Elemental Composition and Flue Gas Emissions of Different Components from Five Semi-Arid Woody Species in Pyrolysed and Non-Pyrolysed Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Selection of Species and Sampling Design
2.3. Biofuels Elemental Characterization
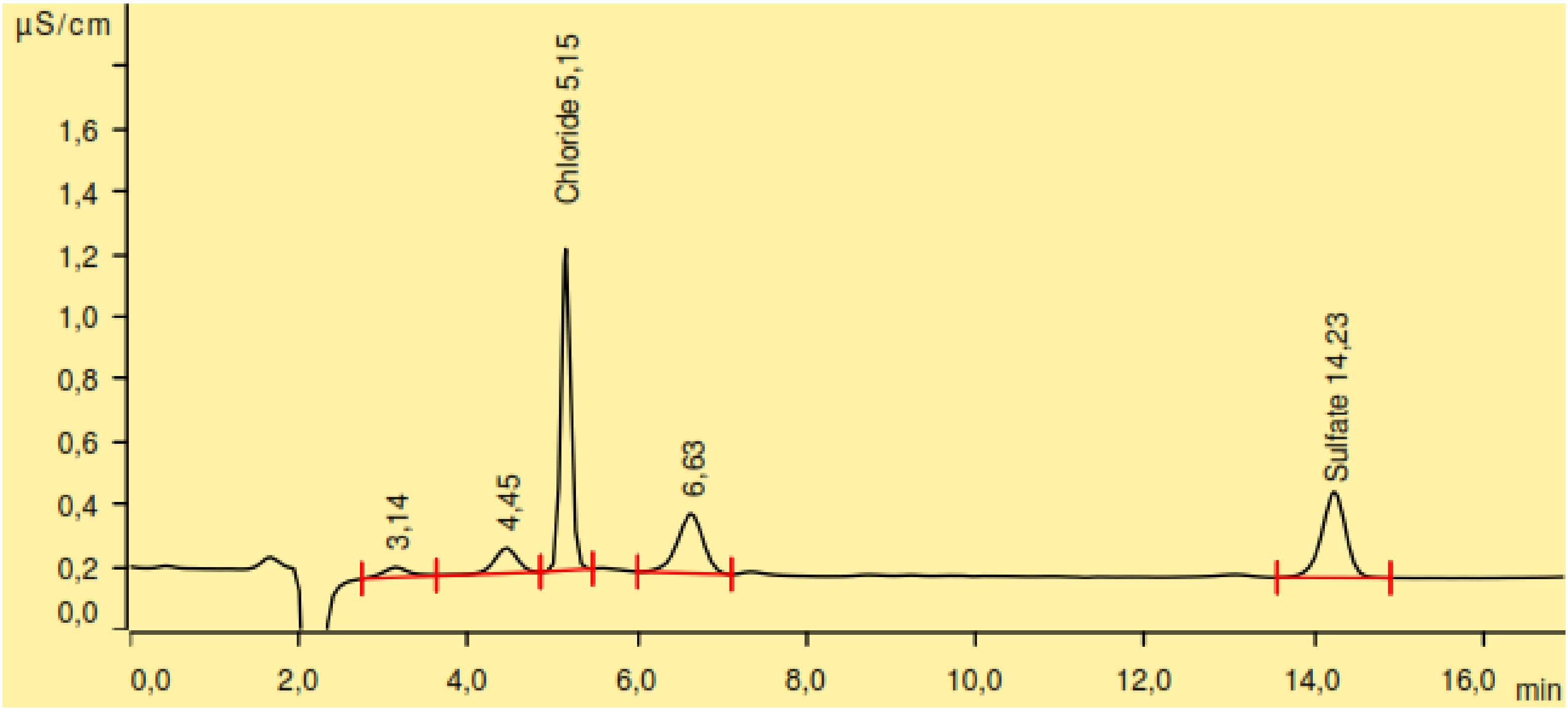
2.4. Sulphur and Chlorine Determination
2.5. Statistical Analysis
3. Results
3.1. Carbon, Hydrogen and Nitrogen in Pyrolysed and Non-Pyrolysed Material from Different Components of Semi-Arid Tree Species
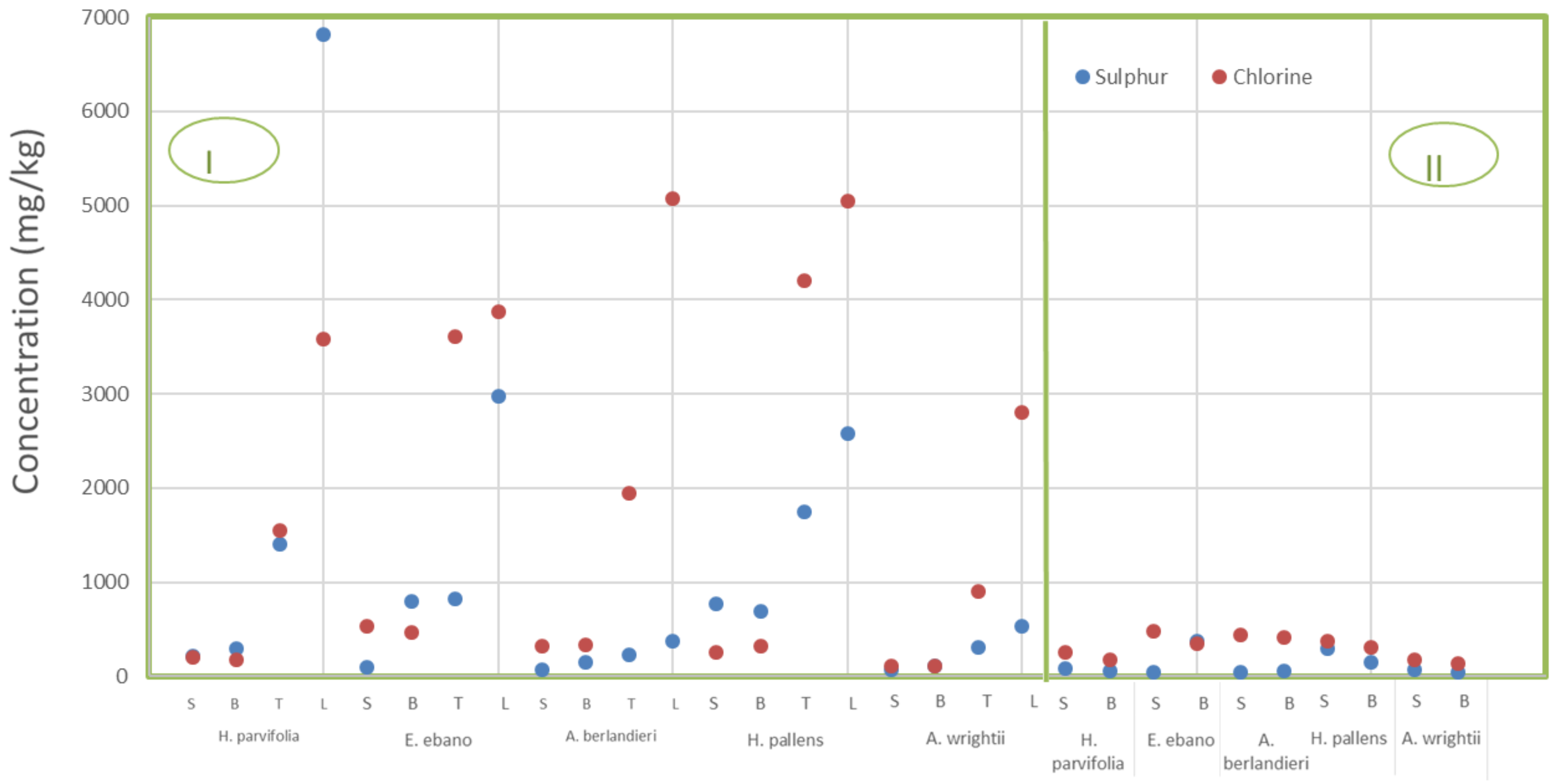
3.2. Chlorine and Sulphur in Pyrolysed and Non-Pyrolysed Biomass
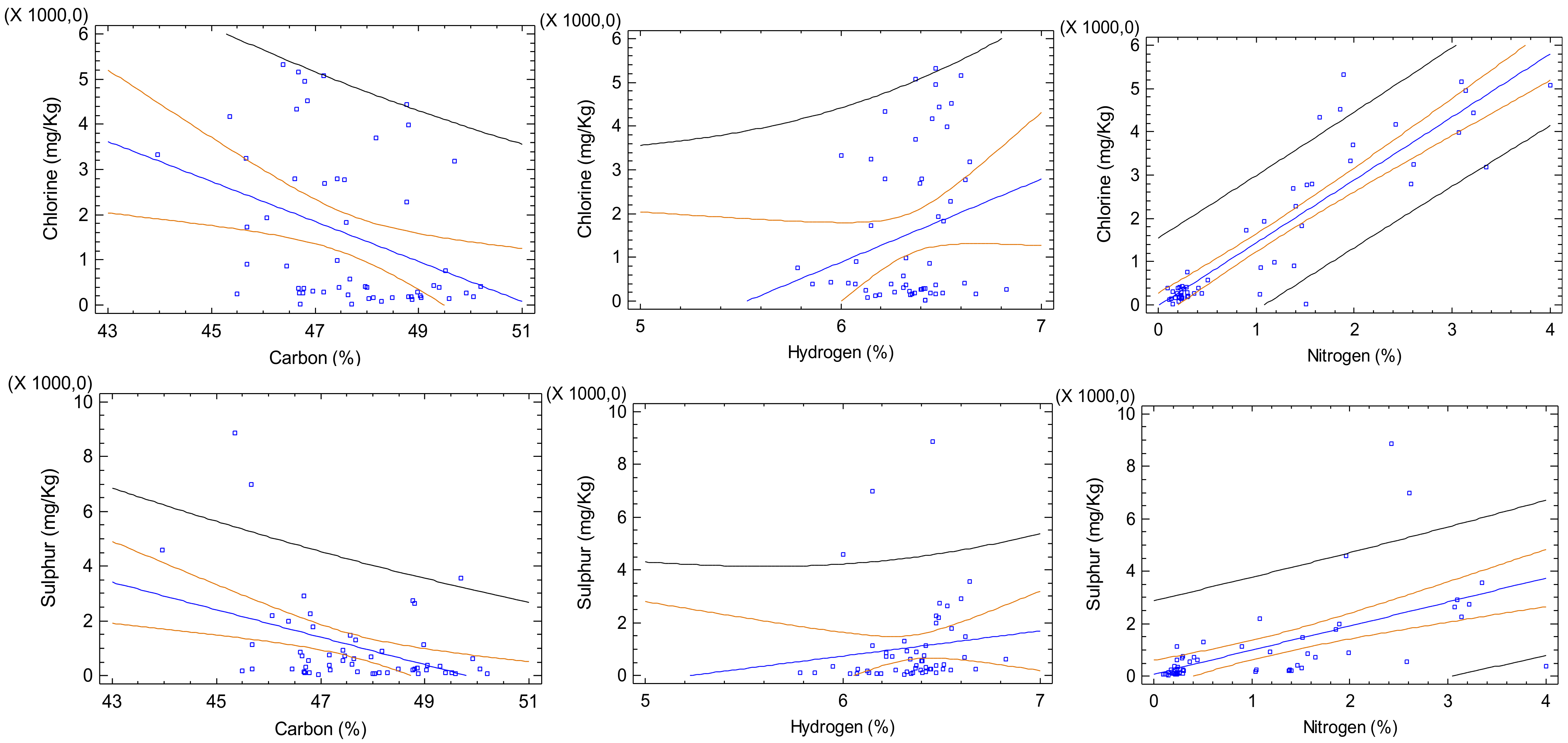
3.3. Correlations between Carbon, Hydrogen and Nitrogen and the Elements Sulphur and Chlorine in Solid Biofuels before and after Treatment by Pyrolysis
4. Discussion
4.1. Carbon, Hydrogen and Nitrogen in Pyrolysed and Non-Pyrolysed Forest Biomass
4.2. Sulphur and Chloride in Pyrolyzed and Non-Pyrolyzed Forest Biomass Combustion
4.3. Correlations Between Carbon, Hydrogen and Nitrogen and the Elements Sulphur and Chlorine in Solid Biofuels before and after Treatment by Pyrolysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brian, K.V.; Adhikari, S.; Taylor, S. Modeling for proximate analysis and heating value of torrefied biomass with vibration spectroscopy. Bioresour. Technol. 2013, 133, 1–8. [Google Scholar]
- McIlveen-Wright, D.R.; Williams, B.C.; McMullan, J.T. A re-appraisal of woodfired combustion, Northern Ireland Centre for Energy Research and Technology. Bioresour. Technol. 2001, 76, 183–190. [Google Scholar] [CrossRef]
- Valle, P.J.A.; Ortega, N.E.O. Prospectivas de Energías Renovables 2012–2026. Secretaría de Energía (SENER). México. 2012. Available online: http://sener.gob.mx/res/PE_y_DT/pub/2012/PER_2012-2026.pdf (accessed on 24 December 2018).
- André, F.J.; de Castro, L.M.; Cerda, E. Las energías renovables en el ámbito internacional. Cuadernos Económicos 2012, 83, 11–36. [Google Scholar]
- Renewable Fuels Agency. The Gallagher Review of the Indirect Effects of Biofuls Production. 2008. Available online: https://www.unido.org/fileadmin/user_media/UNIDO_Header_Site/Subsites/Green_Industry_Asia_Conference__Maanila_/GC13/Gallagher_Report.pdf (accessed on 23 September 2013).
- European Parliament. Report on the implementation of the Circular Economy Plan. 2015. Available online: http://ec.europa.eu/environment/circular-economy/index_en.htm (accessed on 16 May 2018).
- Castellani, B.; Rinaldi, S.; Morini, E.; Nastasi, B.; Rossi, F. Flue gas treatment by power-to-gas integration for methane and ammonia synthesis—Energy and environmental analysis. Energy Convers. Manag. 2018, 171, 626–634. [Google Scholar] [CrossRef]
- Chen, J.; Li, C.; Ristovski, Z.; Milic, A.; Gu, Y.; Islam, M.S.; Guo, H. A review of biomass burning: Emissions and impacts on air quality, health and climate in China. Sci. Total Environ. 2017, 579, 1000–1034. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.; Solomon, G. Pollution prevention at ports: Clearing the air. Environ. Impact Assess. 2004, 24, 749–774. [Google Scholar] [CrossRef]
- Wyrzykowska-Ceradini, B.; Gullett, B.K.; Tabor, D.; Touati, A. Waste combustion as a source of ambient air polybrominated diphenylethers (PBDEs). Atmos. Environ. 2011, 45, 4008–4014. [Google Scholar] [CrossRef]
- Wyrzykowska-Ceradini, B.; Gullett, B.K.; Tabor, D.; Touati, A. PBDDs/Fs and PCDDs/Fs in the raw and clean flue gas during steady state and transient operation of a municipal waste combustor. Environ. Sci. Technol. 2011, 45, 5853–5860. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Sarkar, A. Reaction kinetics and product distribution of slow pyrolysis of Indian textile wastes. Int. J. Chem. React. Eng. 2012, 10. [Google Scholar] [CrossRef]
- Biswal, B.; Kumar, S.; Singh, R.K. Production of hydrocarbon liquid by thermal pyrolysis of paper cup waste. J. Waste Manag. 2013, 1–7. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the composition and application of biomass ash. Part 2. Potential utilization, technological and ecological advantages and challenges. Fuel 2013, 105, 19–39. [Google Scholar] [CrossRef]
- Blumberg, K.O.; Walsh, M.P.; Pera, C. Low-Sufur Gasoine and Diesel: The Key to Lowe Vehicle Emissions; The International Council on Clean Transportation: California, CA, USA, 2003. [Google Scholar]
- Monedero, E.; Hernández, J.J.; Collado, R. Combustion-Related Properties of Poplar, Willow and Black Locust to be used as Fuels in Power Plants. Energies 2017, 10, 997. [Google Scholar] [CrossRef]
- Foroughbakhch, R. Establishment and Growth potential of fuel wood species in northeastern Mexico. Agrofor. Syst. 1992, 19, 95–108. [Google Scholar] [CrossRef]
- Köppen, W. Grundriss der Klimakunde (Outline of Climate Science); Walter de Gruyter: Berlin, Germany, 1931; p. 388. [Google Scholar]
- García, E. Modificaciones al Sistema de Clasificación Climática de Koppen Para Adaptarlo a las Condiciones de la República Mexicana, 3rd ed.; UNAM: Mexico City, Mexico, 2004; p. 252. [Google Scholar]
- Cabral, I.; Treviño, B. Efecto de corte en la dinámica de crecimiento de especies de uso múltiples del matorral espinoso tamaulipeco en el noreste de México. In Simposio Agroforestal de México: Sistemas y Métodos de uso Múltiple del Suelo; UANL (Memorias); tomo 2; Facultad de Ciencias Forestales: Linares, Mexico, 1989; pp. 457–469. [Google Scholar]
- Briseño-Uribe, K.C.; Carrillo-Parra, A.; Bustamante-García, V.; González-Rodríguez, H.; Foroughbachk, R. Firewood Production, Yield and Quality of Charcoal from Eucalyptus camaldulensis and E. microtheca Planted in the Semiarid Land of Northeast Mexico. Int. J. Green Energy 2015, 12, 961–969. [Google Scholar] [CrossRef]
- Technical Association for Pulp and Paper Industry (TAPPI). TAPPI Test Methods (1994–1995); TAPPI Press: Atlanta, GA, USA, 2000. [Google Scholar]
- Schefler, W. Bioestadística; Fondo Educativo Interamericano: Mexico City, DF, Mexico, 1981; 267p. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics, 2nd ed.; McGraw-Hill Book Co.: New York, NY, USA, 1980. [Google Scholar]
- Marcos, M.F. El Carbón Vegetal: Propiedades y Obtención; Agroguías Mundiprensa; Editorial Mundi-Prensa: Logroño, Spain, 1989; p. 114. [Google Scholar]
- Bustamante-García, V.; Carrillo-Parra, A.; Corral-Rivas, J.J. Densificación Energética por Torrefacción de la Biomasa Vegetal. In Biocombustibles Sólidos; Carrillo-Parra, A., Rutiaga-Quiñones, J.G., Eds.; Universidad Autonoma de Nuevo Leon: Monterrey, Mexico, 2015; pp. 160–183. ISBN 978-607-27 0376-6. [Google Scholar]
- Demirbas, A. Sustainable cofiring of biomass with coal. Energy Convers. Manag. 2003, 44, 1465–1479. [Google Scholar] [CrossRef]
- Serrano, A.L.O. Producción de Carbón Vegetal Mediante Carboneras en Zonas Rurales Empobrecidas; Departamento de Ingeniería Térmica y de Fluidos, Universidad Carlos III de Madrid: Madrid, Spain, 2009; 153p. [Google Scholar]
- Valantinavičius, M.; Vonžodas, T. Proximate and Elemental Analysis of Solid Biofuel and Influence to Combustion Process; Lithuanian Energy Institute: Kaunas, Lithuania, 2012; ISSN 1822-7554. Available online: https://www.researchgate.net/publication/259703985_Proximate_and_elemental_analysis_of_solid_biofuel_and_influence_to_combustion_process (accessed on 15 August 2018).
- Hu, Y.; Naito, S.; Kobayashi, N.; Hasatani, M. CO2, NOx and SO2 emissions from the combustion of coal with high oxygen concentration gases. Fuel 2000, 79, 1925–1932. [Google Scholar] [CrossRef]
- Raju, A.I.; Ramya, K.J.; Satya, M.; Praveena, U. Studies on development of fuel briquettes for household and industrial purpose. Int. J. Res. Eng. Technol. 2014, 3, 2. [Google Scholar]
- Orozco, C.; Pérez, A.; González, M.; Rodríguez, F.; Alfayate, J. Contaminación Ambiental. Una Visión Desde la Química; Editorial Paraninfo: Madrid, Spain, 2005; pp. 631–650. [Google Scholar]
- Abdoli, M.A.; Golzary, A.; Hosseini, A.; Sadeghi, P. Wood Pellet Characteristics (Definition, Determination and Internal Relation). In Wood Pellet as a Renewable Source of Energy; Springer: Cham, Switzerland, 2018; pp. 111–138. [Google Scholar]
- Alakangas, E.; Valtanen, J.; Levlin, J.E. CEN technical specification for solid biofuels—Fuel specification and classes. Biomass Bioenergy 2006, 30, 908–914. [Google Scholar] [CrossRef]
- Obernberger, I.; Brunner, T.; Barnthaler, G. Chemical properties of solid biofuels—Significance and impact. Biomass Bioenergy 2006, 30, 973–982. [Google Scholar] [CrossRef]
- Guzmán, G. Evaluación espacio-temporal de la calidad del agua del río San Pedro en el Estado de Aguascalientes, México. Rev. Int. Contam. Ambient. 2011, 27, 89–102. [Google Scholar]
- Ríos, F.; Maroto, A.; Bosque, R. La validación de Métodos analíticos. Rev. Tec. Lab. 2001, 22, 12–17. [Google Scholar]
- Miles, T.R. Alkali Deposits Found in Biomass Power Plants; Research Report NREI/TP-443-8142-SAND 96-8225; National Renewable Energy Laboratory: Oakridge, TN, USA, 1996; Volumes I and II.
- Zhang, X.; Qiu, J.; Dong, X.; Wang, H.; Liu, H. Experimental study on SO2 removal in O2/CO2 coal combustión. Fuel Chem. Div. Prepr. 2003, 48, 418. [Google Scholar]
- Van Loo, S.; Koppejan, J. Handbook of Biomass Combustion and Co-Firing; Bioenergy Task 32; International Energy Agency (IEA): Amsterdam, The Netherlands, 2002. [Google Scholar]
- Jiancheng, Y.; Rui, S.; Shaozeng, S.; Ningbo, Z.; Ning, H.; Hong, C. Experimental study on NOx reduction from staging combustion of high volatile pulverized coals. Part 1. Air staging. Fuel Process. Technol. 2014, 126, 166–275. [Google Scholar]
- Guardado, G.M.B.; Rodríguez, R.J.A.; Monge, H.L.E. Evaluación de la Calidad del Carbón Vegetal Producido en Hornos de Retorta y Hornos Metálicos Portátiles en el Salvador. Engineer’s Thesis, Facultad de Ingeniería y Arquitectura, Universidad Centroamericana “José Simeón Cañas”, Antiguo Cuscatlán, El Salvador, 2010; 67p. [Google Scholar]
- Nguyen, T.V.A.; García-Quintana, Y.; Madelén, C.; Novo, G. Modelo de gestión estratégica para producción de carbón vegetal por programación lineal en la EFI Pinar del Río. Revista Avances 2014, 16, 211–220. [Google Scholar]
- Abascal, F.J.I. Estudio de Factibilidad para la Producción de Carbón Vegetal Corral Viejo en Honduras. Bachelor’s Thesis, Carrera de Administración de Agronegocios, Escuela Agrícola Panamericana, Zamorano, Tegucigalpa, Honduras, 2011; 21p. [Google Scholar]
- Galaz, M.I.M. Caracterización del Sistema de Producción de Carbón de Espino Acacia Caven (Mol.) Mol, en la Comuna de Pumanque, VI Región. Engineer’s Thesis, Facultad de Ciencias Forestales, Universidad de Chile, Santiago, Chile, 2004; 60p. [Google Scholar]

| Non-Pyrolysed Biomass | Pyrolysed Biomass | ||||||
|---|---|---|---|---|---|---|---|
| Stems | Branches | Twigs | Leaves | Stems | Branches | ||
| [%] | [%] | [%] | [%] | [%] | [%] | ||
| Carbon | H. parvifolia | 49.29 ± 0.66 | 48.80 ± 0.28 | 46.39 ± 0.85 | 44.99 ± 0.87 | 84.81 ± 1.23 | 85.89 ± 0.83 |
| E. ebano | 49.70 ± 0.44 | 48.14 ± 0.83 | 47.14 ± 0.81 | 46.09 ± 0.52 | 84.54 ± 0.92 | 83.19 ± 1.09 | |
| A. berlandieri | 47.21 ± 0.69 | 46.32 ± 0.71 | 47.21 ± 0.95 | 47.17 ± 0.24 | 84.17 ± 0.45 | 80.77 ± 0.23 | |
| H. pallens | 47.79 ± 0.72 | 48.35 ± 0.94 | 46.93 ± 0.56 | 46.74 ± 0.08 | 81.99 ± 0.33 | 82.76 ± 0.77 | |
| A. wrghtii | 48.92 ± 0.65 | 47.96 ± 0.22 | 46.92 ± 0.59 | 47.43 ± 0.19 | 85.84 ± 1.25 | 89.30 ± 1.37 | |
| Hydrogen | H. parvifolia | 6.41 ± 0.12 | 6.51 ± 0.15 | 6.32 ± 0.17 | 6.20 ± 0.23 | 2.55 ± 0.05 | 2.54 ± 0.11 |
| E. ebano | 5.89 ± 0.13 | 6.17 ± 0.19 | 6.27 ± 0.09 | 6.55 ± 0.08 | 2.38 ± 0.09 | 2.42 ± 0.07 | |
| A. berlandieri | 6.26 ± 0.17 | 6.31 ± 0.17 | 6.34 ± 0.24 | 6.37 ± 0.09 | 2.49 ± 0.17 | 2.51 ± 0.04 | |
| H. pallens | 6.39 ± 0.04 | 6.62 ± 0.21 | 6.55 ± 0.07 | 6.54 ± 0.09 | 2.69 ± 0.02 | 2.61 ± 0.09 | |
| A. wrghtii | 6.17 ± 0.03 | 6.37 ± 0.04 | 6.46 ± 0.05 | 6.40 ± 0.14 | 2.62 ± 0.14 | 2.69 ± 0.08 | |
| Nitrogen | H. parvifolia | 0.27 ± 0.05 | 0.21 ± 0.03 | 1.06 ± 0.15 | 2.33 ± 0.33 | 0.64 ± 0.02 | 0.52 ± 0.11 |
| E. ebano | 0.24 ± 0.05 | 0.39 ± 0.13 | 1.73 ± 0.22 | 3.21 ± 0.14 | 0.48 ± 0.03 | 0.65 ± 0.12 | |
| A. berlandieri | 0.15 ± 0.05 | 0.52 ± 0.45 | 1.39 ± 0.01 | 4.00 ± 0.09 | 0.39 ± 0.07 | 0.51 ± 0.05 | |
| H. pallens | 0.28 ± 0.08 | 0.34 ± 0.09 | 1.76 ± 0.15 | 3.12 ± 0.03 | 0.57 ± 0.04 | 0.52 ± 0.06 | |
| A. wrghtii | 0.15 ± 0.05 | 0.21 ± 0.05 | 1.34 ± 0.16 | 2.58 ± 0.06 | 0.32 ± 0.08 | 0.39 ± 0.02 | |
| Dependent Variable | Indepen-Dent Variable | Linear Regression | Correlation Coefficient | R-Squared | p-Value | ||
|---|---|---|---|---|---|---|---|
| Non-pyrolysed biomass | 1 | Chlorine | Carbon | Chlorine = 22660.7 − 442.8 × Carbon | −0.35 | 12.28 | 0.0087 * |
| 2 | Chlorine | Hydrogen | Chlorine = −10511.2 + 1900.54 × Hydrogen | 0.23 | 5.20 | 0.0941 | |
| 3 | Chlorine | Nitrogen | Chlorine = −20.6675 + 1455.23 × Nitrogen | 0.90 | 80.59 | 0.0000 ** | |
| 4 | Sulphur | Carbon | Sulphur = 24991 − 501.86 × Carbon | −0.41 | 16.76 | 0.0019 * | |
| 5 | Sulphur | Hydrogen | Sulphur = −4989.95 + 954.51 × Hydrogen | 0.12 | 1.39 | 0.3907 | |
| 6 | Sulphur | Nitrogen | Sulphur = 76.59 + 917.61 × Nitrogen | 0.58 | 34.04 | 0.0000 ** | |
| Pyrolysed biomass | 7 | Chlorine | Carbon | Chlorine = 2947.89 − 31.24 × Carbon | −0.59 | 34.43 | 0.0007 * |
| 8 | Chlorine | Hydrogen | Chlorine = 1702.6 − 544.50 × Hydrogen | −0.46 | 21.44 | 0.0100 * | |
| 9 | Chlorine | Nitrogen | Chlorine = 192.56 + 242.70 × Nitrogen | 0.20 | 3.84 | 0.2995 | |
| 10 | Sulphur | Carbon | Sulphur = 1458.54 − 15.84 × Carbon | −0.31 | 9.66 | 0.0945 | |
| 11 | Sulphur | Hydrogen | Sulphur = 288.58 − 64.83 × Hydrogen | −0.06 | 0.33 | 0.7623 | |
| 12 | Sulphur | Nitrogen | Sulphur = −182.55 + 613.08 × Nitrogen | 0.52 | 26.75 | 0.0034 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngangyo Heya, M.; Foroughbakhch Pournavab, R.; Carrillo Parra, A.; Zelinski, V.; Salas Cruz, L.R. Elemental Composition and Flue Gas Emissions of Different Components from Five Semi-Arid Woody Species in Pyrolysed and Non-Pyrolysed Material. Sustainability 2019, 11, 1245. https://doi.org/10.3390/su11051245
Ngangyo Heya M, Foroughbakhch Pournavab R, Carrillo Parra A, Zelinski V, Salas Cruz LR. Elemental Composition and Flue Gas Emissions of Different Components from Five Semi-Arid Woody Species in Pyrolysed and Non-Pyrolysed Material. Sustainability. 2019; 11(5):1245. https://doi.org/10.3390/su11051245
Chicago/Turabian StyleNgangyo Heya, Maginot, Rahim Foroughbakhch Pournavab, Artemio Carrillo Parra, Volker Zelinski, and Lidia Rosaura Salas Cruz. 2019. "Elemental Composition and Flue Gas Emissions of Different Components from Five Semi-Arid Woody Species in Pyrolysed and Non-Pyrolysed Material" Sustainability 11, no. 5: 1245. https://doi.org/10.3390/su11051245
APA StyleNgangyo Heya, M., Foroughbakhch Pournavab, R., Carrillo Parra, A., Zelinski, V., & Salas Cruz, L. R. (2019). Elemental Composition and Flue Gas Emissions of Different Components from Five Semi-Arid Woody Species in Pyrolysed and Non-Pyrolysed Material. Sustainability, 11(5), 1245. https://doi.org/10.3390/su11051245






