Opportunities for Mineral Carbonation in Australia’s Mining Industry
Abstract
1. Introduction
2. Mineral Carbonation as a Route for Reducing CO2 Emission
3. Mineral Carbonation in Australia
4. Drivers of Utilisation of Mineral Carbonation in the Australian Mining Industry
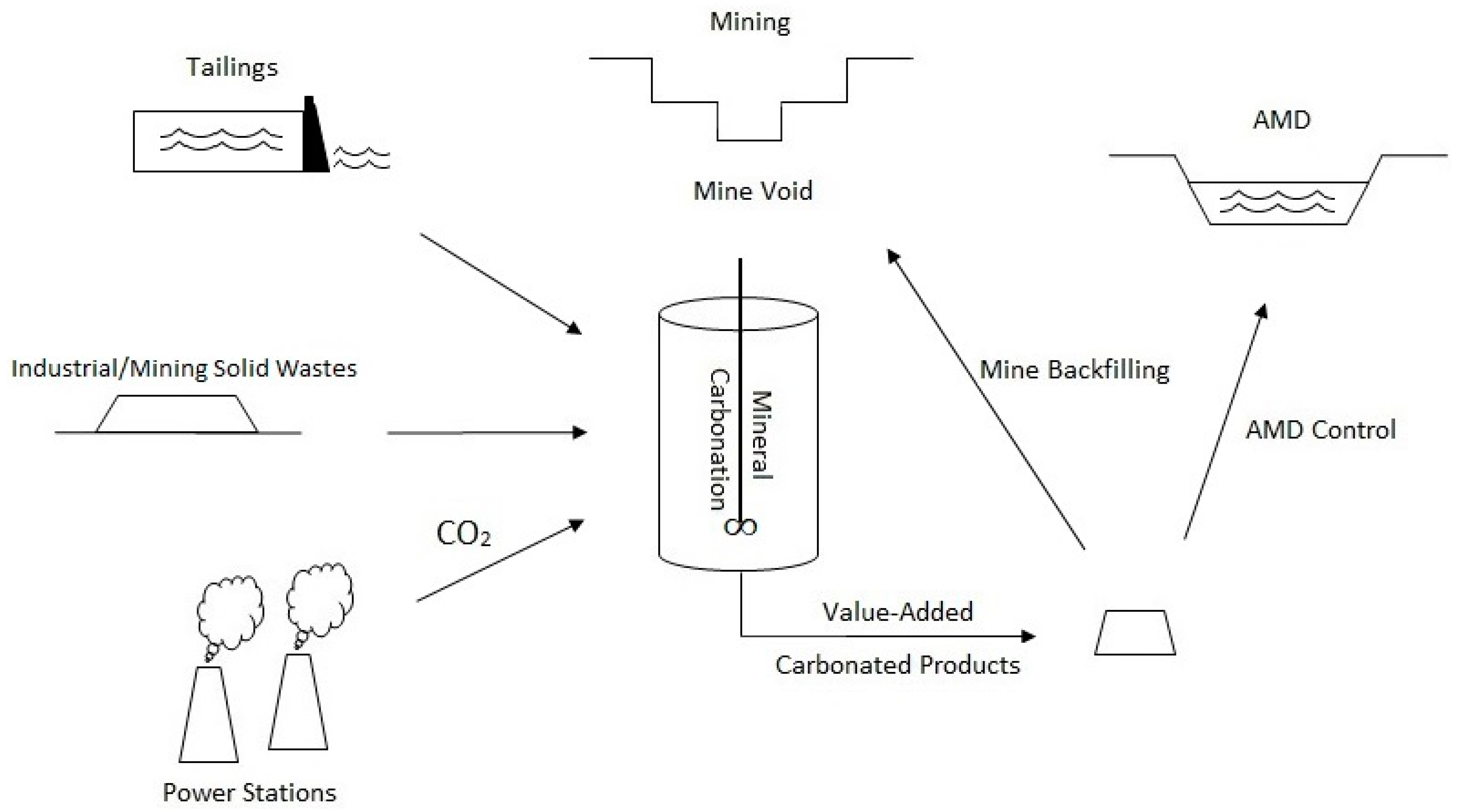
4.1. Integrated Mineral Carbonation Model for Australia’s Mining Industry
4.2. Synergies between Coal Mines and Coal-Fired Power Stations in Close Proximity
4.3. Red Mud
4.4. Ultramafic Mine Tailings
5. Barriers to Utilisation of Integrated Mineral Carbonation
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scoping of the IPCC Sixth Assessment Report (AR6). Background, Cross Cutting Issues and the AR6 Synthesis Report. Available online: https://www.ipcc.ch/site/assets/uploads/2018/04/040820171122-Doc.-6-SYR_Scoping.pdf (accessed on 20 January 2019).
- Trends in Atomospheric Carbon Dioxide. 2018. Available online: https://www.esrl.noaa.gov/gmd/ccgg/trends/data.html (accessed on 20 January 2019).
- Climate Change 2014: Synthesis Report. Summary for Policymaker; Working Groups of the Intergovernmental Panel on Climate Change, IPCC: Geneva, Switzerland, 2014.
- Australian Government, Department of the Environment and Energy. Australia’s 2030 Climate Change Target. 2015. Available online: http://www.environment.gov.au/system/files/resources/c42c11a8-4df7-4d4f-bf92-4f14735c9baa/files/factsheet-australias-2030-climate-change-target.pdf (accessed on 20 January 2019).
- Australian Government, Department of the Environment and Energy. Quarterly Update of Australia’s National Greenhouse Gas Inventory for June 2018. Available online: http://www.environment.gov.au/system/files/resources/e2b0a880-74b9-436b-9ddd-941a74d81fad/files/nggi-quarterly-update-june-2018.pdf (accessed on 20 January 2019).
- SunSHIFT Pty Ltd. Renewable Energy in the Australian Mining Sector; Australian Government White Paper; Australian Renewable Energy Agency: Sydney, Australia, 2017.
- United Nations. The Paris Agreement; United Nations: Paris, France, 2015. [Google Scholar]
- The Parliament of the Commonwealth of Australia. Retirement of Coal Fired Power Stations. Interim Report. 2016. Available online: https://www.aph.gov.au/Parliamentary_Business/Committees/Senate/Environment_and_Communications/Coal_fired_power_stations/Interim_Report (accessed on 20 January 2019).
- Jewell, J.; Cherp, A.; Riahi, K. Energy security under de-carbonization scenarios: An assessment framework and evaluation under different technology and policy choices. Energy Policy 2014, 65, 743–760. [Google Scholar] [CrossRef]
- IEA COP24 Brochure. International Energy Agency at COP24. 2018. Available online: https://www.iea.org/media/topics/climatechange/IEA-COP24-brochure.pdf?utm_campaign=IEA%20newsletters&utm_source=SendGrid&utm_medium=Email (accessed on 20 January 2019).
- International Energy Agency (IEA). Energy Technology Perspectives; OECD/IEA: Paris, France, 2010. [Google Scholar]
- International Energy Agency (IEA). CCS Retrofit, Analysis of the Global Installed Power Plant Fleet; OECD/IEA: Paris, France, 2012. [Google Scholar]
- International Energy Agency (IEA). Sustainable Development Scenario. 2015. Available online: https://www.iea.org/weo/weomodel/sds/ (accessed on 20 January 2019).
- United Nations. The Sustainable Development Goals. 2015. Available online: https://www.un.org/sustainabledevelopment/sustainable-development-goals/ (accessed on 20 January 2019).
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A review of mineral carbonation technologies to sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [CrossRef] [PubMed]
- Bobicki, E.R.; Liu, Q.; Xu, Z.; Zeng, H. Carbon capture and storage using alkaline industrial wastes. Prog. Energy Combust. Sci. 2012, 38, 302–320. [Google Scholar] [CrossRef]
- Bobicki, E.R.; Liu, Q.; Xu, Z. Mineral carbon storage in pre-treated ultramafic ores. Miner. Eng. 2015, 70, 43–54. [Google Scholar] [CrossRef]
- Sarvaramini, A.; Assima, G.P.; Beaudoin, G.; Larachi, F. Biomass torrefaction and CO2 capture using mining wastes—A new approach for reducing greenhouse gas emissions of co-firing plants. Fuel 2014, 115, 749–757. [Google Scholar] [CrossRef]
- Sanna, A.; Dri, M.; Hall, M.R.; Maroto-Valer, M. Waste materials for carbon capture and storage by mineralisation (CCSM)—A UK perspective. Appl. Energy 2012, 99, 545–554. [Google Scholar] [CrossRef]
- Li, J.; Hitch, M.; Power, I.M.; Pan, Y. Integrated mineral carbonation of ultramafic mine deposits—A review. Minerals 2018, 8, 147. [Google Scholar] [CrossRef]
- Araizi, P.-K. Accelerated Carbonation of Wastes and Minerals. Ph.D. Thesis, University of Greenwich, London, UK, 2015. [Google Scholar]
- BHP Sustainability Report; BHP plc: Melbourne, Australia, 2017.
- Glencore Sustainability Report; Glencore plc: Baar, Switzerland, 2017.
- AngloAmerican Sustainability Report; Anglo American plc: London, UK, 2017.
- CCUS Council. Available online: https://www.gov.uk/government/groups/ccus-council (accessed on 20 January 2019).
- Xu, X.; Liu, W.; Chu, G.; Zhang, G.; Luo, D.; Yue, H.; Liang, B.; Li, C. Energy-efficient mineral carbonation of CaSO4 derived from wollastonite via a roasting-leaching route. Hydrometallurgy 2019, 184, 151–161. [Google Scholar] [CrossRef]
- Deng, C.; Liu, W.; Chu, G.; Luo, D.; Zhang, G.; Wang, L.; Yue, H.; Liang, B.; Li, C. Aqueous carbonation of MgSO4 with (NH4)2CO3 for CO2 sequestration. Greenh. Gases Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Wang, F.; Dreisinger, D.; Jarvis, M.; Hitchins, T. Kinetics and mechanism of mineral carbonation of olivine for CO2 sequestration. Miner. Eng. 2019, 131, 185–197. [Google Scholar] [CrossRef]
- Liu, W.; Yin, S.; Luo, D.; Zhang, G.; Yue, H.; Liang, B.; Wang, L.; Li, C. Optimising the recovery of high-value-added ammonium alum during mineral carbonation of blast furnace slag. J. Alloys Compd. 2019, 774, 1151–1159. [Google Scholar] [CrossRef]
- Ragipani, R.; Bhattacharya, S.; Suresh, A.K. Towards efficient calcium extraction from steel slag and carbon dioxide utilisation via pressure-swing mineral carbonation. React. Chem. Eng. 2019, 4, 52–66. [Google Scholar] [CrossRef]
- Rio Tinto Sustainable Development Report; Rio Tinto plc: London, UK, 2017.
- BHP Annual Report; BHP plc: Melbourne, Australia, 2017.
- Glencore Annual Report; Glencore plc: Baar, Switzerland, 2017.
- Nguyen, D. Carbon Dioxide Geological Sequestration: Technical and Economic Reviews; Society of Petroleum Engineers: San Antonio, TX, USA, 2003. [Google Scholar] [CrossRef]
- Ennis-King, J.; Paterson, L. Engineering aspects of geological sequestration of carbon dioxide. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, Melbourne, Australia, 8–10 October 2002. [Google Scholar]
- Holloway, S. Underground sequestration of carbon dioxide—A viable greenhouse gas mitigation option. Energy 2005, 30, 2318–2333. [Google Scholar] [CrossRef]
- Marini, L. Geological Sequestration of Carbon Dioxide: Thermodynamics, Kinetics, and Reaction Path Modeling; Elsevier: Amsterdam, The Netherlands, 2006; Volume 11. [Google Scholar]
- Wilson, T. The deep ocean disposal of carbon dioxide. Energy Convers. Manag. 1992, 33, 627–633. [Google Scholar] [CrossRef]
- Handa, N.; Ohsumi, T. Direct ocean Disposal of Carbon Dioxide; Terrapub: Tokyo, Japan, 1995. [Google Scholar]
- Brewer, P.G.; Friederich, G.; Peltzer, E.T.; Orr, F.M. Direct experiments on the ocean disposal of fossil fuel CO2. Science 1999, 284, 943–945. [Google Scholar] [CrossRef] [PubMed]
- Palmgren, C.R.; Morgan, M.G.; Bruine de Bruin, W.; Keith, D.W. Initial Public Perceptions of Deep Geological and Oceanic Disposal of Carbon Dioxide. Environ. Sci. Technol. 2004, 38, 6441–6450. [Google Scholar] [CrossRef] [PubMed]
- Usui, N.; Ikenouchi, M. The biological CO2 fixation and utilization project by RITE (1)—Highly-effective photobioreactor system—. Energy Convers. Manag. 1997, 38, S487–S492. [Google Scholar] [CrossRef]
- Yun, Y.S.; Lee, S.B.; Park, J.M.; Lee, C.I.; Yang, J.W. Carbon dioxide fixation by algal cultivation using wastewater nutrients. J. Chem. Technol. Biotechnol. 1997, 69, 451–455. [Google Scholar] [CrossRef]
- Aresta, M. Carbon Dioxide as Chemical Feedstock; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Park, A.-H.A. Carbon Dioxide Sequestration: Chemical and Physical Activation of Aqueous Carbonation of Mg-Bearing Minerals and pH Swing Process. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2005. [Google Scholar]
- Werner, M.S. Combined CO2 Capture and Storage by Mineralization: Dissolution and Carbonation of Activated Serpentine. Ph.D. Thesis, ETH Zurich, Zürich, Switzerland, 2014. [Google Scholar]
- Lackner, K.S.; Wendt, C.H.; Butt, D.P.; Joyce, E.L., Jr.; Sharp, D.H. Carbon dioxide disposal in carbonate minerals. Energy 1995, 20, 1153–1170. [Google Scholar] [CrossRef]
- Metz, B.; Davidson, O.; De Coninck, H. Carbon Dioxide Capture and Storage: Special Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Loughnan, F.C. Chemical Weathering of the Silicate Minerals; Elsevier: New York, NY, USA, 1969; p. 154. [Google Scholar]
- Garrels, R.M. The carbonate-silicate geochemical cycle and its effect on atmospheric carbon dioxide over the past 100 million years. Am. J. Sci. 1983, 283, 641–683. [Google Scholar]
- Möller, P. Magnesite: Geology, Mineralogy, Geochemistry, Formation of Mg-Carbonates; Gebrüder Borntraeger: Berlin, Germany, 1989. [Google Scholar]
- Seifritz, W. CO2 disposal by means of silicates. Nature 1990, 345, 486. [Google Scholar] [CrossRef]
- Hasan, S.N.M.S.; Kusin, F.M.; Jusop, S.; Yusuff, F.M. Potential of soil, sludge and sediment for mineral carbonation process in selinsing gold mine, Malaysia. Minerals 2018, 8, 257. [Google Scholar] [CrossRef]
- Lackner, K.S. Carbonate chemistry for sequestering fossil carbon. Annu. Rev. Energy Environ. 2002, 27, 193–232. [Google Scholar] [CrossRef]
- Goff, F.; Lackner, K. Carbon dioxide sequestering using ultramafic rocks. Environ. Geosci. 1998, 5, 89–101. [Google Scholar] [CrossRef]
- Zafaranloo, A. Kinetics of Magnesium Extraction from Activated Serpentine by Carbonic Acid. Ph.D. Thesis, University of Sydney, Sydney, Australia, 2015. [Google Scholar]
- O’Connor, W.; Dahlin, D.; Rush, G.; Gerdemann, S.; Penner, L.; Nilsen, D. Aqueous Mineral Carbonation: Mineral Availability, Pretreatment, Reaction Parametrics, and Process Studies; Office of Process Development, National Energy Technology Laboratory (Formerly Albany Research Center) Office of Fossil Energy, US DOE: Albany, OR, USA, 2005; p. 462.
- Brent, G.F.; Petrie, J.G. CO2 sequestration by mineral carbonation in the Australian context. In Proceedings of the Chemeca 2008: Towards a Sustainable Australasia, Newcastle, Australia, 28 September–1 October 2008; p. 1273. [Google Scholar]
- Lackner, K.S.; Butt, D.P.; Wendt, C.H. Progress on binding CO2 in mineral substrates. Energy Convers. Manag. 1997, 38, S259–S264. [Google Scholar] [CrossRef]
- Fagerlund, J. Carbonation of Mg(OH)2 in a Pressurised Fluidised Bed for CO2 Sequestration. Ph.D Thesis, Åbo Akademi University, Turku, Finland, 2012. [Google Scholar]
- Teir, S.; Revitzer, H.; Eloneva, S.; Fogelholm, C.-J.; Zevenhoven, R. Dissolution of natural serpentinite in mineral and organic acids. Int. J. Miner. Process. 2007, 83, 36–46. [Google Scholar] [CrossRef]
- Drief, A.; Nieto, F. The effect of dry grinding on antigorite from Mulhacen, Spain. Clays Clay Miner. 1999, 47, 417–424. [Google Scholar] [CrossRef]
- Kim, D.-J.; Chung, H.-S. Effect of grinding on the structure and chemical extraction of metals from serpentine. Part. Sci. Technol. 2002, 20, 159–168. [Google Scholar] [CrossRef]
- Van Essendelft, D.T.; Schobert, H.H. Kinetics of the Acid Digestion of Serpentine with Concurrent Grinding. 3. Model Validation and Prediction. Ind. Eng. Chem. Res. 2010, 49, 1588–1590. [Google Scholar] [CrossRef]
- Kleiv, R.A.; Thornhill, M. The effect of mechanical activation in the production of olivine surface area. Miner. Eng. 2016, 89, 19–23. [Google Scholar] [CrossRef]
- Rashid, M.I.; Benhelal, E.; Farhang, F.; Oliver, T.K.; Rayson, M.S.; Brent, G.F.; Stockenhuber, M.; Kennedy, E.M. Development of Concurrent grinding for application in aqueous mineral carbonation. J. Clean. Prod. 2019, 212, 151–161. [Google Scholar] [CrossRef]
- Balucan, R.D.; Dlugogorski, B.Z. Thermal Activation of Antigorite for Mineralization of CO2. Environ. Sci. Technol. 2013, 47, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Balucan, R.D.; Kennedy, E.M.; Mackie, J.F.; Dlugogorski, B.Z. Optimization of antigorite heat pre-treatment via kinetic modeling of the dehydroxylation reaction for CO2 mineralization. Greenh. Gases Sci. Technol. 2011, 1, 294–304. [Google Scholar] [CrossRef]
- Farhang, F.; Kennedy, E.; Stockenhuber, M.; Brent, G.; Rayson, M. Dehydroxylation of Magnesium Silicate Minerals for Carbonation. WIPO Patent No.: WO/2017/106929, 29 June 2017. [Google Scholar]
- Brindley, G.W.; Hayami, R. Kinetics and mechanisms of dehydration and recrystallization of serpentine: II Spectrum of activation energies for recrystallization. In Proceedings of the 12th National Conference on Clays and Clay Minerals, Atlanta, GA, USA, 30 September–2 October 1963; pp. 49–54. [Google Scholar]
- Mann, J.P. Serpentine Activation for CO2 Sequestration. Ph.D. Thesis, University of Sydney, Faculty of Engineering & IT, School of Chemical & Biomolecular Engineering, Sydney, Australia, 2014. [Google Scholar]
- Maroto-Valer, M.M.; Fauth, D.J.; Kuchta, M.E.; Zhang, Y.; Andrésen, J.M. Activation of magnesium rich minerals as carbonation feedstock materials for CO2 sequestration. Fuel Process. Technol. 2005, 86, 1627–1645. [Google Scholar] [CrossRef]
- McKelvy, M.J.; Chizmeshya, A.V.G.; Diefenbacher, J.; Béarat, H.; Wolf, G. Exploration of the Role of Heat Activation in Enhancing Serpentine Carbon Sequestration Reactions. Environ. Sci. Technol. 2004, 38, 6897–6903. [Google Scholar] [CrossRef] [PubMed]
- Daval, D.; Hellmann, R.; Martinez, I.; Gangloff, S.; Guyot, F. Lizardite serpentine dissolution kinetics as a function of pH and temperature, including effects of elevated pCO2. Chem. Geol. 2013, 351, 245–256. [Google Scholar] [CrossRef]
- Prigiobbe, V.; Hänchen, M.; Costa, G.; Baciocchi, R.; Mazzotti, M. Analysis of the effect of temperature, pH, CO2 pressure and salinity on the olivine dissolution kinetics. Energy Procedia 2009, 1, 4881–4884. [Google Scholar] [CrossRef]
- Felmy, A.R.; Qafoku, O.; Arey, B.W.; Hu, J.Z.; Hu, M.; Todd Schaef, H.; Ilton, E.S.; Hess, N.J.; Pearce, C.I.; Feng, J.; et al. Reaction of water-saturated supercritical CO2 with forsterite: Evidence for magnesite formation at low temperatures. Geochim. Cosmochim. Acta 2012, 91, 271–282. [Google Scholar] [CrossRef]
- Hanchen, M.; Prigiobbe, V.; Baciocchi, R.; Mazzotti, M. Precipitation in the Mg-carbonate system—Effects of temperature and CO2 pressure. Chem. Eng. Sci. 2008, 63, 1012–1028. [Google Scholar] [CrossRef]
- Bonfils, B.; Julcour-Lebigue, C.; Guyot, F.; Bodénan, F.; Chiquet, P.; Bourgeois, F. Comprehensive analysis of direct aqueous mineral carbonation using dissolution enhancing organic additives. Int. J. Greenh. Gas Control 2012, 9, 334–346. [Google Scholar] [CrossRef]
- Swanson, E.J. Catalytic Enhancement of Silicate Mineral Weathering for Direct Carbon Capture and Storage. Ph.D. Thesis, Columbia University, New York City, NY, USA, 2014. [Google Scholar]
- Park, A.-H.A.; Fan, L.-S. CO2 mineral sequestration: Physically activated dissolution of serpentine and pH swing process. Chem. Eng. Sci. 2004, 59, 5241–5247. [Google Scholar] [CrossRef]
- Werner, M.; Hariharan, S.; Mazzotti, M. Flue gas CO2 mineralization using thermally activated serpentine: From single-to double-step carbonation. Phys. Chem. Chem. Phys. 2014, 16, 24978–24993. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y. Research and Education of CO2 Separation from Coal Combustion Flue Gases with Regenerable Magnesium Solutions; Chemical Engineering Program Department of Biomedical, Chemical, and Environmental Engineering, University of Cincinnati: Cincinnati, OH, USA, 2013. [Google Scholar]
- Huijgen, W.J.J.; Comans, R.N.J. Mineral CO2 Sequestration by Steel Slag Carbonation. Environ. Sci. Technol. 2005, 39, 9676–9682. [Google Scholar] [CrossRef] [PubMed]
- Baciocchi, R.; Costa, G.; Di Gianfilippo, M.; Polettini, A.; Pomi, R.; Stramazzo, A. Thin-film versus slurry-phase carbonation of steel slag: CO2 uptake and effects on mineralogy. J. Hazard. Mater. 2015, 283, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Huijgen, W.J.J.; Comans, R.N.J. Carbonation of Steel Slag for CO2 Sequestration: Leaching of Products and Reaction Mechanisms. Environ. Sci. Technol. 2006, 40, 2790–2796. [Google Scholar] [CrossRef] [PubMed]
- Morone, M.; Costa, G.; Polettini, A.; Pomi, R.; Baciocchi, R. Valorization of steel slag by a combined carbonation and granulation treatment. Miner. Eng. 2014, 59, 82–90. [Google Scholar] [CrossRef]
- Librandi, P.; Costa, G.; Souza, A.C.B.D.; Stendardo, S.; Luna, A.S.; Baciocchi, R. Carbonation of Steel Slag: Testing of the Wet Route in a Pilot-scale Reactor. Energy Procedia 2017, 114, 5381–5392. [Google Scholar] [CrossRef]
- Montes-Hernandez, G.; Pérez-López, R.; Renard, F.; Nieto, J.M.; Charlet, L. Mineral sequestration of CO2 by aqueous carbonation of coal combustion fly-ash. J. Hazard. Mater. 2009, 161, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Dindi, A.; Quang, D.V.; Vega, L.F.; Nashef, E.; Abu-Zahra, M.R.M. Applications of fly ash for CO2 capture, utilization, and storage. J. CO2 Util. 2019, 29, 82–102. [Google Scholar] [CrossRef]
- Ji, L.; Yu, H.; Yu, B.; Zhang, R.; French, D.; Grigore, M.; Wang, X.; Chen, Z.; Zhao, S. Insights into Carbonation Kinetics of Fly Ash from Victorian Lignite for CO2 Sequestration. Energy Fuels 2018, 32, 4569–4578. [Google Scholar] [CrossRef]
- Jacobs, A.D. Quantifying the Mineral Carbonation Potential of Mine Waste Material: A New Parameter for Geospatial Estimation. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2014. [Google Scholar]
- Hitch, M.; Ballantyne, S.M.; Hindle, S.R. Revaluing mine waste rock for carbon capture and storage. Int. J. Min. Reclam. Environ. 2010, 24, 64–79. [Google Scholar] [CrossRef]
- Alcoa. Long Term Residue Management Strategy. Alcoa Australia Aluminium Kwainana 2012 Report. Available online: https://www.alcoa.com/australia/en/pdf/kwinana_refinery_ltrms_report_2012.pdf (accessed on 20 January 2019).
- Brent, G.F.; Allen, D.J.; Eichler, B.R.; Petrie, J.G.; Mann, J.P.; Haynes, B.S. Mineral Carbonation as the Core of an Industrial Symbiosis for Energy-Intensive Minerals Conversion. J. Ind. Ecol. 2012, 16, 94–104. [Google Scholar] [CrossRef]
- Farhang, F.; Oliver, T.K.; Rayson, M.; Brent, G.; Stockenhuber, M.; Kennedy, E. Experimental study on the precipitation of magnesite from thermally activated serpentine for CO2 sequestration. Chem. Eng. J. 2016, 303, 349–449. [Google Scholar] [CrossRef]
- Farhang, F.; Rayson, M.; Brent, G.; Hodgins, T.; Stockenhuber, M.; Kennedy, E. Insights into the dissolution kinetics of thermally activated serpentine for CO2 sequestration. Chem. Eng. J. 2017, 330, 1174–1186. [Google Scholar] [CrossRef]
- Oliver, T.K.; Farhang, F.; Hodgins, T.W.; Rayson, M.S.; Brent, G.F.; Molloy, T.S.; Stockenhuber, M.; Kennedy, E.M. CO2 Capture Modeling Using Heat-Activated Serpentinite Slurries. Energy Fuels 2018. [Google Scholar] [CrossRef]
- Rashid, M.I.; Benhelal, E.; Farhang, F.; Oliver, T.K.; Rayson, M.S.; Brent, G.F.; Stockenhuber, M.; Kennedy, E.M. ACEME: Direct Aqueous Mineral Carbonation of Dunite Rock. Environ. Prog. Sustain. Energy 2018. [Google Scholar] [CrossRef]
- Benhelal, E.; Rashid, M.I.; Holt, C.; Rayson, M.S.; Brent, G.; Hook, J.M.; Stockenhuber, M.; Kennedy, E.M. The utilisation of feed and byproducts of mineral carbonation processes as pozzolanic cement replacements. J. Clean. Prod. 2018, 186, 499–513. [Google Scholar] [CrossRef]
- Farhang, F.; Oliver, T.K.; Rayson, M.S.; Brent, G.F.; Molloy, T.S.; Stockenhuber, M.; Kennedy, E.M. Dissolution of heat activated serpentine for CO2 sequestration: The effect of silica precipitation at different temperature and pH values. J. CO2 Util. 2019, 30, 123–129. [Google Scholar] [CrossRef]
- Benhelal, E.; Rashid, M.I.; Rayson, M.S.; Prigge, J.-D.; Molloy, S.; Brent, G.F.; Cote, A.; Stockenhuber, M.; Kennedy, E.M. Study on mineral carbonation of heat activated lizardite at pilot and laboratory scale. J. CO2 Util. 2018, 26, 230–238. [Google Scholar] [CrossRef]
- Brent, G.; Rayson, M.; Kennedy, E.; Stockenhuber, M.; Collins, W.; Prigge, J.; Hynes, R.; Molloy, S.; Zulfiqar, H.; Farhang, F.; et al. Mineral carbonation of serpentinite: From the laboratory to pilot scale—The MCi Project. In Proceedings of the 5th International Conference on Accelerated Carbonation for Environmental and Material Engineering ACEME, New York, NY, USA, 21–24 January 2015. [Google Scholar]
- McCutcheon, J.; Wilson, S.A.; Southam, G. Microbially Accelerated Carbonate Mineral Precipitation as a Strategy for in Situ Carbon Sequestration and Rehabilitation of Asbestos Mine Sites. Environ. Sci. Technol. 2016, 50, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, J.; Dipple, G.M.; Wilson, S.A.; Southam, G. Production of magnesium-rich solutions by acid leaching of chrysotile: A precursor to field-scale deployment of microbially enabled carbonate mineral precipitation. Chem. Geol. 2015, 413, 119–131. [Google Scholar] [CrossRef]
- Hamilton, J.L.; Wilson, S.A.; Morgan, B.; Turvey, C.C.; Paterson, D.J.; MacRae, C.; McCutcheon, J.; Southam, G. Nesquehonite sequesters transition metals and CO2 during accelerated carbon mineralisation. Int. J. Greenh. Gas Control 2016, 55, 73–81. [Google Scholar] [CrossRef]
- Turvey, C.C.; Wilson, S.A.; Hamilton, J.L.; Tait, A.W.; McCutcheon, J.; Beinlich, A.; Fallon, S.J.; Dipple, G.M.; Southam, G. Hydrotalcites and hydrated Mg-carbonates as carbon sinks in serpentinite mineral wastes from the Woodsreef chrysotile mine, New South Wales, Australia: Controls on carbonate mineralogy and efficiency of CO2 air capture in mine tailings. Int. J. Greenh. Gas Control 2018, 79, 38–60. [Google Scholar] [CrossRef]
- Hamilton, J.L.; Wilson, S.A.; Morgan, B.; Turvey, C.C.; Paterson, D.J.; Jowitt, S.M.; McCutcheon, J.; Southam, G. Fate of transition metals during passive carbonation of ultramafic mine tailings via air capture with potential for metal resource recovery. Int. J. Greenh. Gas Control 2018, 71, 155–167. [Google Scholar] [CrossRef]
- Steel, K.M.; Alizadehhesari, K.; Balucan, R.D.; Bašić, B. Conversion of CO2 into mineral carbonates using a regenerable buffer to control solution pH. Fuel 2013, 111, 40–47. [Google Scholar] [CrossRef]
- Steel, K.; Alizadehhesari, K.; Fox, K.; Balucan, R. Potential for CO2 sequestration as mineral carbonate within Ni laterite processing. In Proceedings of the ALTA 2013: Nickel-Cobalt-Copper, Uranium-REE and Gold Conference & Exhibition, Perth, Australia, 25 May–1 June 2013; pp. 424–431. [Google Scholar]
- Morgan, B.; Wilson, S.A.; Madsen, I.C.; Gozukara, Y.M.; Habsuda, J. Increased thermal stability of nesquehonite (MgCO3·3H2O) in the presence of humidity and CO2: Implications for low-temperature CO2 storage. Int. J. Greenh. Gas Control 2015, 39, 366–376. [Google Scholar] [CrossRef]
- Australian Government, Geoscience Australia. Australia’s Identified Mineral Resources. 2017. Available online: http://www.ga.gov.au/__data/assets/pdf_file/0005/58874/Australias-Identified-Mineral-Resources-2017.pdf (accessed on 20 January 2019).
- Burgess, J.; Jefery, L.; Lowe, A.; Schuck, S.; Flentje, W. Novel CO2 Capture Task Force Report; Global CCS Institute: Melbourne, Australia, 2011. [Google Scholar]
- Kemache, N.; Pasquier, L.-C.; Cecchi, E.; Mouedhen, I.; Blais, J.-F.; Mercier, G. Aqueous mineral carbonation for CO2 sequestration: From laboratory to pilot scale. Fuel Process. Technol. 2017, 166, 209–216. [Google Scholar] [CrossRef]
- Coelho, P.C.S.; Teixeira, J.P.F.; Gonçalves, O.N.B.S.M. Mining Activities: Health Impacts. In Encyclopedia of Environmental Health; Nriagu, J.O., Ed.; Elsevier: Burlington, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Mining Report Finds 60,000 Abandoned Sites, Lack of Rehabilitation and Unreliable Data. 2017. Available online: https://www.abc.net.au/news/2017-02-15/australia-institute-report-raises-concerns-on-mine-rehab/8270558 (accessed on 20 January 2019).
- The Parliament of the Commonwealth of Australia. Between a Rock and a Hard Place the Science of Geosequestration. 2007. Available online: https://www.aph.gov.au/Parliamentary_Business/Committees/House_of_Representatives_Committees?url=scin/geosequestration/report.htm (accessed on 20 January 2019).
- Heidrich, C.; Feuerborn, H.-J.; Weir, A. Coal Combustion Products: A Global Perspective. In Proceedings of the 2013 World of Coal Ash (WOCA) Conference, Lexington, KY, USA, 22–25 April 2013. [Google Scholar]
- The Australian Government, Department of the Environment and Energy. National Waste Report. 2018. Available online: http://www.environment.gov.au/system/files/resources/7381c1de-31d0-429b-912c-91a6dbc83af7/files/national-waste-report-2018.pdf (accessed on 20 January 2019).
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Stanwell Corporation. 2018. Available online: https://www.stanwell.com/energy-assets/our-power-stations/coal/ (accessed on 20 January 2019).
- USGS. Mineral Commodity Summaries; U.S. Department of the Interior U.S. Geological Survey: Reston, VA, USA, 2018.
- Gelencsér, A.; Kováts, N.; Turóczi, B.; Rostási, A.; Hoffer, A.; Imre, K.; Nyiró-Kósa, I.; Csákberényi-Malasics, D.; Tóth, A.; Czitrovszky, A.; et al. The red mud accident in Ajka (Hungary): Characterization and potential health effects of fugitive dust. Environ. Sci. Technol. 2011, 45, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Ruyters, S.; Mertens, J.; Vassilieva, E.; Dehandschutter, B.; Poffijn, A.; Smolders, E. The red mud accident in Ajka (Hungary): Plant toxicity and trace metal bioavailability in red mud contaminated soil. Environ. Sci. Technol. 2011, 45, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Winkler, D.; Bidló, A.; Bolodár-Varga, B.; Erdő, Á.; Horváth, A. Long-term ecological effects of the red mud disaster in Hungary: Regeneration of red mud flooded areas in a contaminated industrial region. Sci. Total Environ. 2018, 644, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.P. Red Mud Minimisation and Management for the Alumina Industry by the Carbonation Method. Ph.D. Thesis, The University of Adelaide, Adelaide, Australia, 2016. [Google Scholar]
- Lu, G.; Zhang, T.A.; Zhu, X.; Liu, Y.; Wang, Y.; Guo, F.; Zhao, Q.; Zheng, C. Calcification–carbonation Method for Cleaner Alumina Production and CO2 Utilization. JOM 2014, 66, 1616–1621. [Google Scholar] [CrossRef]
- Ahn, J.W.; Thriveni, T.; Nam, S.Y. Sustainable recycling technologies for bauxite residue (red mud) utilization. In Energy Technology 2015: Carbon Dioxide Management and Other Technologies; Springer International Publishing: Basel, Switzerland, 2016. [Google Scholar] [CrossRef]
- Carter, C.M.; Van Der Sloot, H.A.; Cooling, D.; Van Zomeren, A.; Matheson, T. Characterization of untreated and neutralized bauxite residue for improved waste management. Environ. Eng. Sci. 2008, 25, 475–488. [Google Scholar] [CrossRef]
- Nikraz, H.R.; Bodley, A.J.; Cooling, D.J.; Kong, P.Y.; Soomro, M. Comparison of physical properties between treated and untreated bauxite residue mud. J. Mater. Civ. Eng. 2007, 19, 2–9. [Google Scholar] [CrossRef]
- Cooling, D.J.; Hay, P.S.; Guilfoyle, L. Carbonation of Bauxite Residue. In Proceedings of the 6th International Alumina Quality Workshop, Brisbane, Australia, 8–13 September 2002. [Google Scholar]
- Stringer, D. World’s Top Miner Charges into Cobalt. 2018. Available online: https://www.bloomberg.com/news/articles/2018-08-06/world-s-top-miner-charges-into-cobalt-as-battery-ambitions-grow (accessed on 20 January 2019).
- Mao, F. Wittenoom: Tourists Urged to Stay Away from Asbestos Town. 2018. Available online: https://www.bbc.com/news/world-australia-44816907 (accessed on 20 January 2019).
- Revathy, T.D.R.; Palanivelu, K.; Ramachandran, A. Sequestration of Carbon dioxide by red mud through direct mineral carbonation at room temperature. Int. J. Glob. Warm. 2017, 11, 23–37. [Google Scholar] [CrossRef]
- Liang, G.; Chen, W.; Nguyen, A.V.; Nguyen, T.A.H. Red mud carbonation using carbon dioxide: Effects of carbonate and calcium ions on goethite surface properties and settling. J. Colloid Interface Sci. 2018, 517, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.C.; Patel, R.K.; Ray, B.C. Neutralization of red mud using CO2 sequestration cycle. J. Hazard. Mater. 2010, 179, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Su, S.; Xu, K.; Chen, Q.; Xu, J.; Sun, Z.; Wang, Y.; Hu, S.; Wang, X.; Xue, Y.; et al. CO2 sequestration by direct gas–solid carbonation of fly ash with steam addition. J. Clean. Prod. 2018, 178, 98–107. [Google Scholar] [CrossRef]
- Jaschik, J.; Jaschik, M.; Warmuzinski, K. The utilisation of fly ash in CO2 mineral carbonation. Chem. Process Eng. Inzynieria Chemiczna i Procesowa 2016, 37, 29–39. [Google Scholar] [CrossRef]
- Tamilselvi Dananjayan, R.R.; Kandasamy, P.; Andimuthu, R. Direct mineral carbonation of coal fly ash for CO2 sequestration. J. Clean. Prod. 2016, 112, 4173–4182. [Google Scholar] [CrossRef]
- Ukwattage, N.L.; Ranjith, P.G.; Yellishetty, M.; Bui, H.H.; Xu, T. A laboratory-scale study of the aqueous mineral carbonation of coal fly ash for CO2 sequestration. J. Clean. Prod. 2015, 103, 665–674. [Google Scholar] [CrossRef]
- Teir, S.; Kuusik, R.; Fogelholm, C.J.; Zevenhoven, R. Production of magnesium carbonates from serpentinite for long-term storage of CO2. Int. J. Miner. Process. 2007, 85, 1–15. [Google Scholar] [CrossRef]
- Teir, S.; Eloneva, S.; Fogelholm, C.J.; Zevenhoven, R. Fixation of carbon dioxide by producing hydromagnesite from serpentinite. Appl. Energy 2009, 86, 214–218. [Google Scholar] [CrossRef]
- Mervine, E.M.; Wilson, S.A.; Power, I.M.; Dipple, G.M.; Turvey, C.C.; Hamilton, J.L.; Vanderzee, S.; Raudsepp, M.; Southam, C.; Matter, J.M.; et al. Potential for offsetting diamond mine carbon emissions through mineral carbonation of processed kimberlite: An assessment of De Beers mine sites in South Africa and Canada. Mineral. Petrol. 2018, 112, 755–765. [Google Scholar] [CrossRef]
- Meyer, N.A.; Vögeli, J.U.; Becker, M.; Broadhurst, J.L.; Reid, D.L.; Franzidis, J.P. Mineral carbonation of PGM mine tailings for CO2 storage in South Africa: A case study. Miner. Eng. 2014, 59, 45–51. [Google Scholar] [CrossRef]
- Vogeli, J.; Reid, D.L.; Becker, M.; Broadhurst, J.; Franzidis, J.P. Investigation of the potential for mineral carbonation of PGM tailings in South Africa. Miner. Eng. 2011, 24, 1348–1356. [Google Scholar] [CrossRef]
- McCutcheon, J.; Turvey, C.C.; Wilson, S.A.; Hamilton, J.L.; Southam, G. Experimental deployment of microbial mineral carbonation at an asbestos mine: Potential applications to carbon storage and tailings stabilization. Minerals 2017, 7, 191. [Google Scholar] [CrossRef]
- Oskierski, H.C.; Dlugogorski, B.Z.; Oliver, T.K.; Jacobsen, G. Chemical and isotopic signatures of waters associated with the carbonation of ultramafic mine tailings, Woodsreef Asbestos Mine, Australia. Chem. Geol. 2016, 436, 11–23. [Google Scholar] [CrossRef]
- Newall, P. CO2 Storage as Carbonate Minerals; IEA Greenhouse R&D Programme Report; Report Number PH3/17; CSMA Consultants: Cheltenham, UK, 2000. [Google Scholar]
- Penner, L.; O’Connor, W.K.; Dahlin, D.C.; Gerdemann, S.; Rush, G.E. Mineral carbonation: Energy costs of pretreatment options and insights gained from flow loop reaction studies. In Proceedings of the Third Annual Conference on Carbon Capture & Sequestration, Alexandria, VA, USA, 3–6 May 2004. [Google Scholar]
- Gerdemann, S.J.; O’Connor, W.K.; Dahlin, D.C.; Penner, L.R.; Rush, H. Ex Situ Aqueous Mineral Carbonation. Environ. Sci. Technol. 2007, 41, 2587–2593. [Google Scholar] [CrossRef] [PubMed]
- Huijgen, W.J.J.; Comans, R.N.J. Carbon Dioxide Sequestration by Mineral Carbonation. Literature Review; ECN-C-03-016; Energy research Centre of the Netherlands ECN: Petten, The Netherlands, 2003. [Google Scholar]
- Huijgen, W.J.J.; Comans, R.N.J.; Witkamp, G.-J. Cost evaluation of CO2 sequestration by aqueous mineral carbonation. Energy Convers. Manag. 2007, 48, 1923–1935. [Google Scholar] [CrossRef]
- Haug, T.A.; Kleiv, R.A.; Munz, I.A. Investigating dissolution of mechanically activated olivine for carbonation purposes. Appl. Geochem. 2010, 25, 1547–1563. [Google Scholar] [CrossRef]
- Geerlings, J.J.C.; Wesker, E. Process for Sequestration of Carbon Dioxide by Mineral Carbonation. U.S. Patent 7722850, 25 May 2010. [Google Scholar]
- Park, A.-H.A.; Jadhav, R.; Fan, L.-S. CO2 mineral sequestration: Chemically enhanced aqueous carbonation of serpentine. Can. J. Chem. Eng. 2003, 81, 885–890. [Google Scholar] [CrossRef]
- Daval, D.; Sissmann, O.; Menguy, N.; Saldi, G.D.; Guyot, F.; Martinez, I.; Corvisier, J.; Garcia, B.; Machouk, I.; Knauss, K.G.; et al. Influence of amorphous silica layer formation on the dissolution rate of olivine at 90 °C and elevated pCO2. Chem. Geol. 2011, 284, 193–209. [Google Scholar] [CrossRef]
- Julcour, C.; Bourgeois, F.; Bonfils, B.; Benhamed, I.; Guyot, F.; Bodenan, F.; Petiot, C.; Gaucher, E.C. Development of an attrition-leaching hybrid process for direct aqueous mineral carbonation. Chem. Eng. J. 2015, 262, 716–726. [Google Scholar] [CrossRef]
- Carbon Dioxide Storage by Mineral Carbonation; IEA Greenhouse R&D Programme Report; Report Number 2005/11; IEA Greenhouse Gas R&D Programme: Cheltenham, UK, 2005.

| Strategy | Reference | Method | Benefit |
|---|---|---|---|
| Mechanical pretreatment of serpentine rock | [62,63,64,65,66] | High-energy crushing and grinding | Increases the overall specific surface area of the minerals, i.e., the surface per unit mass available for Mg/Ca-extraction |
| Thermal activation of the hydrated Mg-silicate serpentine | [67,68,69,70,71,72,73] | Heating the mineral to 630 °C or above | Destabilises the crystal lattice, thus increasing the reactivity of the mineral Heat activation also creates an even higher specific surface area [46] |
| Increasing reaction temperatures | [74,75] | Applying operating temperatures above 100 °C | Accelerate the kinetics of the reaction |
| Increasing reaction pressure | [76,77] | Applying operating CO2 pressure above 150 bar | Increases the activity of protons needed for metal extraction. Also counteracts the low solubility of CO2 at high temperature |
| Using organic and inorganic chemicals | [61,78,79,80] | Adding different substances as catalysts and additives to the reactants | Enhances the kinetics of the carbonation process and precipitation of magnesium carbonate |
| Applying New double-step and multistep process designs | [81] | The operating conditions are changed between the different stages | Allows to control and promote extraction and precipitation separately |
| Direct capture of CO2 from flue gas stream | [81,82] | Capturing CO2 directly from a flue gas stream by mineral carbonation | Provides a simple and straightforward process route |
| Institute/Industry | Research Focus | Goal |
|---|---|---|
| Alcoa’s Kwinana Aluminium Refinery | Bauxite residue treatment | To reduce long-term storage risk and adverse environmental effects |
| Mineral Carbonation International (MCi) | Carbonation of serpentine as an abundant mineral in New South Wales (NSW), Australia and globally | integration with power generation and CO2 capture processes, carbonation, value-adding and final product storage |
| The University of Queensland | field studies | use of waste materials and ultramafic mine tailings as feedstock |
| Monash University | field studies | use of waste materials and ultramafic mine tailings as feedstock |
| Australia’s Mineral Resources (Alphabetic Order) | Can Be Found in Mafic-Ultramafic Ores | Mineral Carbonation Potential Elements in Waste Products | Examples of Mineral Phases Prone to Carbonation | Australia’s World Ranking for Resources | % of World Resources | Australia’s World Ranking for Production | % of World Production | Australia’s Production (Mt) | World’s Production (Mt) |
|---|---|---|---|---|---|---|---|---|---|
| Antimony | 4 | 9 | 4 | 4 | 5.5 (kt) | 130 (kt) | |||
| Bauxite | Ca, Mg, Fe, Na | Magnetite (Fe3O4), Sodium aluminosilicate (Na(AlSiO4)) | 2 | 22 | 1 | 31 | 82.152 | 271.5 | |
| Black Coal | Ca, Mg | Hydrocalumite (Ca2Al(OH)6Cl·2H2O), Ettringite (Ca6Al2(SO4)3(OH)12·26H2O), Portlandite (Ca(OH)2) | 4 | 10 | 4 | 7 | 566.3 | 7795 | |
| Brown Coal | Ca, Mg | Hydrocalumite (Ca2Al(OH)6Cl·2H2O), Ettringite (Ca6Al2(SO4)3(OH)12·26H2O), Portlandite (Ca(OH)2) | 2 | 24 | 5 | 6 | 63.3 | 783.3 | |
| Chromium | X | Mg | n.a. | n.a. | n.a. | n.a. | n.a. | 30.4 | |
| Cobalt | X | Ca, Fe | 2 | 14 | 5 | 4 | 5.47 (kt) | 123 (kt) | |
| Copper | X | Ca, Mg, Fe | 2 | 12 | 5 | 5 | 0.948 | 19.4 | |
| Diamond | X | Ca, Mg, Fe | Serpentine-Group minerals (Mg3(Si2O5)(OH)4), Forsterite (Mg2SiO4) | 3 | 18 | 2 | 24 | 13.958 (Mc) | 127 (Mc) |
| Flourine | n.a. | n.a. | n.a. | n.a. | n.a. | 3.1 | |||
| Gold | X | Ca, Mg, Fe | 1 | 17 | 2 | 9 | 288 (t) | 3255 (t) | |
| IImenite | 2 | 19 | 3 | 13 | 1.4 | 11.6 | |||
| Iron Ore | X | Fe, Ca, Mg | Magnetite (Fe3O4), goethite (FeO(OH)), biotite (K(Mg,Fe)3AlSi3O10(F,OH)2) | 1 | 29 | 1 | 38 | 858 | 2230 |
| Lead | 1 | 40 | 2 | 9 | 0.45 | 4.82 | |||
| Lithium | 3 | 18 | 1 | 41 | 14 (kt) | 34.7 (kt) | |||
| Magnesite | Ca, Mg, Fe | 5 | 4 | 8 | 2 | 0.554 | 27.7 | ||
| Manganese Ore | 4 | 13 | 4 | 9 | 3.2 | 44 | |||
| Molybdenum | X | 7 | 1 | n.a. | n.a. | n.a. | 227 (kt) | ||
| Nickel | X | Mg, Ca, | Enstatite (MgSiO3), Diopside (MgCaSi2O6), Talc (Mg3Si4O10(OH)2), Serpentine-Group minerals (Mg3(Si2O5)(OH)4) | 1 | 24 | 5 | 9 | 0.204 | 2.25 |
| Niobium | 2 | 6 | minor | minor | minor | 64 (kt) | |||
| Phosphate | 10 | 2 | minor | minor | 1.54 | 264 | |||
| PGEs (Platinum-group elements) | X | Mg, Ca, Fe, Na | Enstatite (MgSiO3), Talc (Mg3Si4O10(OH)2), Bytownite [(Ca, Na)[Al(Al, Si)Si2O8], Diopside (MgCaSi2O6) | n.a. | n.a. | n.a. | n.a. | 678 (kg) | 380 (t) |
| Potash | minor | 2 | minor | minor | minor | 39 | |||
| Rare Earths (REO & Y2O3) | 6 | 3 | 2 | 11 | 0.014 | 0.126 | |||
| Rutile | 1 | 50 | 1 | 42 | 0.3 | 0.7 | |||
| Silver | 2 | 16 | 5 | 5 | 1.418 (kt) | 27 (kt) | |||
| Tantalum | 1 | n.a. | n.a. | n.a. | 183 (t) | 1.1 (kt) | |||
| Tin | 4 | 10 | 7 | 2 | 6.635 | 278 | |||
| Tungsten | 2 | 12 | n.a. | n.a. | 0.11 | 86.5 | |||
| Uranium | 1 | 29 | 3 | 10 | 6.314 | 62 (kt) | |||
| Vanadium | X | Ca, Mg, Fe | 4 | 11 | minor | minor | minor | 76 (kt) | |
| Zinc | 1 | 28 | 3 | 7 | 0.884 | 11.9 | |||
| Zircon | 1 | 67 | 1 | 31 | 0.6 | 2.4 |
| Mine Waste | Carbonation Advantages | Product Applicability | Carbonation References |
|---|---|---|---|
| Red mud |
|
| Cooling et al. 2002 [130] Tran 2016 [125] Revathy et al. 2017 [133] Liang et al. 2018 [134] Sahu et al. 2010 [135] |
| Fly ash |
|
| Liu et al. 2018 [136] Jaschik et al. 2016 [137] Tamilselvi Dananjayan et al. 2016 [138] Ukwattage et al. 2015 [139] |
| Ultramafic mine tailings (nickel, diamond, PGE and asbestos tailings) |
|
| Nickel tailings: Teir et al. 2007 [140] Teir et al. 2009 [141] Diamond tailings: Mervine et al. 2018 [142] PGE tailings: Meyer et al. 2014 [143] Vogeli et al. 2011 [144] Asbestos tailings: McCutcheon et al. 2017 [145] Oskierski et al. 2016 [146] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azadi, M.; Edraki, M.; Farhang, F.; Ahn, J. Opportunities for Mineral Carbonation in Australia’s Mining Industry. Sustainability 2019, 11, 1250. https://doi.org/10.3390/su11051250
Azadi M, Edraki M, Farhang F, Ahn J. Opportunities for Mineral Carbonation in Australia’s Mining Industry. Sustainability. 2019; 11(5):1250. https://doi.org/10.3390/su11051250
Chicago/Turabian StyleAzadi, Mehdi, Mansour Edraki, Faezeh Farhang, and Jiwhan Ahn. 2019. "Opportunities for Mineral Carbonation in Australia’s Mining Industry" Sustainability 11, no. 5: 1250. https://doi.org/10.3390/su11051250
APA StyleAzadi, M., Edraki, M., Farhang, F., & Ahn, J. (2019). Opportunities for Mineral Carbonation in Australia’s Mining Industry. Sustainability, 11(5), 1250. https://doi.org/10.3390/su11051250







