In this section, the mechanism of each surfactant collector addition on improving the extraction from water to isooctane phase of the cerium oxide abrasive and the glass powder, as well as the mutual separation of these two powders, is discussed.
4.1. The Mechanism of Surfactant Collector Addition on Improving Extraction
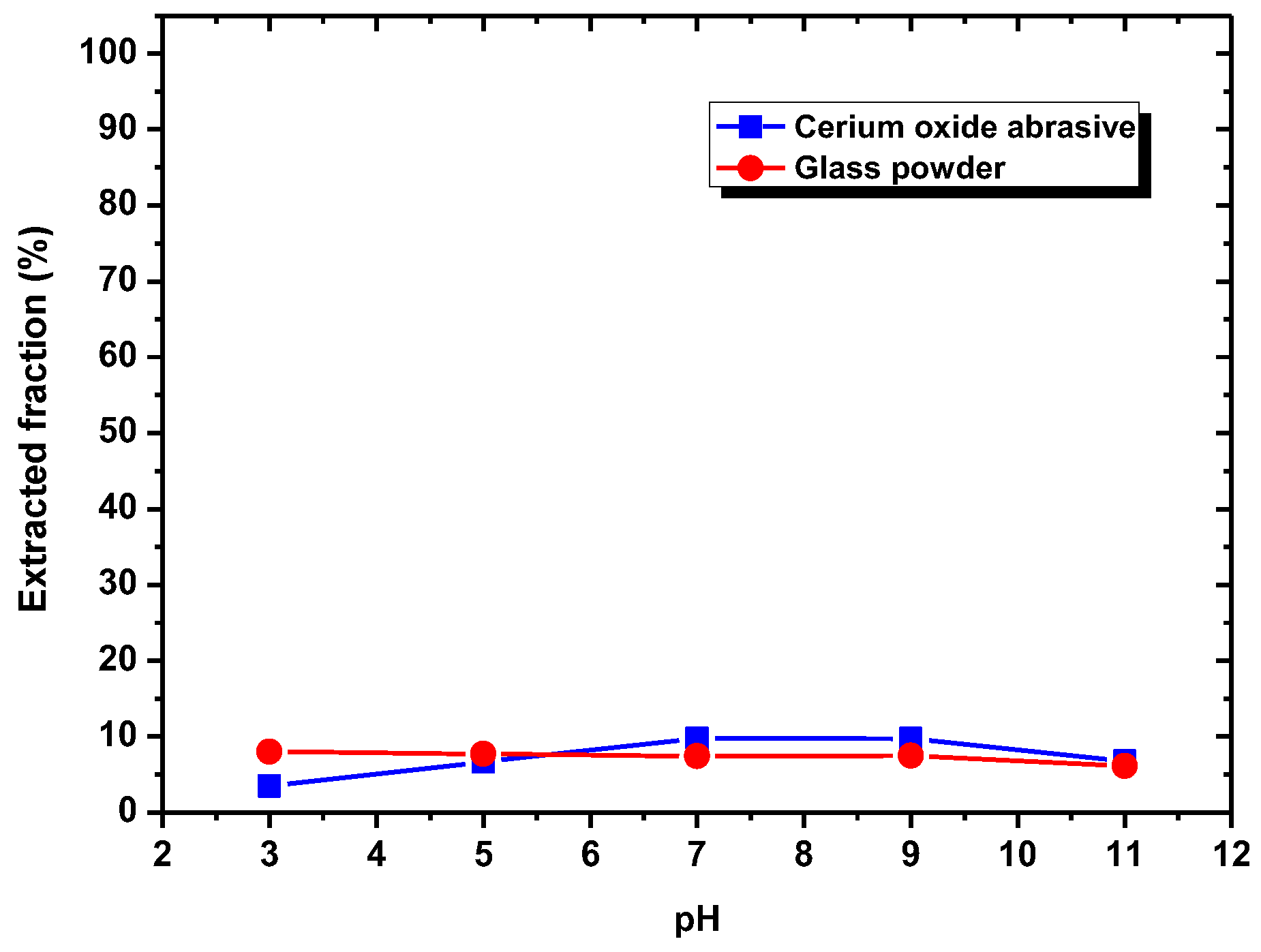
According to the results shown in
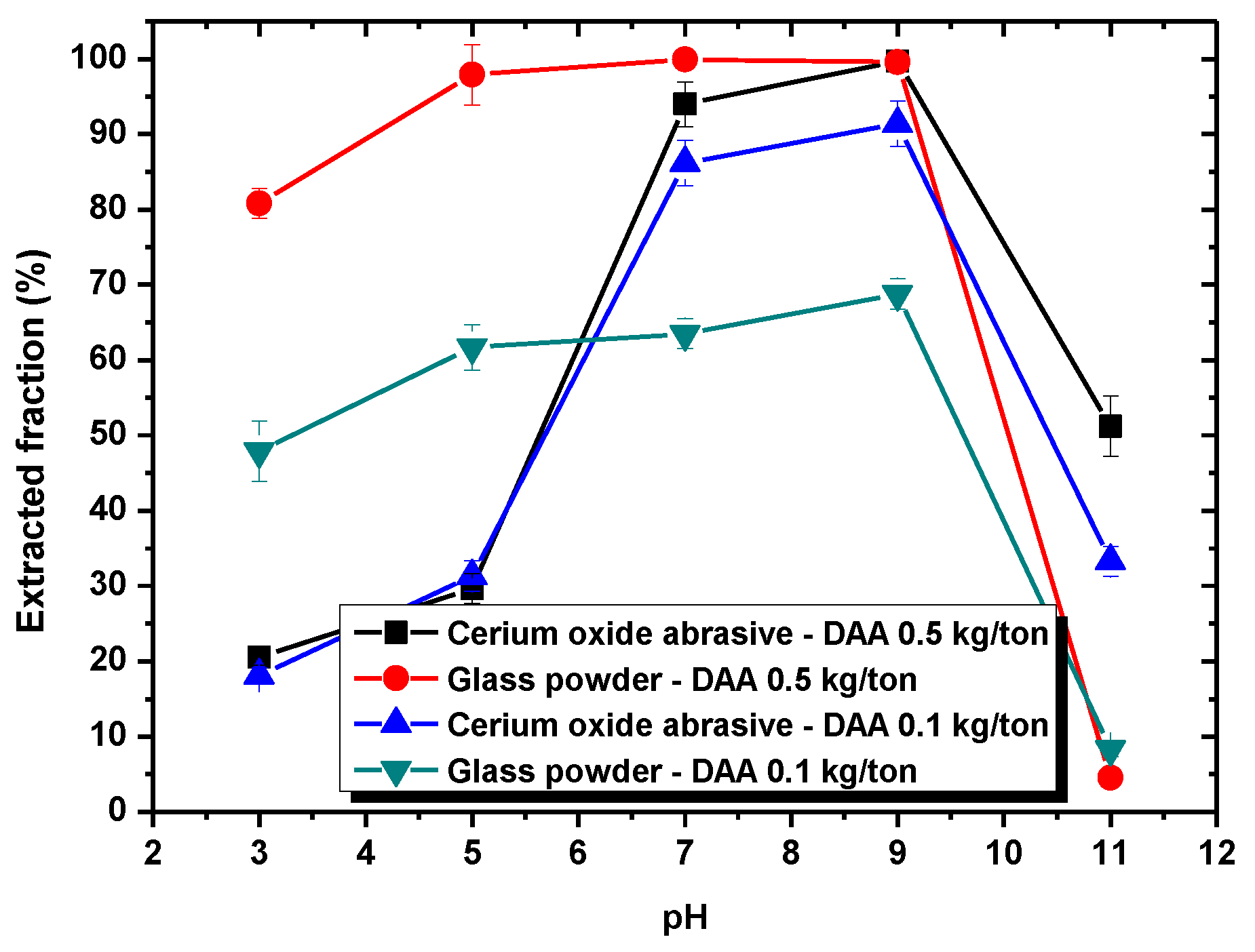
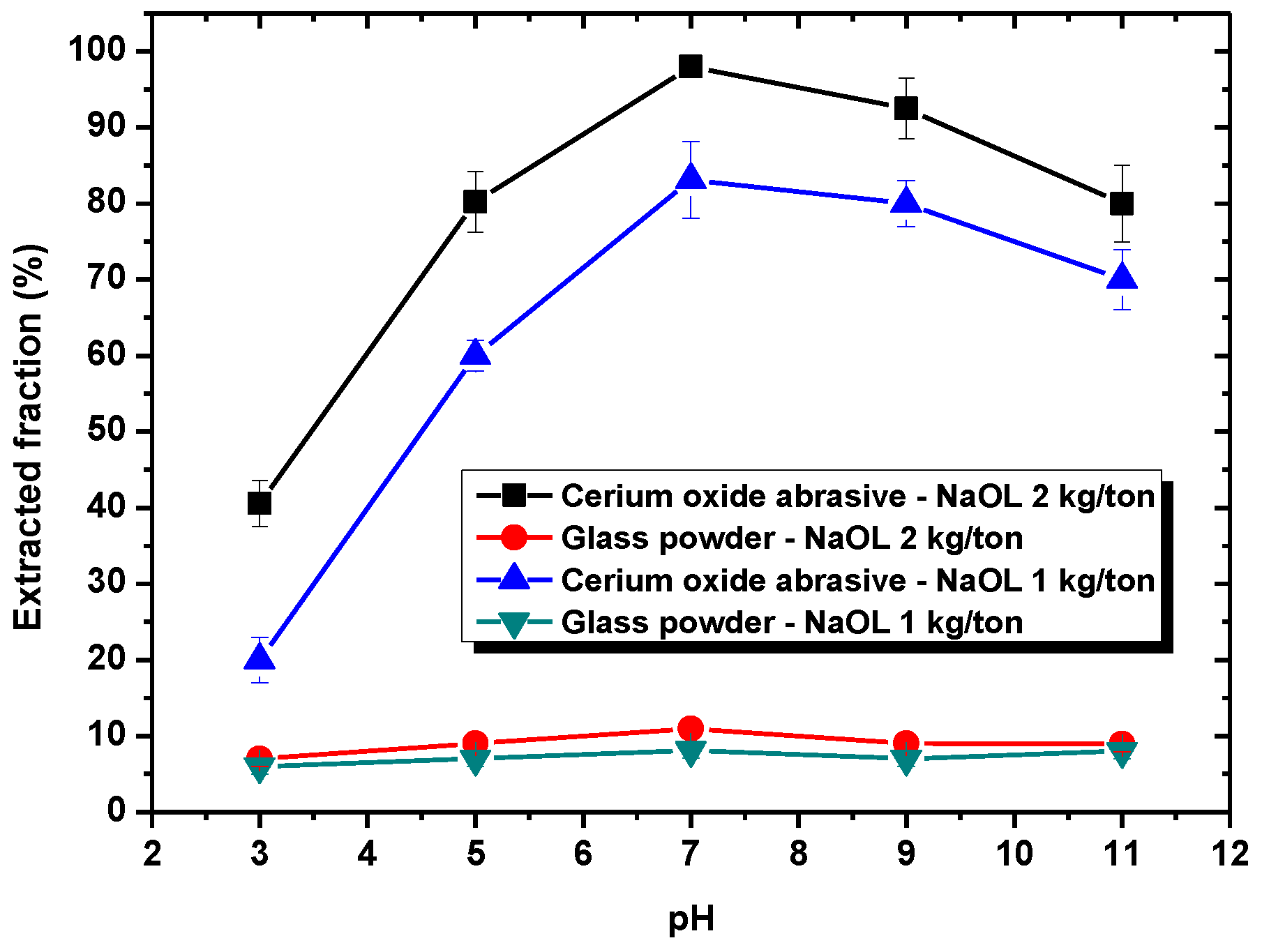
Section 3.1, DAA addition could improve the extracted fraction from water to isooctane phase of both cerium oxide abrasive and glass powder at pH 7–9 and pH 3–9, respectively, whereas NaOL addition could only improve that of cerium oxide abrasive across a broad pH range. A surfactant collector contains a hydrophilic group and a hydrophobic group. In general, a surfactant collector reacts with the particle through either electrostatic physical adsorption or chemical adsorption occurred between its hydrophilic group and the particle, and thus the surface of the particle becomes hydrophobic. By contrast, the hydrophobic group of the surfactant collector contacts with the air bubble or oil droplet, and thus the particle floats upwards. The occurrence of electrostatic physical adsorption and chemical adsorption is related to the surface charge of the particle and the dissociation status of the surfactant collector in aqueous solution. Usually, both of them vary with the solution pH.
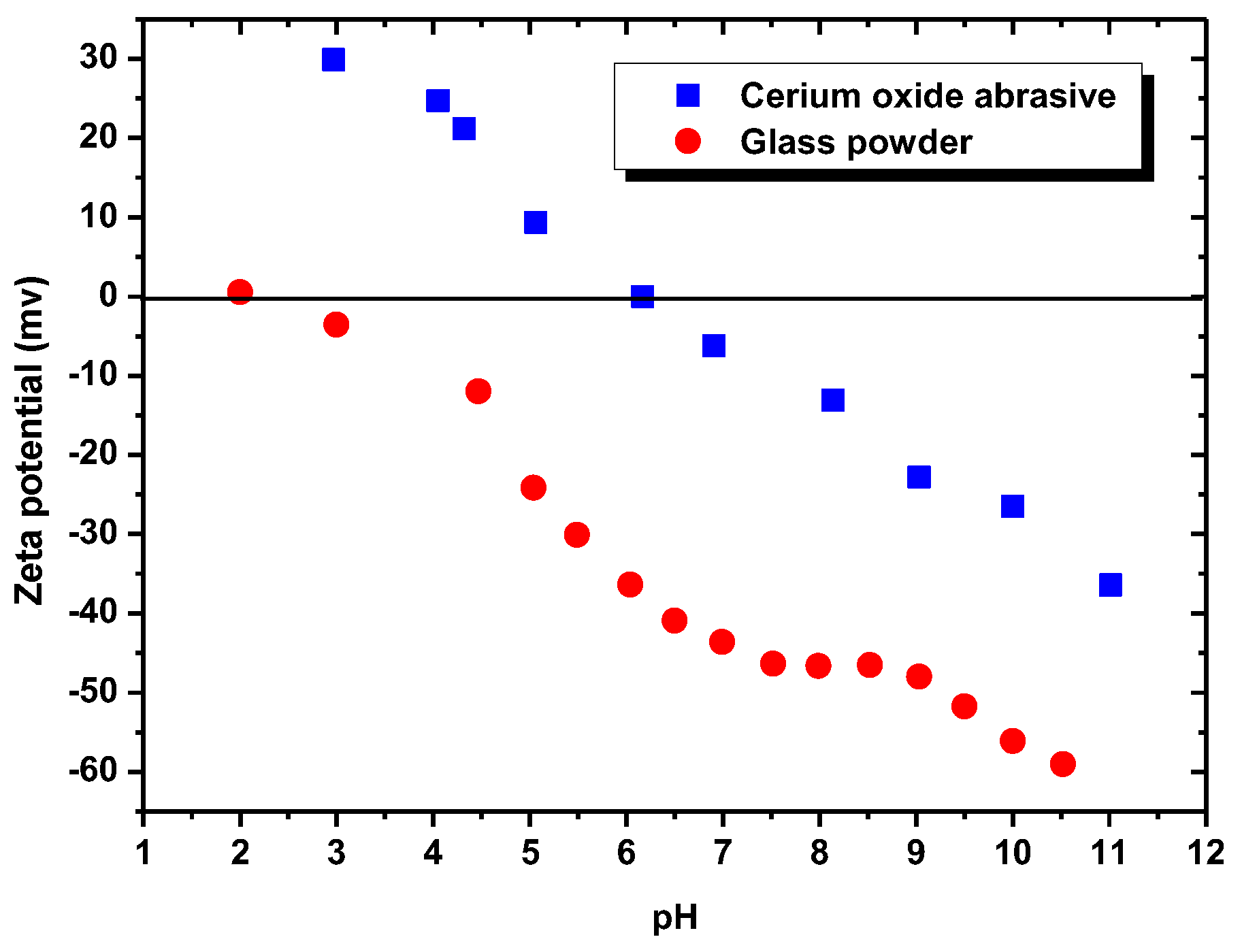
Figure 9 illustrates the zeta potentials (i.e., the surface charges) of the cerium oxide abrasive and the glass powder, as measured by a zeta potential analyzer (Stabino, Particle Metrix GmbH, Meerbusch, Germany). Distilled water was used as the solvent. Potassium nitrate (KNO
3) solution of 1 × 10
−3 M was used as the supporting electrolyte. The isoelectric point (IEP) of cerium oxide abrasive was observed at pH 6.2, and the surface of cerium oxide abrasive was determined to be negatively and positively charged above and below pH 6.2, respectively. By contrast, the IEP of the glass powder was observed at pH 2, and the surface of glass powder was determined to be negatively charged across a broad pH range above pH 2. The cerium oxide abrasive is produced from rare earth minerals such as monazite. The IEP and zeta potentials of the cerium oxide abrasive are similar to the reported analysis results of the monazite [
20].
With respect to the surfactant collector of DAA, DAA (C
14H
31NO
2) is substantially composed of two components: dodecylamine (C
12H
25NH
2) and acetic acid (CH
3COOH). Dodecylamine and acetate acid are weak base and weak acid, respectively, which have acid dissociation constants (pKa) of 10.63 and 4.8, respectively [
19]. When DAA is in an aqueous solution, the following reactions occur
The species of DAA in an aqueous depends on the solution pH. Between pH 4.8 and 10.63, the acetic acid dissociates to form the acetate ion (CH3COO−) and releases a hydrogen ion (H+). The dodecylamine accepts the hydrogen ion to form C12H25NH3+. The reaction of DAA with particle is mainly through its hydrophilic group, i.e., the amine group NH3+, and thus DAA acts as a cationic surfactant collector. Below pH 4.8, the acetic acid dissociates less, and thus the hydrogen ion is less released. The hydrophilic group of dodecylamine exists as NH2, and thus the capability of DAA as a cationic surfactant collector reduces. Above pH 10.63, the Equation (1) progresses inversely so that the hydrophilic group of dodecylamine exists as NH2, and thus the capability of DAA as a cationic surfactant collector also decreases.
Below pH 6, the surface of cerium oxide abrasive is positively charged. Meanwhile, the DAA acts as a cationic surfactant collector. Therefore, the electrostatic physical adsorption between the positively charged cerium oxide abrasive and the cationic surfactant collector DAA cannot occur. Hence, the extracted fraction of cerium oxide abrasive is not substantially improved. Above pH 6, the surface of cerium oxide abrasive becomes negatively charged, and the electrostatic physical adsorption with the cationic surfactant collector DAA is able to occur. Therefore, the extracted fraction of cerium oxide abrasive is significantly improved. The higher the pH, the higher extracted fraction of cerium oxide abrasive is obtained. Above pH 10, the capability of DAA as a cationic surfactant collector reduces, and thus the effectiveness in improving the extracted fraction of cerium oxide abrasive is decreased. By contrast, the surface of glass powder is negatively charged above pH 2. The electrostatic physical adsorption between glass powder and the cationic surfactant collector DAA is likely to occur, and thus the extracted fraction of glass powder is improved. However, below pH 4.8 and above pH 10.63, the capability of DAA as a cationic surfactant collector reduces, and thus the effectiveness in improving the extracted fraction of glass powder is decreased. Consequently, the effectiveness of DAA addition in improving the extracted fraction of the cerium oxide abrasive and the glass powder is attributed to the electrostatic physical adsorption between DAA and them.
With respect to the surfactant collector of NaOL, NaOL (C
17H
33COONa) is a strong electrolyte which fully ionizes to form the anionic oleic ion (C
17H
33COO
−) in an aqueous solution and thus acts as an anionic surfactant collector. Below a pH of 6, the electrostatic physical attraction between positively charged cerium oxide abrasive and anionic oleic ion is likely to occur. Therefore, the extracted fraction of cerium oxide abrasive is improved. However, it is observed that the extracted fraction decreased with the decrease in solution pH below pH 6. It is considered that the oleic ion can react with hydrogen ion to form the oleic acid (C
17H
33COOH) according to the following Equation (3)
The pKa of oleic acid is 5 [
21]. Therefore, in a weak acid medium near and below pH 5, the oleic ion starts to react with the hydrogen ion to form the oleic acid. Hence, the amount of oleic ion reduces, and thus its improvement of the extraction of the cerium oxide abrasive decreases. On the contrary, above a pH of 6, the cerium oxide abrasive becomes negatively charged and the electrostatic physical adsorption with the anionic oleic ion is not likely to occur. However, the extracted fraction of cerium oxide abrasive is still improved. It is considered that chemical adsorption between cerium oxide abrasive and oleic ion may occur. It was reported that oleic ion can react with metal (Me) to form Me(C
17H
33COO)
2 [
22]. In addition, oleic ion can also absorb to the surface of monazite chemically [
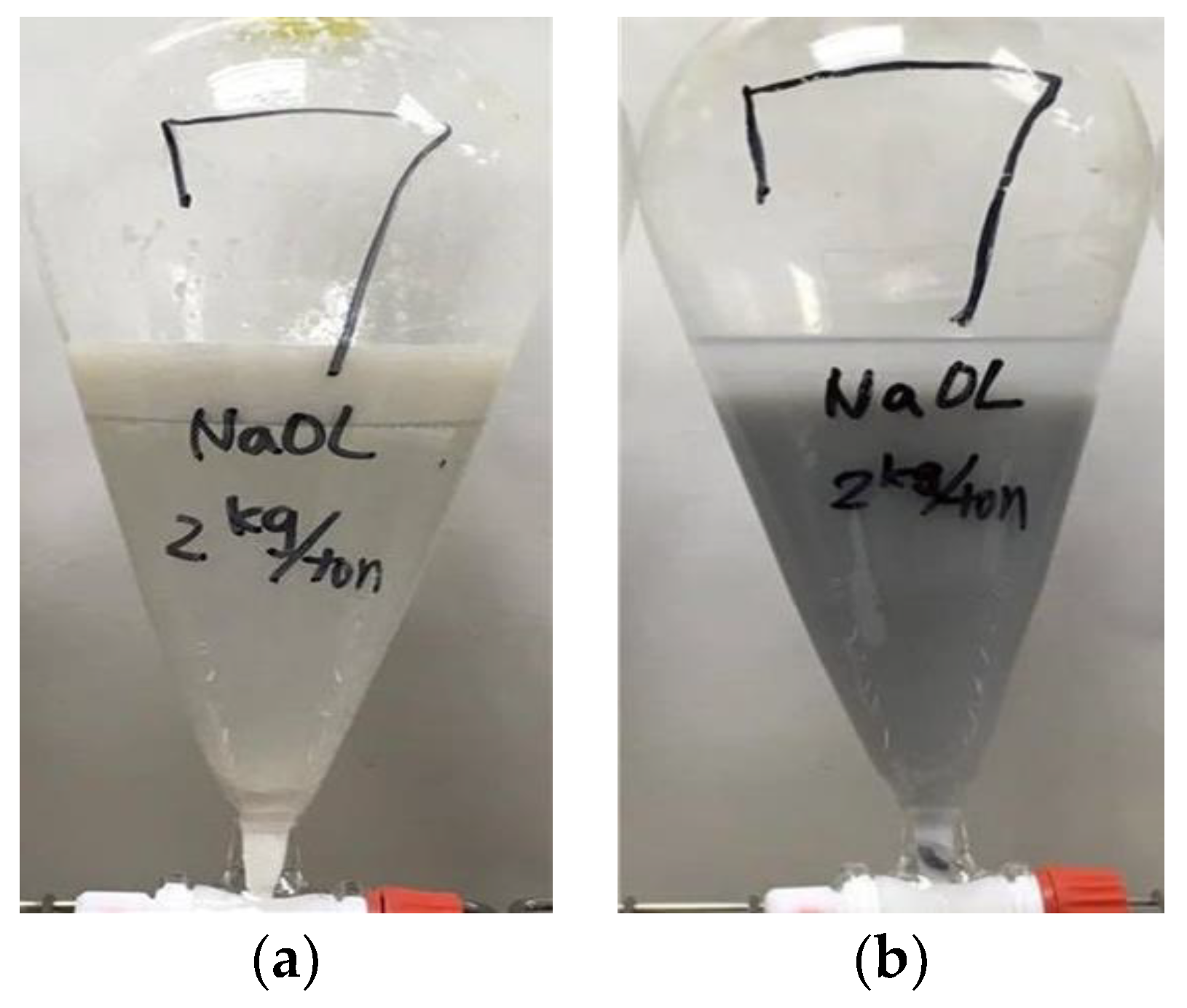
23]. Hence, the extracted fraction of the cerium oxide abrasive can be improved with NaOL addition. At higher pH values, the negative charge of cerium oxide abrasive increases. The electrostatic repulsion between cerium oxide abrasive and anionic oleic ion also increases; thus, the extracted fraction of cerium oxide abrasive decreases. Consequently, the effectiveness of NaOL addition in improving the extracted fraction of the cerium oxide abrasive is attributed to the synergistic effect of electrostatic physical adsorption and chemical adsorption between NaOL and cerium oxide abrasive. A higher extracted fraction of the cerium oxide abrasive is obtained at pH 7. By contrast, the glass powder cannot react with NaOL by means of either electrostatic attraction or chemical adsorption; thus, its extracted fraction cannot be improved with NaOL addition. Therefore, NaOL addition can selectively improve the extraction of the cerium oxide abrasive.
4.2. The Mechanism of Surfactant Collector Addition on Improving Separation
According to the results shown in
Section 3.2, the cerium oxide abrasive and the polished glass in the abrasive-glass polishing waste could not be separated without surfactant collector addition. In addition, DAA addition could not improve their separation very well, whereas NaOL could with a specific pH range and dosage. The results are considered to be caused by the combined effects of the extraction of the cerium oxide abrasive and the glass powder, as well as the surface charge of these two powders.
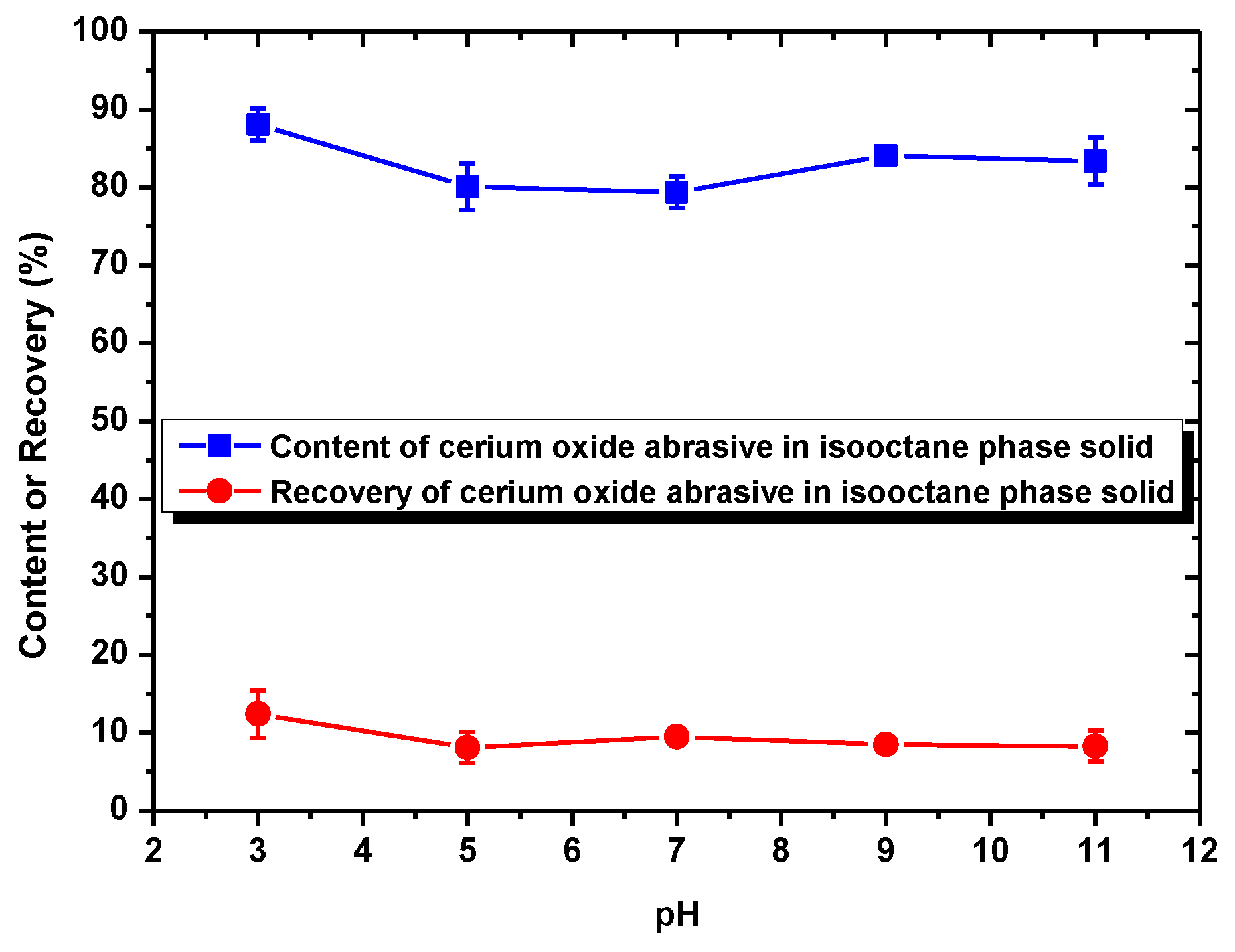
When surfactant collector was not added, the recovery of cerium oxide abrasive in the solid recovered from the isooctane phase was ca. 10%, and the content of that was near its initial content (80.5%) in the polishing waste. This is because neither cerium oxide abrasive nor glass powder can be extracted to the isooctane phase without a surfactant collector addition. Most of these two powders remain in the water phase and therefore cannot be separated.
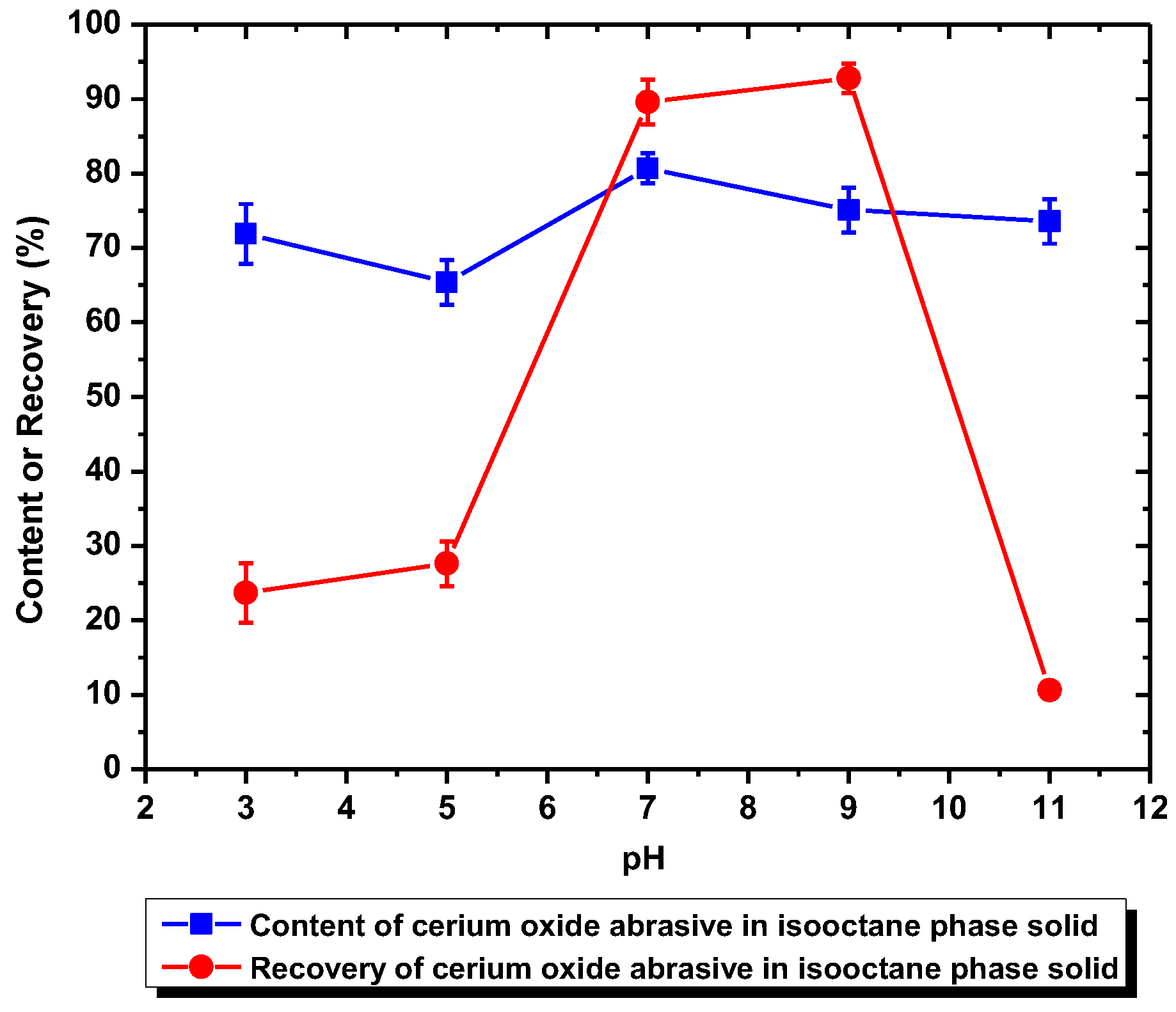
When using DAA as the surfactant collector, the extraction of the cerium oxide abrasive could be improved at pH 7–9, and that of the glass powder could be improved at pH 3–9. When the separation was conducted at pH 7–9, although a higher recovery (more than 90%) of cerium oxide abrasive in the solid recovered from the isooctane phase can be obtained, the content was near its initial content in the polishing waste. This is because both of these two powders were extracted from water to the isooctane phase at pH 7–9 simultaneously, which means the mutual separation of these two powders cannot be achieved. On the other hand, when the separation was conducted at pH 3–5, where most of the polished glass was significantly extracted to the isooctane phase whereas most of cerium oxide abrasive was not, the cerium oxide abrasive might be recovered from the water phase. However, the content of cerium oxide abrasive in the solid recovered from the water phase at pH 3–5 was near its initial content and its recovery was relatively low, which suggested that the ideal separation also cannot be achieved. It is considered that the results are caused by the heteroaggregation between the cerium oxide abrasive and glass powder.
According to the zeta potentials shown in
Figure 9, the surface charge levels of the cerium oxide abrasive and the glass powder were positive and negative, respectively, below a pH of 6.2. Consequently, the cerium oxide abrasive aggregated with the glass powder below pH 6.2, owing to electrostatic attraction. This might result in two outcomes. The first one is that the cerium oxide abrasive is extracted to the isooctane phase with the glass powder, leading to a decrease in the recovery of cerium oxide abrasive in the solid recovered from the water phase. The second one is that the extraction of the glass powder is hindered by the cerium oxide abrasive, resulting in a decrease in the content of cerium oxide abrasive in the solid recovered from the water phase. According to the experimental results, the content and recovery of cerium oxide abrasive in the solid recovered from the water phase was near its initial content and its recovery was relatively low at pH 3–5, implying that both outcomes occurred simultaneously. Therefore, these two powders cannot be well separated.
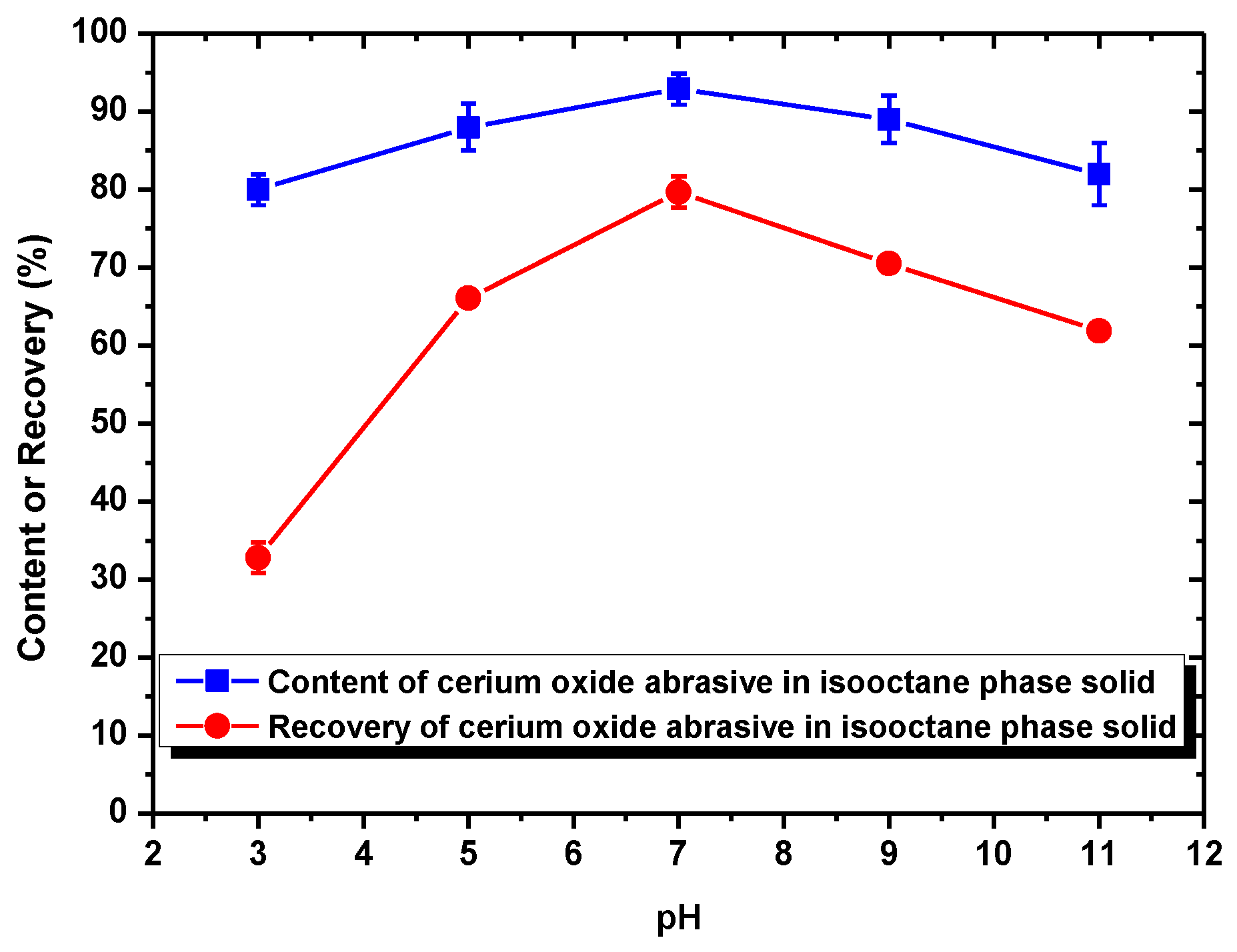
When using NaOL as the surfactant collector, only the extraction of the cerium oxide abrasive could be improved with NaOL addition across a wide pH range, whereas that of glass powder could not. Below the pH of 6.2, the content and recovery of cerium oxide abrasive in the solid recovered from the isooctane phase both decreased. It is considered that the aforementioned two outcomes of heteroaggregation, i.e., the cerium oxide abrasive extracted to the isooctane phase with the glass powder, leading to a decrease in the content of cerium oxide abrasive in the solid recovered from the isooctane phase, as well as the extraction of the cerium oxide abrasive being hindered by the glass powder, resulting in a decrease in the recovery of cerium oxide abrasive in the solid recovered from the isooctane phase, occurred simultaneously. Above a pH of 6.2, the heteroaggregation between the two powders became weak, and only the cerium oxide abrasive was extracted to the isooctane phase; therefore, a higher content and recovery of cerium oxide abrasive in the solid recovered from the isooctane phase was obtained. Above a pH of 7, the cerium oxide abrasive showed lower content and recovery owing to the decrease in its extracted fraction. Therefore, the ideal mutual separation of cerium oxide abrasive and polished glass is accomplished at a pH of 7.
4.3. The Strategy for Recovering Cerium Oxide Abrasive from Abrasive-Glass Polishing Waste
Concerning the mutual separation of cerium oxide abrasive and glass powder in an abrasive-glass polishing powder waste for recovering cerium oxide abrasive, there are two approaches to achieve this goal, applying the liquid–liquid–powder extraction method. The first one is to extract the glass powder to the isooctane phase and leave the cerium oxide abrasive in the water phase. The second one is to extract the cerium oxide abrasive to the isooctane phase and leave the glass powder in the water phase. Actually, an abrasive-glass polishing waste is generated from applying the cerium oxide abrasive to polish the glass component. The polishing ability of cerium oxide abrasive diminishes with the increase in polished glass. In general, the amount of polished glass should be less than that of the cerium oxide abrasive, otherwise the quality of polishing product cannot be ensured. Therefore, the content of the cerium oxide abrasive in a polishing waste is often more than that of the polished glass. Similar to the concept in the froth flotation method, in which the smaller component is floated to the froth, the recovery of cerium oxide abrasive from abrasive-glass polishing waste using the liquid–liquid–powder extraction method can ideally be carried out through extracting the lesser component, i.e., the polished glass, to the isooctane phase and leaving the cerium oxide abrasive in the water phase. Therefore, the aforementioned first approach is preferred, i.e., using a cationic surfactant collector of DAA and conducting the separation at pH 3–5 to selectively improve the extraction of polished glass. The improvement by DAA addition is based on physical electrostatic adsorption with the polished glass. However, the heteroaggregation between cerium oxide abrasive and polished glass occurs at pH 3–5, which leads to the extraction of the cerium oxide abrasive to the isooctane phase with the polished glass, and thus these two powders cannot be well separated. Consequently, the optimal approach for the recovery of cerium oxide abrasive is through the aforementioned second approach, i.e., using an anionic surfactant collector of NaOL and conducting the separation at pH 7 to selectively improve the extraction of cerium oxide abrasive. The improvement by NaOL addition is based on chemical adsorption with the cerium oxide abrasive. In addition, the heteroaggregation between cerium oxide abrasive and polished glass does not occur at pH 7. Only the cerium oxide abrasive is extracted to the isooctane phase, whereas the polished glass remains in the water phase, and therefore good separation and recovery of the cerium oxide abrasive can be accomplished.