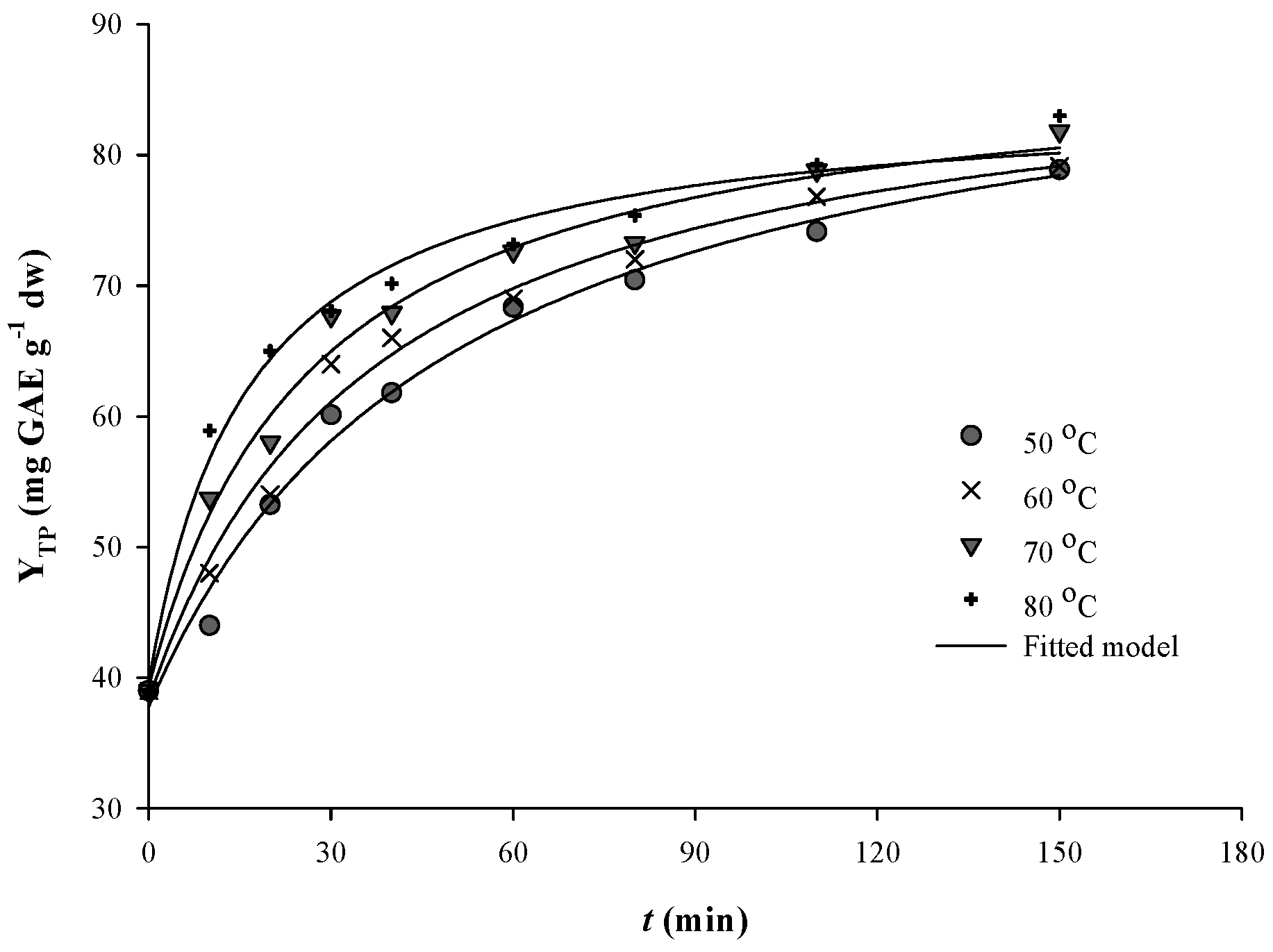


| T (°C) | Kinetic Parameters | |||
|---|---|---|---|---|
| k (×10−3) (g mg−1 min−1) | h (mg g−1 min−1) | YTP(s) (mg GAE g−1) | t0.5 (min) | |
| 50 | 0.369 | 1.838 | 92.00 | 50.06 |
| 60 | 0.528 | 2.400 | 89.27 | 37.19 |
| 70 | 0.768 | 3.278 | 87.91 | 26.82 |
| 80 | 1.370 | 5.194 | 84.53 | 16.27 |
| Extract | YTP (mg GAE g−1 dm) | YTFn (mg RtE g−1 dm) | AAR (μmol DPPH g−1 dm) | PR (μmol AAE g−1 dm) |
|---|---|---|---|---|
| m-β-CD | 108.14 ± 2.70 | 53.62 ± 1.61 | 820.93 ± 16.42 | 590.66 ± 14.77 |
| GL | 83.86 ± 2.10 | 51.46 ± 2.57 | 817.58 ± 8.18 | 709.12 ± 17.73 |
| No | Rt (min) | UV-Vis (λmax) | [M − H]+ (m/z) | Other Ions (m/z) | Tentative Identity |
|---|---|---|---|---|---|
| 1 | 15.77 | 246, 318 | 353 | 179 | Chlorogenic acid |
| 2 | 17.40 | 248, 318 | 253 | - | Unknown |
| 3 | 21.00 | 270, 340 | 593 | - | Unknown |
| 4 | 23.57 | 280, 344 | 477 | 301 | 6-Hydroxy luteolin 7-O-glucoside |
| 5 | 25.78 | 256, 352 | 461 | 285 | Luteolin 7-O-glucuronide |
| 6 | 27.12 | 258, 348 | 593 | 285 | Luteolin 7-O-rutinoside |
| 7 | 27.90 | 270, 352 | 491 | 299 | 6-Methoxyluteolin 7-O-glucoside (nepitrin) |
| 8 | 28.65 | 246, 316 | 359 | 161 | Rosmarinic acid |
| 9 | 29.55 | 264, 346 | 445 | 269 | Apigenin 7-O-glucuronide |
| 10 | 32.15 | 270, 352 | 475 | 299 | 6-Methoxyluteolin derivative |
| 11 | 32.82 | 274, 332 | 461 | 299, 283 | 6-Methoxyluteolin derivative |
| Compound | Yield (mg g−1 dm) ± sd | ||
|---|---|---|---|
| m-β-CD | GL | % Difference | |
| Chlorogenic acid | 0.15 ± 0.02 | 0.24 ± 0.05 | 37.5 |
| Luteolin 7-O-glucuronide | 6.96 ± 1.12 | 5.51 ± 1.57 | 20.8 |
| Rosmarinic acid | 10.57 ± 1.37 | 10.63 ± 0.98 | 0.57 |
| Sum | 17.68 | 16.38 | 7.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigorakis, S.; Halahlah, A.; Makris, D.P. Hydroglycerolic Solvent and Ultrasonication Pretreatment: A Green Blend for High-Efficiency Extraction of Salvia fruticosa Polyphenols. Sustainability 2020, 12, 4840. https://doi.org/10.3390/su12124840
Grigorakis S, Halahlah A, Makris DP. Hydroglycerolic Solvent and Ultrasonication Pretreatment: A Green Blend for High-Efficiency Extraction of Salvia fruticosa Polyphenols. Sustainability. 2020; 12(12):4840. https://doi.org/10.3390/su12124840
Chicago/Turabian StyleGrigorakis, Spyros, Abedalghani Halahlah, and Dimitris P. Makris. 2020. "Hydroglycerolic Solvent and Ultrasonication Pretreatment: A Green Blend for High-Efficiency Extraction of Salvia fruticosa Polyphenols" Sustainability 12, no. 12: 4840. https://doi.org/10.3390/su12124840
APA StyleGrigorakis, S., Halahlah, A., & Makris, D. P. (2020). Hydroglycerolic Solvent and Ultrasonication Pretreatment: A Green Blend for High-Efficiency Extraction of Salvia fruticosa Polyphenols. Sustainability, 12(12), 4840. https://doi.org/10.3390/su12124840







