A Review on the Feedstocks for the Sustainable Production of Bioactive Compounds in Biorefineries
Abstract
1. Introduction
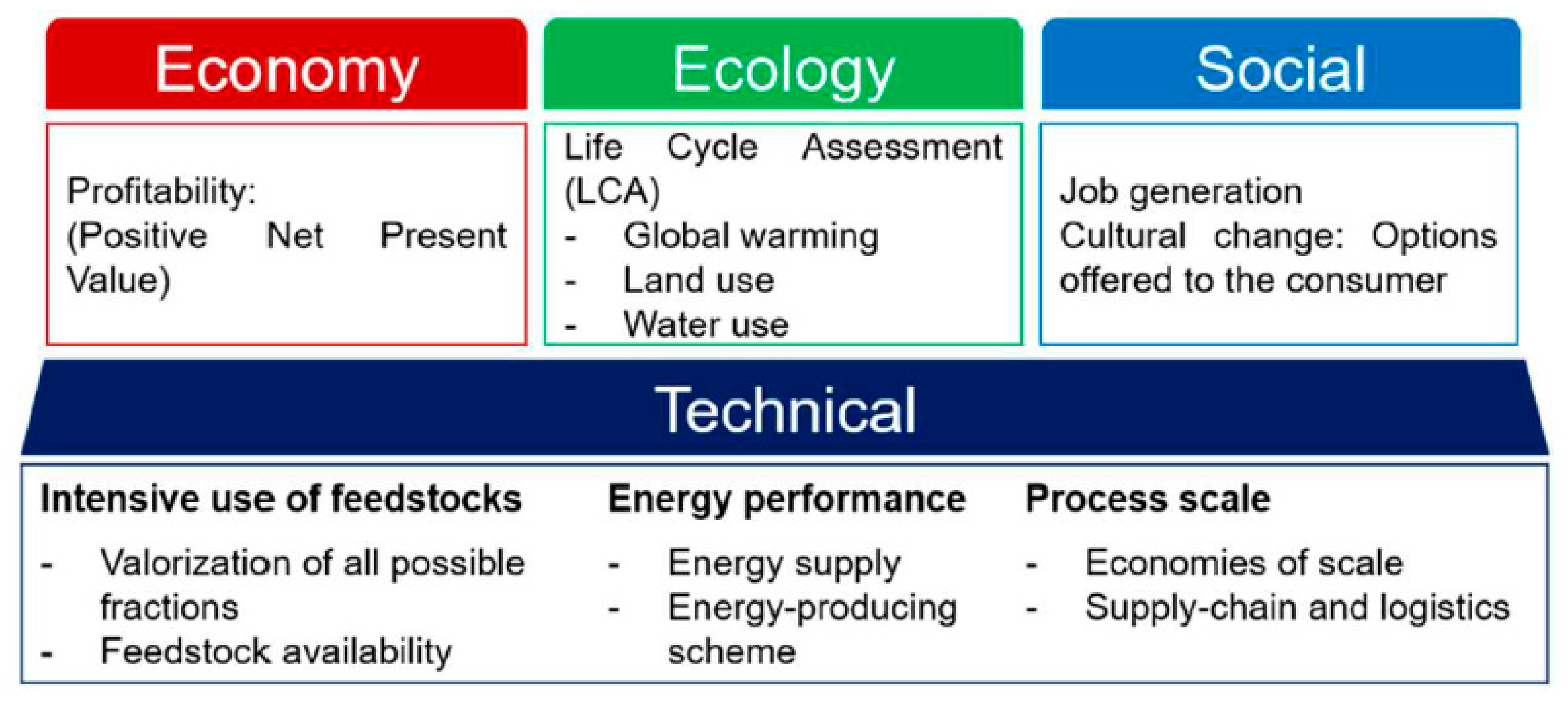
2. Important Aspects to Consider for the Sustainability of a Biorefinery
3. Bioactive Compounds and Raw Materials
3.1. Definition of “Bioactive”
3.2. Types and Sources of BAC
3.2.1. Types
Phenolic Compounds
Antibiotics
Other Possible Bioactive Compounds
3.2.2. Sources
3.3. Technologies
3.3.1. Extraction Techniques
Conventional Extraction Techniques
Non-Conventional Extraction Techniques: Conventional-Technique Enhancers
Non-Conventional Extraction Techniques: Extraction Techniques
3.3.2. Production Techniques
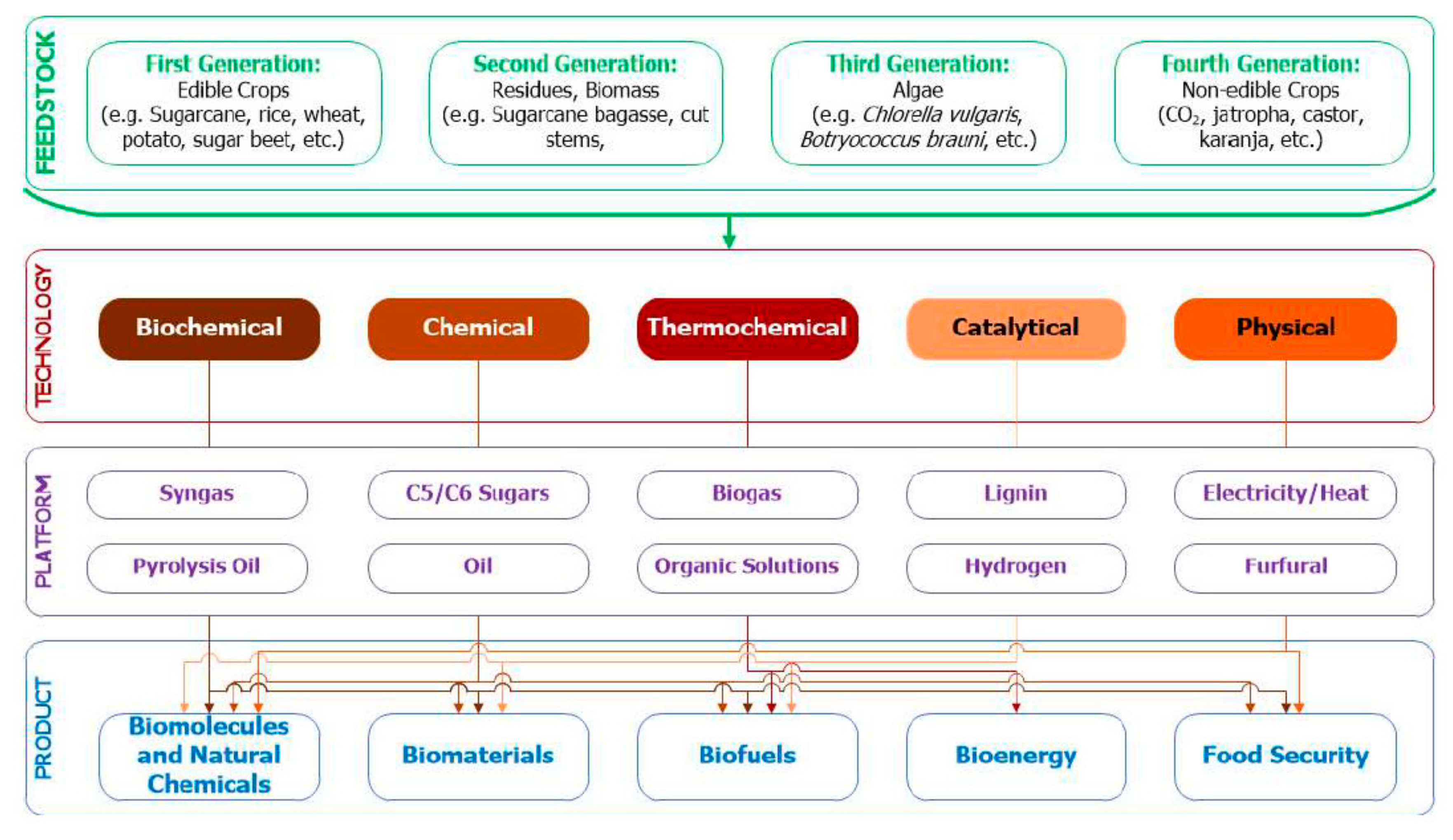
4. Sustainable Production: Feedstock Composition and Further Valorization
4.1. Feedstock Composition
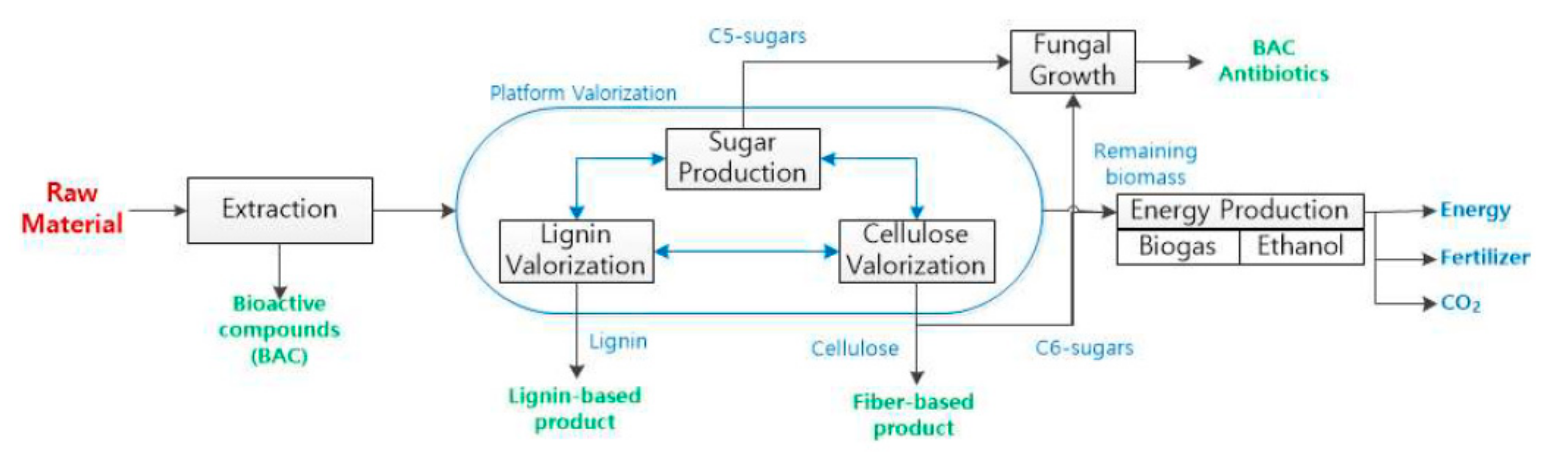
4.2. Further Valorization
4.3. Final Valorization: Energy Production and Nutrient Recovery
4.4. Examples of Feedstock Valorization According to Different Composition Fractions
5. Study Case: Selection of a Feedstock for a Sustainable Biorefinery for the Production of Bioactive Compounds
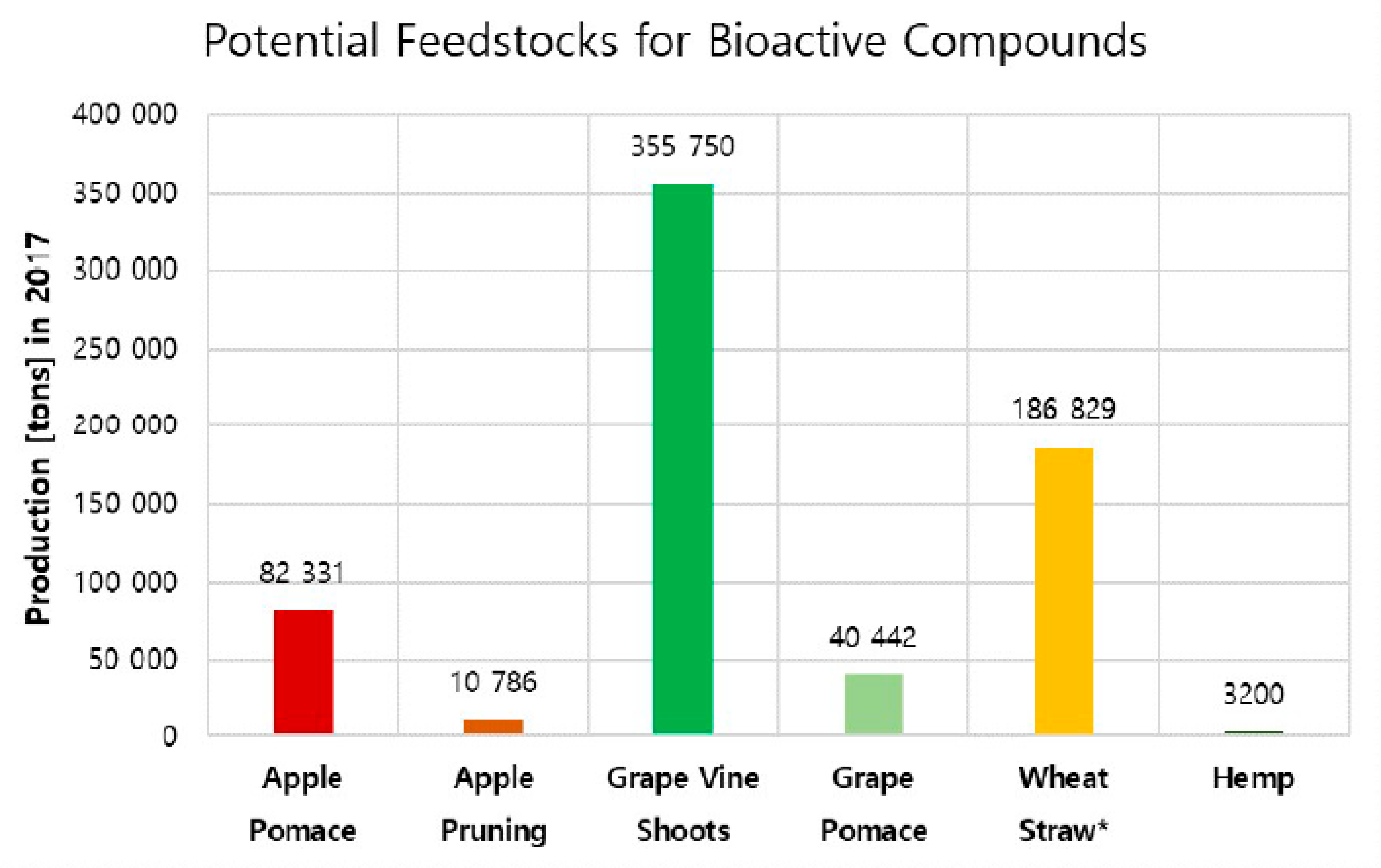
5.1. Raw Material Selection
5.2. Proposed Biorefineries
6. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United Nations Development Program the 2030 Agenda for Sustainable Development. Available online: https://sustainabledevelopment.un.org/?menu=1300 (accessed on 22 May 2019).
- Palmeros Parada, M.; Osseweijer, P.; Posada Duque, J.A. Sustainable biorefineries, an analysis of practices for incorporating sustainability in biorefinery design. Ind. Crops Prod. 2017, 106, 105–123. [Google Scholar] [CrossRef]
- Brundtland, G.; Khalid, M.; Agnelli, S.; Al-Athel, S.A.; Chidzero, B.; Fadika, L.M. Report of the World Commission on Environment and Development: Our Common Future; Oxford University Press: Oxford, UK, 1987. [Google Scholar]
- Serna-Loaiza, S.; García-Velásquez, C.A.; Cardona, C.A. Strategy for the selection of the minimum processing scale for the economic feasibility of biorefineries. Biofuels Bioprod. Biorefining 2019, 13, 107–119. [Google Scholar] [CrossRef]
- Kamm, B.; Kamm, M.; Gruber, P.; Kromus, S. Biorefinery Systems—An Overview. In Biorefineries—Industrial Processes and Products: Status Quo and Future Directions; Kamm, B., Gruber, P.R., Kamm, M., Eds.; WILEY-VCH: Weinheim, Germany, 2010; pp. 3–40. ISBN 978-3-527-32953-3. [Google Scholar]
- Huang, H.J.; Ramaswamy, S.; Tschirner, U.W.; Ramarao, B.V. A review of separation technologies in current and future biorefineries. Sep. Purif. Technol. 2008, 62, 1–21. [Google Scholar] [CrossRef]
- Cardona Alzate, C.A.; Serna-Loaiza, S.; Ortiz-Sanchez, M. Sustainable Biorefineries: What was learned from the design, analysis and implementation. J. Sustain. Dev. Energy Water Environ. Syst. 2020, 8, 88–117. [Google Scholar] [CrossRef]
- Kamm, B.; Kamm, M. Principles of biorefineries. Appl. Microbiol. Biotechnol. 2004, 64, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Oreopoulou, V.; Tzia, C. Utilization of Plant By-Products for the Recovery of Proteins, Dietary Fibers, Antioxidants, and Colorants. In Utilization of By-Products and Treatment of Waste in the Food Industry; Springer: Berlin, Germany, 2007; pp. 209–232. [Google Scholar]
- Guaadaoui, A.; Benaicha, S.; Elmajdoub, N.; Bellaoui, M.; Hamal, A. What is a bioactive compound? A combined definition for a preliminary consensus. Int. J. Food Sci. Nutr. 2014, 3, 17–179. [Google Scholar]
- Gwehenberger, G.; Narodoslawsky, M.; Liebmann, B.; Friedl, A. Ecology of scale versus economy of scale for bioethanol production. Biofuels Bioprod. Biorefining 2007, 1, 264–269. [Google Scholar] [CrossRef]
- Reap, J.; Roman, F.; Duncan, S.; Bras, B. A survey of unresolved problems in life cycle assessment. Int. J. Life Cycle Assess. 2008, 13, 290–300. [Google Scholar] [CrossRef]
- Siebert, A.; Bezama, A.; O’Keeffe, S.; Thrän, D. Social life cycle assessment indices and indicators to monitor the social implications of wood-based products. J. Clean. Prod. 2018, 172, 4074–4084. [Google Scholar] [CrossRef]
- Cadena, E.; Rocca, F.; Gutierrez, J.A.; Carvalho, A. Social life cycle assessment methodology for evaluating production process design: Biorefinery case study. J. Clean. Prod. 2019, 238, 117718. [Google Scholar] [CrossRef]
- Martinkus, N.; Latta, G.; Rijkhoff, S.A.M.; Mueller, D.; Hoard, S.; Sasatani, D.; Pierobon, F.; Wolcott, M. A multi-criteria decision support tool for biorefinery siting: Using economic, environmental, and social metrics for a refined siting analysis. Biomass Bioenergy 2019, 128, 105330. [Google Scholar] [CrossRef]
- National Cancer Institute NCI Dictionary of Cancer Terms. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/bioactive-compound (accessed on 28 August 2019).
- Kris-Etherton, P.M.; Hecker, K.D.; Bonanome, A.; Coval, S.M.; Binkoski, A.E.; Hilpert, K.F.; Griel, A.E.; Etherton, T.D. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002, 113, 71–88. [Google Scholar] [CrossRef]
- Biesalski, H.-K.; Dragsted, L.O.; Elmadfa, I.; Grossklaus, R.; Müller, M.; Schrenk, D.; Walter, P.; Weber, P. Bioactive compounds: Safety and efficacy. Nutrition 2009, 25, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Aslam, K.; Khosa, M.K.; Jahan, N.; Nosheen, S. Synthesis and applications of coumarin. Pak. J. Pharm. Sci. 2010, 23, 449–454. [Google Scholar]
- Shukla, H.D.; Sharma, S.K. Clostridium botulinum: A Bug with Beauty and Weapon. Crit. Rev. Microbiol. 2005, 31, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, G.; Balachandran, L. Biodiversity in Production of Antibiotics and Other Bioactive Compounds. In Biotechnological Applications of Biodiversity; Mukherjee, J., Ed.; Springer: Berlin, Germany, 2014; pp. 37–58. ISBN 978-3-662-45096-3. [Google Scholar]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J.D. Bioactivities of phenolics by focusing on suppression of chronic diseases: A review. Int. J. Mol. Sci. 2018, 19, 1–16. [Google Scholar] [CrossRef]
- Izzi, V.; Masuelli, L.; Tresoldi, I.; Sacchetti, P.; Modesti, A.; Galvano, F.; Bei, R. The effects of dietary flavonoids on the regulation of redox inflammatory networks. Front. Biosci. 2012, 17, 2396–2418. [Google Scholar] [CrossRef]
- Kawabata, J.; Mishima, M.; Kurihara, H.; Mizutani, J.; Kobophenol, B. A tetrastilbene from Carex pumila. Phytochemistry 1991, 30, 645–647. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Nitiema, L.; Savadogo, A.; Simpore, J.; Dianou, D.; Traore, A. In vitro Antimicrobial Activity of Some Phenolic Compounds (Coumarin and Quercetin) Against Gastroenteritis Bacterial Strains. Int. J. Microbiol. Res. 2012, 3, 183–187. [Google Scholar]
- Kostova, I.; Bhatia, S.; Grigorov, P.; Balkansky, S.; Parmar, V.S.; Prasad, A.K.; Saso, L. Coumarins as Antioxidants. Curr. Med. Chem. 2011, 18, 3929–3951. [Google Scholar] [CrossRef] [PubMed]
- De Hoyos-Martínez, P.L.; Merle, J.; Labidi, J.; Charrier–El Bouhtoury, F. Tannins extraction: A key point for their valorization and cleaner production. J. Clean. Prod. 2019, 206, 1138–1155. [Google Scholar] [CrossRef]
- Beisl, S.; Friedl, A.; Miltner, A. Lignin from micro- to nanosize: Applications. Int. J. Mol. Sci. 2017, 18, 2367. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Charusanti, P.; Musiol-Kroll, E.M.; Jiang, X.; Tong, Y.; Kim, H.U.; Lee, S.Y. Metabolic engineering of antibiotic factories: New tools for antibiotic production in actinomycetes. Trends Biotechnol. 2015, 33, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Kim, H.U. The secondary metabolite bioinformatics portal: Computational tools to facilitate synthetic biology of secondary metabolite production. Synth. Syst. Biotechnol. 2016, 1, 69–79. [Google Scholar] [CrossRef]
- Dubourg, G.; Elsawi, Z.; Raoult, D. Assessment of the in vitro antimicrobial activity of Lactobacillus species for identifying new potential antibiotics. Int. J. Antimicrob. Agents 2015, 46, 590–593. [Google Scholar] [CrossRef]
- Seiple, I.B.; Zhang, Z.; Jakubec, P.; Langlois-Mercier, A.; Wright, P.M.; Hog, D.T.; Yabu, K.; Allu, S.R.; Fukuzaki, T.; Carlsen, P.N.; et al. A platform for the discovery of new macrolide antibiotics. Nature 2016, 533, 338. [Google Scholar] [CrossRef]
- Vieira, A.R.; Abar, L.; Vingeliene, S.; Chan, D.S.M.; Aune, D.; Navarro-Rosenblatt, D.; Stevens, C.; Greenwood, D.; Norat, T. Fruits, vegetables and lung cancer risk: A systematic review and meta-analysis. Ann. Oncol. 2015, 27, 81–96. [Google Scholar] [CrossRef]
- Leoncini, E.; Nedovic, D.; Panic, N.; Pastorino, R.; Edefonti, V.; Boccia, S. Carotenoid Intake from Natural Sources and Head and Neck Cancer: A Systematic Review and Meta-analysis of Epidemiological Studies. Cancer Epidemiol. Prev. Biomark. 2015, 24, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Tiyaboonchai, W.; Tungpradit, W.; Plianbangchang, P. Formulation and characterization of curcuminoids loaded solid lipid nanoparticles. Int. J. Pharm. 2007, 337, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Messina, F.; Rosati, O.; Curini, M.; Marcotullio, M.C. Cannabis and Bioactive Cannabinoids; Elsevier: Amsterdam, Netherlands, 2015; Volume 45, ISBN 9780444634733. [Google Scholar]
- Mintz, C.S.; Nison, E.; Fabrizio, A.J. Cannabis-derived pharmaceuticals. J. Commer. Biotechnol. 2015, 21, 16–31. [Google Scholar] [CrossRef]
- Kitrytė, V.; Bagdonaitė, D.; Rimantas Venskutonis, P. Biorefining of industrial hemp (Cannabis sativa L.) threshing residues into cannabinoid and antioxidant fractions by supercritical carbon dioxide, pressurized liquid and enzyme-assisted extractions. Food Chem. 2018, 267, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.M.; Barroso, M.F.; Porto, J.V.; Ramalhosa, M.J.; Švarc-Gajić, J.; Estevinho, L.; Morais, S.; Delerue-Matos, C. Potential of Portuguese vine shoot wastes as natural resources of bioactive compounds. Sci. Total Environ. 2018, 634, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Luque-Rodríguez, J.M.; Pérez-Juan, P.; Luque De Castro, M.D. Extraction of polyphenols from vine shoots of Vitis vinifera by superheated ethanol-water mixtures. J. Agric. Food Chem. 2006, 54, 8775–8781. [Google Scholar] [CrossRef]
- Kalinowska, M.; Bielawska, A.; Lewandowska-Siwkiewicz, H.; Priebe, W.; Lewandowski, W. Apples: Content of phenolic compounds vs. variety, part of apple and cultivation model, extraction of phenolic compounds, biological properties. Plant Physiol. Biochem. 2014, 84, 169–188. [Google Scholar] [CrossRef]
- Tangney, C.C.; Rasmussen, H.E. Polyphenols, inflammation, and cardiovascular disease. Curr. Atheroscler. Rep. 2013, 15, 324. [Google Scholar] [CrossRef]
- Cappello, A.R.; Dolce, V.; Iacopetta, D.; Martello, M.; Fiorillo, M.; Curcio, R.; Dhanyalayam, D. Bergamot (Citrus bergamia Risso) Flavonoids and Their Potential Benefits in Human Hyperlipidemia and Atherosclerosis: An Overview. Mini Rev. Med. Chem. 2016, 16, 619–629. [Google Scholar] [CrossRef]
- Machado, A.P.D.F.; Pasquel-Reátegui, J.L.; Barbero, G.F.; Martínez, J. Pressurized liquid extraction of bioactive compounds from blackberry (Rubus fruticosus L.) residues: A comparison with conventional methods. Food Res. Int. 2015, 77, 675–683. [Google Scholar] [CrossRef]
- Sohn, D.W.; Bae, W.J.; Kim, H.S.; Kim, S.W.; Kim, S.W. The Anti-inflammatory and Antifibrosis Effects of Anthocyanin Extracted from Black Soybean on a Peyronie Disease Rat Model. Urology 2014, 84, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Majumder, R.; Das, C.K.; Mandal, M. Lead bioactive compounds of Aloe vera as potential anticancer agent. Pharmacol. Res. 2019, 148, 104416. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Galván, A.S.; Restrepo-Serna, D.L.; Ortiz-Sánchez, M.; Cardona-Alzate, C.A. Analysis of Extraction Kinetics of Bioactive Compounds from Spent Coffee Grounds (Coffea arábica). Waste Biomass Valorization 2018, 9, 2381–2389. [Google Scholar] [CrossRef]
- Pillai, M.K.; Young, D.J.; Majid, M.B.A. Therapeutic Potential of Alpinia officinarum. Mini Rev. Med. Chem. 2018, 18, 1220–1232. [Google Scholar] [CrossRef] [PubMed]
- Gullón, B.; Gullón, P.; Eibes, G.; Cara, C.; De Torres, A.; López-Linares, J.C.; Ruiz, E.; Castro, E. Valorisation of olive agro-industrial by-products as a source of bioactive compounds. Sci. Total Environ. 2018, 645, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.S.; Coelho, M.S.; de las Mercedes Salas-Mellado, M. Bioactive Compounds as Ingredients of Functional Foods: Polyphenols, Carotenoids, Peptides from Animal and Plant Sources New. In Bioactive Compounds; Segura Campos, M.R., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 129–142. ISBN 978-0-12-814774-0. [Google Scholar]
- Li, R.; Liu, F.; Yang, X.; Chen, L.; Wang, F.; Zhang, G.; Zhang, Q.; Zhang, L.; He, Y.; Li, Y.; et al. Analysis of bisabolocurcumin ether (a terpene-conjugated curcuminoid) and three curcuminoids in Curcuma species from different regions by UPLC-ESI MS/MS and their in vitro anti-inflammatory activities. J. Funct. Foods 2019, 52, 186–195. [Google Scholar] [CrossRef]
- Derntl, C.; Kiesenhofer, D.P.; Mach, R.L.; Mach-Aigner, A.R. Novel Strategies for Genomic Manipulation of Trichoderma reesei with the Purpose of Strain Engineering. Appl. Environ. Microbiol. 2015, 81, 6314–6323. [Google Scholar] [CrossRef]
- De Boer, C.; Peterson, D.H. Geldanamycin and Process for Producing Same; Patent and Trademark Office: Washington, DC, USA, 1971; Volume 7. [Google Scholar]
- Kim, W.S.; Davis, S.; Wong, G.; Demain, A.L. Nutritional studies on the growth of the rapamycin-producing Streptomyces hygroscopicus. J. Microbiol. Biotechnol. 2003, 13, 560–563. [Google Scholar]
- Demain, A.L.; Zhang, J. Cephalosporin C Production by Cephalosporium acremonium: The Methionine Story. Crit. Rev. Biotechnol. 1998, 18, 283–294. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Q.; Deng, W.; Qian, J.; Zhang, S.; Liu, W. Toward improvement of erythromycin A production in an industrial Saccharopolyspora erythraea strain via facilitation of genetic manipulation with an artificial attB site for specific recombination. Appl. Environ. Microbiol. 2011, 77, 7508–7516. [Google Scholar] [CrossRef]
- De Lima Procópio, R.E.; da Silva, I.R.; Martins, M.K.; de Azevedo, J.L.; de Araújo, J.M. Antibiotics produced by Streptomyces. Brazilian J. Infect. Dis. 2012, 16, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Lee, B.-R.; Sathiyanarayanan, G.; Song, H.-S.; Kim, J.; Jeon, J.-M.; Kim, J.-H.; Park, S.-H.; Yu, J.-H.; Park, K.; et al. Medium engineering for enhanced production of undecylprodigiosin antibiotic in Streptomyces coelicolor using oil palm biomass hydrolysate as a carbon source. Bioresour. Technol. 2016, 217, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical Practice Guidelines by the Infectious Diseases Society of America for the Treatment of Methicillin-Resistant Staphylococcus aureus Infections in Adults and Children: Executive Summary. Clin. Infect. Dis. 2011, 52, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.-M.; Kim, S.-Y.; Moon, H.-J.; Oh, D.-K.; Lee, J.-K. Optimization of culture conditions and scale-up to pilot and plant scales for vancomycin production by Amycolatopsis orientalis. Appl. Microbiol. Biotechnol. 2008, 77, 789–795. [Google Scholar] [CrossRef]
- Pintać, D.; Majkić, T.; Torović, L.; Orčić, D.; Beara, I.; Simin, N.; Mimica–Dukić, N.; Lesjak, M. Solvent selection for efficient extraction of bioactive compounds from grape pomace. Ind. Crops Prod. 2018, 111, 379–390. [Google Scholar] [CrossRef]
- Zuorro, A.; Iannone, A.; Lavecchia, R. Water–Organic Solvent Extraction of Phenolic Antioxidants from Brewers’ Spent Grain. Processes 2019, 7, 126. [Google Scholar] [CrossRef]
- Venkatesan, T.; Choi, Y.-W.; Kim, Y.-K. Impact of Different Extraction Solvents on Phenolic Content and Antioxidant Potential of Pinus densiflora Bark Extract. Biomed Res. Int. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Subhedar, P.B.; Gogate, P.R. Use of Ultrasound for Pretreatment of Biomass and Subsequent Hydrolysis and Fermentation. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Mussatto, S.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 127–149. ISBN 978-0-12-802323-5. [Google Scholar]
- Barbosa-Pereira, L.; Guglielmetti, A.; Zeppa, G. Pulsed Electric Field Assisted Extraction of Bioactive Compounds from Cocoa Bean Shell and Coffee Silverskin. Food Bioprocess Technol. 2018, 11, 818–835. [Google Scholar] [CrossRef]
- Heinz, V.; Toepfl, S.; Knorr, D. Impact of temperature on lethality and energy efficiency of apple juice pasteurization by pulsed electric fields treatment. Innov. Food Sci. Emerg. Technol. 2003, 4, 167–175. [Google Scholar] [CrossRef]
- Casas, M.P.; Domínguez González, H. Enzyme-Assisted Aqueous Extraction Processes. In Water Extraction of Bioactive Compounds: From Plants to Drug Development; Dominguez González, H., González Muñoz, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 333–368. ISBN 978-0-12-809380-1. [Google Scholar]
- Cravotto, G.; Boffa, L.; Mantegna, S.; Perego, P.; Avogadro, M.; Cintas, P. Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves. Ultrason. Sonochem. 2008, 15, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Passos, C.P.; Silva, R.M.; Da, F.A.; Coimbra, M.A.; Silva, C.M. Supercritical fluid extraction of grape seed (Vitis vinifera L.) oil. Effect of the operating conditions upon oil composition and antioxidant capacity. Chem. Eng. J. 2010, 160, 634–640. [Google Scholar] [CrossRef]
- Narayanaswamy, N.; Faik, A.; Goetz, D.J.; Gu, T. Supercritical carbon dioxide pretreatment of corn stover and switchgrass for lignocellulosic ethanol production. Bioresour. Technol. 2011, 102, 6995–7000. [Google Scholar] [CrossRef]
- Tang, B.; Bi, W.; Tian, M.; Row, K.H. Application of ionic liquid for extraction and separation of bioactive compounds from plants. J. Chromatogr. B 2012, 904, 1–21. [Google Scholar] [CrossRef]
- Choi, Y.H.; Verpoorte, R. Green solvents for the extraction of bioactive compounds from natural products using ionic liquids and deep eutectic solvents. Curr. Opin. Food Sci. 2019, 26, 87–93. [Google Scholar] [CrossRef]
- Mingeot-Leclercq, M.-P.; Glupczynski, Y.; Tulkens, P.M. Aminoglycosides: Activity and Resistance. Antimicrob. Agents Chemother. 1999, 43, 727–737. [Google Scholar] [CrossRef]
- Stapleton, P.D.; Taylor, P.W. Methicillin Resistance in Staphylococcus Aureus: Mechanisms and Modulation. Sci. Prog. 2002, 85, 57–72. [Google Scholar] [CrossRef]
- Von Nussbaum, F.; Brands, M.; Hinzen, B.; Weigand, S.; Häbich, D. Antibacterial natural products in medicinal chemistry—Exodus or revival? Angew. Chemie Int. Ed. 2006, 45, 5072–5129. [Google Scholar] [CrossRef]
- Kuhad, R.C.; Singh, A. Lignocellulose Biotechnology: Current and Future Prospects. Crit. Rev. Biotechnol. 1993, 13, 151–172. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Zhang, M.; Xie, L.; Yin, Z.; Khanal, S.K.; Zhou, Q. Biorefinery approach for cassava-based industrial wastes: Current status and opportunities. Bioresour. Technol. 2016, 215, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Hernández, V.; Romero-García, J.M.; Dávila, J.A.; Castro, E.; Cardona, C.A. Techno-economic and environmental assessment of an olive stone based biorefinery. Resour. Conserv. Recycl. 2014, 92, 145–150. [Google Scholar] [CrossRef]
- Coelho, J.P.; Filipe, R.M.; Robalo, M.P.; Stateva, R.P. Recovering value from organic waste materials: Supercritical fluid extraction of oil from industrial grape seeds. J. Supercrit. Fluids 2018, 141, 68–77. [Google Scholar] [CrossRef]
- Happi Emaga, T.; Robert, C.; Ronkart, S.N.; Wathelet, B.; Paquot, M. Dietary fibre components and pectin chemical features of peels during ripening in banana and plantain varieties. Bioresour. Technol. 2008, 99, 4346–4354. [Google Scholar] [CrossRef] [PubMed]
- Wikiera, A.; Mika, M.; Starzyńska-Janiszewska, A.; Stodolak, B. Endo-xylanase and endo-cellulase-assisted extraction of pectin from apple pomace. Carbohydr. Polym. 2016, 142, 199–205. [Google Scholar] [CrossRef]
- Ma, X.; Chen, W.; Yan, T.; Wang, D.; Hou, F.; Miao, S.; Liu, D. Comparison of citrus pectin and apple pectin in conjugation with soy protein isolate (SPI) under controlled dry-heating conditions. Food Chem. 2019, 125501. [Google Scholar] [CrossRef]
- Sampaolesi, S.; Gamba, R.R.; De Antoni, G.L.; León Peláez, Á.M. Potentiality of yeasts obtained as beer fermentation residue to be used as probiotics. LWT 2019, 113, 108251. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, S.-S.; Kim, J.; Woo, H.C. Fast pyrolysis of fermentation residue derived from Saccharina japonica for a hybrid biological and thermal process. Energy 2019, 170, 239–249. [Google Scholar] [CrossRef]
- Cheng, M.-H.; Rosentrater, K.A. Fractionation of distillers dried grains with solubles (DDGS) by combination of sieving and aspiration. Food Bioprod. Process. 2017, 103, 76–85. [Google Scholar] [CrossRef]
- You, S.; Ok, Y.S.; Chen, S.S.; Tsang, D.C.W.; Kwon, E.E.; Lee, J.; Wang, C.-H. A critical review on sustainable biochar system through gasification: Energy and environmental applications. Bioresour. Technol. 2017, 246, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Morgan, T.J.; Turn, S.Q.; George, A. Fast Pyrolysis Behavior of Banagrass as a Function of Temperature and Volatiles Residence Time in a Fluidized Bed Reactor. PLoS ONE 2015, 10, e0136511. [Google Scholar] [CrossRef] [PubMed]
- Patra, T.K.; Sheth, P.N. Biomass gasification models for downdraft gasifier: A state-of-the-art review. Renew. Sustain. Energy Rev. 2015, 50, 583–593. [Google Scholar] [CrossRef]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef]
- Ma, H.; Guo, Y.; Qin, Y.; Li, Y.-Y. Nutrient recovery technologies integrated with energy recovery by waste biomass anaerobic digestion. Bioresour. Technol. 2018, 269, 520–531. [Google Scholar] [CrossRef]
- van Kernebeek, H.R.J.; Oosting, S.J.; van Ittersum, M.K.; Ripoll-Bosch, R.; de Boer, I.J.M. Closing the phosphorus cycle in a food system: Insights from a modelling exercise. Animal 2018, 12, 1755–1765. [Google Scholar] [CrossRef]
- De Vrieze, J.; Arends, J.B.A.; Verbeeck, K.; Gildemyn, S.; Rabaey, K. Interfacing anaerobic digestion with (bio)electrochemical systems: Potentials and challenges. Water Res. 2018, 146, 244–255. [Google Scholar] [CrossRef]
- Callaway, J.C.; Pate, D.W. Hempseed Oil. In Gourmet and Health-Promoting Specialty Oils; Moreau, R.A., Kamal-Eldin, A., Eds.; AOCS Press: Champaign, IL, USA, 2009; pp. 185–213. ISBN 978-1-893997-97-4. [Google Scholar]
- Kuglarz, M.; Alvarado-Morales, M.; Karakashev, D.; Angelidaki, I. Integrated production of cellulosic bioethanol and succinic acid from industrial hemp in a biorefinery concept. Bioresour. Technol. 2016, 200, 639–647. [Google Scholar] [CrossRef]
- Weinwurm, F.; Turk, T.; Denner, J.; Whitmore, K.; Friedl, A. Combined liquid hot water and ethanol organosolv treatment of wheat straw for extraction and reaction modeling. J. Clean. Prod. 2017, 165, 1473–1484. [Google Scholar] [CrossRef]
- Pérez, J.A.; Ballesteros, I.; Ballesteros, M.; Sáez, F.; Negro, M.J.; Manzanares, P. Optimizing Liquid Hot Water pretreatment conditions to enhance sugar recovery from wheat straw for fuel-ethanol production. Fuel 2008, 87, 3640–3647. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, X.; Li, Z.; Wang, X.; Sun, J. Effects of liquid digestate pretreatment on biogas production for anaerobic digestion of wheat straw. Bioresour. Technol. 2019, 280, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Aristizábal, V.; Gómez, Á.; Cardona, C.A. Biorefineries based on coffee cut-stems and sugarcane bagasse: Furan-based compounds and alkanes as interesting products. Bioresour. Technol. 2015, 196, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Carvalheiro, F.; Duarte, L.C.; Gírio, F.M. Hemicellulose biorefineries: A review on biomass pretreatments. J. Sci. Ind. Res. 2008, 67, 849–864. [Google Scholar]
- Roberto, I.C.; Mussatto, S.I.; Rodrigues, R.C.L. Dilute-acid hydrolysis for optimization of xylose recovery from rice straw in a semi-pilot reactor. Ind. Crops Prod. 2003, 17, 171–176. [Google Scholar] [CrossRef]
- Spiridon, I.; Popa, V.I. Hemicelluloses: Major Sources, Properties and Applications. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 289–304. ISBN 978-0-08-045316-3. [Google Scholar]
- Dávila, I.; Gullón, B.; Labidi, J.; Gullón, P. Multiproduct biorefinery from vine shoots: Bio-ethanol and lignin production. Renew. Energy 2019, 142, 612–623. [Google Scholar] [CrossRef]
- Jiménez Gómez, S.; Cartagena Causapé, M.C.; Arce Martínez, A. Distribution of nutrients in anaerobic digestion of vine shoots. Bioresour. Technol. 1993, 45, 93–97. [Google Scholar] [CrossRef]
- Gañán, J.; Al-Kassir Abdulla, A.; Cuerda Correa, E.M.; Macías-García, A. Energetic exploitation of vine shoot by gasification processes: A preliminary study. Fuel Process. Technol. 2006, 87, 891–897. [Google Scholar] [CrossRef]
- El Achkar, J.H.; Lendormi, T.; Hobaika, Z.; Salameh, D.; Louka, N.; Maroun, R.G.; Lanoisellé, J.-L. Anaerobic digestion of nine varieties of grape pomace: Correlation between biochemical composition and methane production. Biomass Bioenergy 2017, 107, 335–344. [Google Scholar] [CrossRef]
- Perussello, C.A.; Zhang, Z.; Marzocchella, A.; Tiwari, B.K. Valorization of Apple Pomace by Extraction of Valuable Compounds. Compr. Rev. Food Sci. Food Saf. 2017, 16, 776–796. [Google Scholar] [CrossRef]
- Riggio, V.; Comino, E.; Rosso, M. Energy production from anaerobic co-digestion processing of cow slurry, olive pomace and apple pulp. Renew. Energy 2015, 83, 1043–1049. [Google Scholar] [CrossRef]
- Dávila, J.A.; Rosenberg, M.; Cardona, C.A. A biorefinery for efficient processing and utilization of spent pulp of Colombian Andes Berry (Rubus glaucus benth): Experimental, techno-economic and environmental assessment. Bioresour. Technol. 2017, 223, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Oduntan, A.O.; Arueya, G.L. Design, formulation, and characterization of a potential ‘whole food’ using fibre rich orange (Citrus sinensis Lin) pomace as base. Bioact. Carbohydr. Diet. Fibre 2019, 17, 100172. [Google Scholar] [CrossRef]
- Patsalou, M.; Menikea, K.K.; Makri, E.; Vasquez, M.I.; Drouza, C.; Koutinas, M. Development of a citrus peel-based biorefinery strategy for the production of succinic acid. J. Clean. Prod. 2017, 166, 706–716. [Google Scholar] [CrossRef]
- Kwon, D.; Oh, J.-I.; Lam, S.S.; Moon, D.H.; Kwon, E.E. Orange peel valorization by pyrolysis under the carbon dioxide environment. Bioresour. Technol. 2019, 285, 121356. [Google Scholar] [CrossRef]
- European Commission. Report of the EC on the implementation of the Circular Economy Action Plan; European Commission: Brussels, Belgium, 2019.
- OECD. Municipal Waste; OECD Publishing: Paris, France, 2015. [Google Scholar]
- OECD. Material Resources, Productivity and the Environment; OECD Publishing: Paris, France, 2015; ISBN 9789264190504. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO) Crop Production. 2017. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 18 September 2019).
- European Industrial Hemp Association EU Hemp Cultivation Area. 2017. Available online: http://eiha.org/document/eu-hemp-cultivation-area-2017/ (accessed on 18 September 2019).
- Beisl, S.; Loidolt, P.; Miltner, A.; Harasek, M.; Friedl, A. Production of micro- and nanoscale lignin from wheat straw using different precipitation setups. Molecules 2018, 23, 633. [Google Scholar] [CrossRef]
- Väisänen, T.; Batello, P.; Lappalainen, R.; Tomppo, L. Modification of hemp fibers (Cannabis Sativa L.) for composite applications. Ind. Crops Prod. 2018, 111, 422–429. [Google Scholar] [CrossRef]
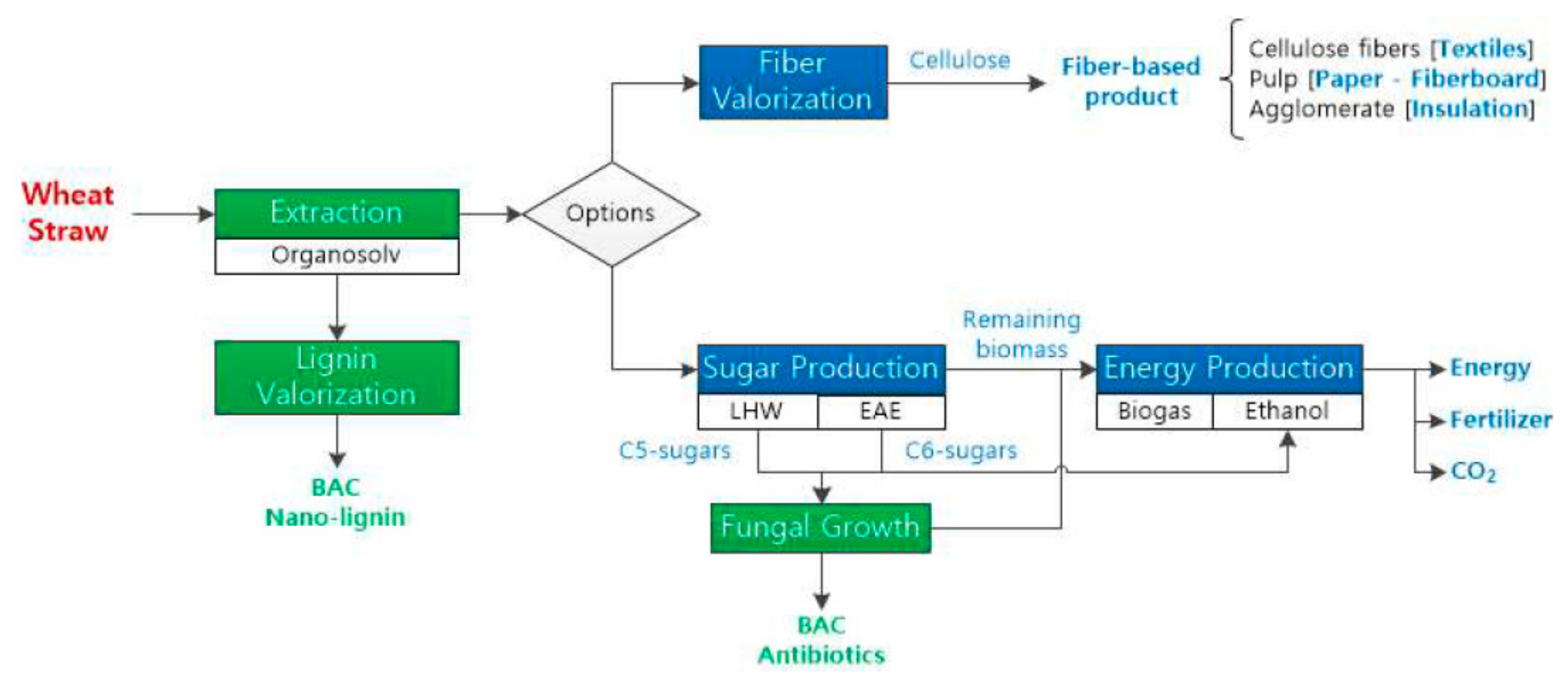
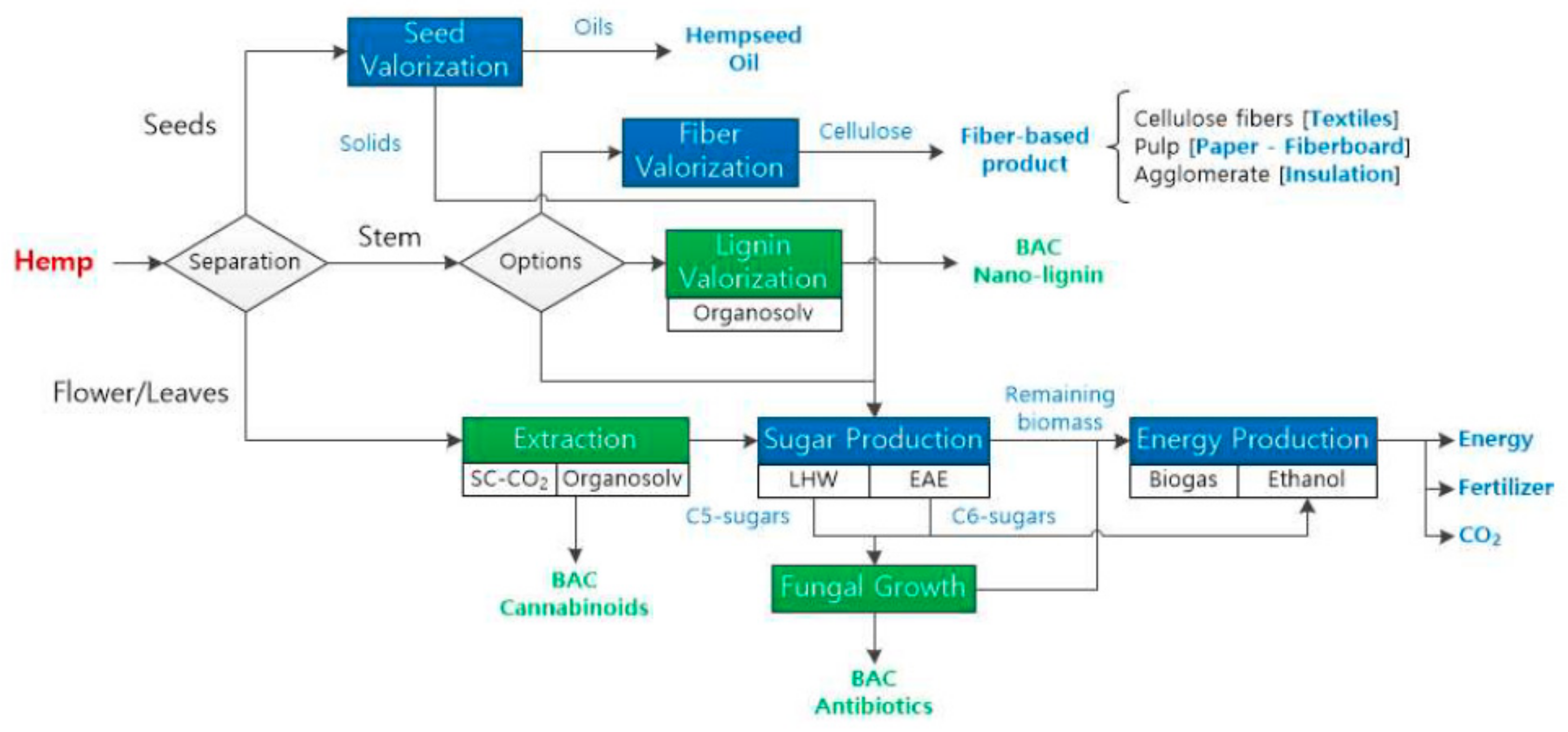

| Source | Type | Bioactivity/Application | Ref. | |
|---|---|---|---|---|
| Phenolic compounds, Carotenoids, Curcuminoids, Cannabinoids | Hemp | Cannabinoids | Relieves convulsion, inflammation, anxiety, and nausea | [40] |
| Wheat straw | Nano-lignin | Bactericidal; Antioxidant | [31] | |
| Grape (vine shoots, seed) | Resveratrol Kaempferol Quercetin | Inhibition of lipase activity; Reduced risk of cardiovascular disease; Bactericidal | [42,43] | |
| Apple | Phenolic acids Flavonols Dihydrochalcones | Reduced risk of cardiovascular disease; Decreased cholesterol level; Reduced risk of type 2 diabetes | [44] | |
| Citrus (orange, lemon, grapefruit) peel | Flavonoids | Antitumor; Antiatherosclerosis; Antibacterial; Reduces blood cholesterol | [9,45,46] | |
| Black rice | Flavones Tannin Anthocyanidins | Antiatherosclerosis; Antitumor; Antiallergic | [24] | |
| Blackberry | Phenolic acids Flavonoids Tannins | Anticancer | [47] | |
| Apricot | Coumarins | Antioxidant; Antimicrobial | [28,29] | |
| Soybean | Anthocyanins | Anti-inflammation | [48] | |
| Aloe vera | Catechin Quercetin | Anticancer; Anti-inflammation | [49] | |
| Coffee | Gallic acid Chlorogenic acid | Antioxidant | [50] | |
| Alpinia officinarum | Phenolic acids Flavonols | Anticancer; Anti-inflammation | [51] | |
| Olive | Flavonoids | Bactericidal; Antioxidant | [52] | |
| Tomato | Lycopene | Antioxidant; Anticancer | [53] | |
| Curcuma | Curcumin Bisabolocurcumin | Anti-inflammation | [54] |
| Microorganism | Antibiotic | Substrate | Bioactivity/Application | Ref. | |
|---|---|---|---|---|---|
| Antibiotics | Streptomyces hygroscopicus | Geldanamycin | Pentoses, Hexoses, Glycerol, Starch | Antitumor; Inhibits nuclear hormone receptors | [56,57] |
| Acremonium chrysogenum | Cephalosporin | Glycerol, Hexoses | Skin and soft tissue infection | [58] | |
| Saccharopolyspora erythraea | Erythromycin | Starch, Hexoses | Respiratory tract infections; Skin infections; Chlamydia; Pelvic inflammatory; Syphilis | [59] | |
| Streptomyces griseus | Streptomycin | Starch, Rice bran, Sucrose | Tuberculosis; Endocarditis; Brucellosis | [60] | |
| Streptomyces aureofaciens | Tetracycline | Hexoses | Acne; Cholera; Brucellosis; Malaria; Syphilis | [60] | |
| Streptomyces coelicolor | Undecylprodigiosin | Pentoses | Antimalarial activity | [61] | |
| Amycolatopsis orientalis | Vancomycin | Starch, Dextrin | Skin infections; Bloodstream infections; Endocarditis; Meningitis | [62,63] |
| Source | Applied Technology | Fraction | Platforms/Products | Ref. |
|---|---|---|---|---|
| Industrial hemp—threshing residues | Pressing | Seeds | Oils | [99] |
| Enzymatic hydrolysis | Lignocellulosics | Glucose (substrate) | [41] | |
| Acid hydrolysis | Lignocellulosics | Ethanol Succinic acid | [100] | |
| Wheat straw | Organosolv | Lignin | Nano-lignin | [31] |
| Liquid hot water | Hemicellulose | C5-sugars (substrate) | [101] | |
| Enzymatic hydrolysis | Cellulose | Glucose (substrate) | [102] | |
| Anaerobic digestion | Solids | Heat and power; Methane | [103] | |
| Coffee residues | Dehydration | Hemicellulose | Furan-based products | [104] |
| Furfural | [105] | |||
| Rice straw | Acid hydrolysis Enzymatic hydrolysis | Lignocellulosics | Xylitol | [106] |
| Liquid hot water Dilute-acid hydrolysis | Hemicellulose | C5-sugars (substrate) | [107] | |
| Grape vine shoots | Liquid hot water Enzymatic hydrolysis | Cellulose Hemicellulose | Ethanol | [108] |
| Alkali hydrolysis | Lignin | Lignin | ||
| Anaerobic digestion | Solids | Heat and power Methane | [109] | |
| Gasification | Solids | Syngas | [110] | |
| Grape pomace | Anaerobic digestion | Solids | Heat and power Methane | [111] |
| Apple pomace | Enzymatic hydrolysis | Pectin | Pectin | [112] |
| Enzymatic hydrolysis | Cellulose | Glucose (substrate) | ||
| Anaerobic digestion | Solids | Heat and power Methane | [113] | |
| Blackberry pulp | Acid hydrolysis | Hemicellulose | Xylitol | [114] |
| Enzymatic hydrolysis | Cellulose | Ethanol | ||
| Orange residues | Enzymatic hydrolysis | Pectin | Food | [115] |
| Acid hydrolysis Enzymatic hydrolysis | Cellulose Hemicellulose | Succinic acid | [116] | |
| Pyrolysis | Solids | Pyrolysis oil Char | [117] | |
| Olive stone | Acid hydrolysis | Hemicellulose | Xylitol Furfural | [113] |
| Enzymatic hydrolysis | Cellulose | Ethanol PHB | ||
| Anaerobic digestion | Solids | Heat and power Methane | ||
| Fermentation by-products (DDGS) | Thermal-mechanical | Protein and fiber | Feed | [91] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serna-Loaiza, S.; Miltner, A.; Miltner, M.; Friedl, A. A Review on the Feedstocks for the Sustainable Production of Bioactive Compounds in Biorefineries. Sustainability 2019, 11, 6765. https://doi.org/10.3390/su11236765
Serna-Loaiza S, Miltner A, Miltner M, Friedl A. A Review on the Feedstocks for the Sustainable Production of Bioactive Compounds in Biorefineries. Sustainability. 2019; 11(23):6765. https://doi.org/10.3390/su11236765
Chicago/Turabian StyleSerna-Loaiza, Sebastián, Angela Miltner, Martin Miltner, and Anton Friedl. 2019. "A Review on the Feedstocks for the Sustainable Production of Bioactive Compounds in Biorefineries" Sustainability 11, no. 23: 6765. https://doi.org/10.3390/su11236765
APA StyleSerna-Loaiza, S., Miltner, A., Miltner, M., & Friedl, A. (2019). A Review on the Feedstocks for the Sustainable Production of Bioactive Compounds in Biorefineries. Sustainability, 11(23), 6765. https://doi.org/10.3390/su11236765





