Rice Husk Silica Enhances Innate Immune in Zebrafish (Danio rerio) and Improves Resistance to Aeromonas hydrophila and Streptococcus iniae Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish and Bacterial Strains
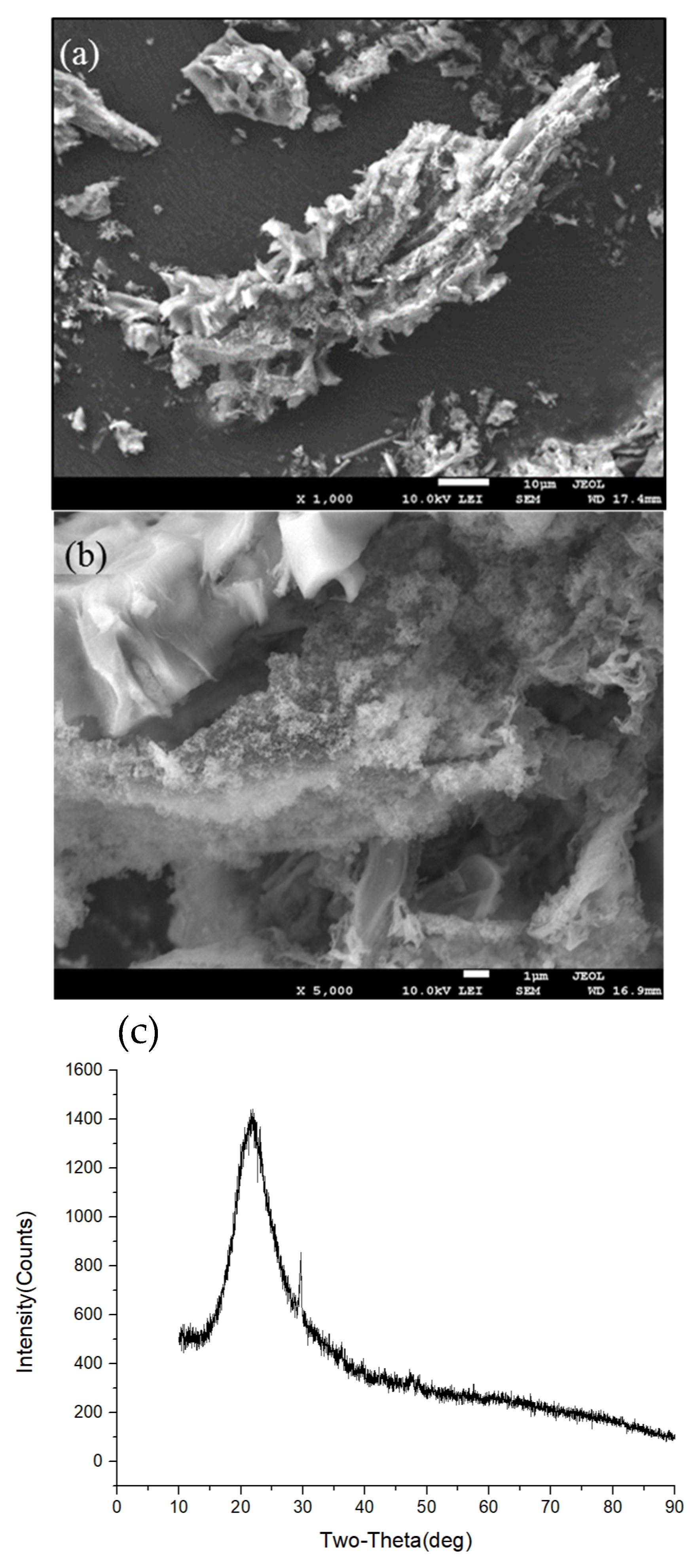
2.2. Preparation of RHS
2.3. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Assay
2.4. Toxicity Assessment of RHS to Zebrafish Embryos and Juveniles
2.5. Analysis of Innate Immune-Related Genes by Real-Time PCR
2.6. Treatment of Adult Zebrafish with RHS and Challenge Experiment
2.7. Statistical Analysis
3. Results
3.1. Toxicity Assay of RHS in Zebrafish
3.2. Antimicrobial Activity of RHS against Diverse Pathogens
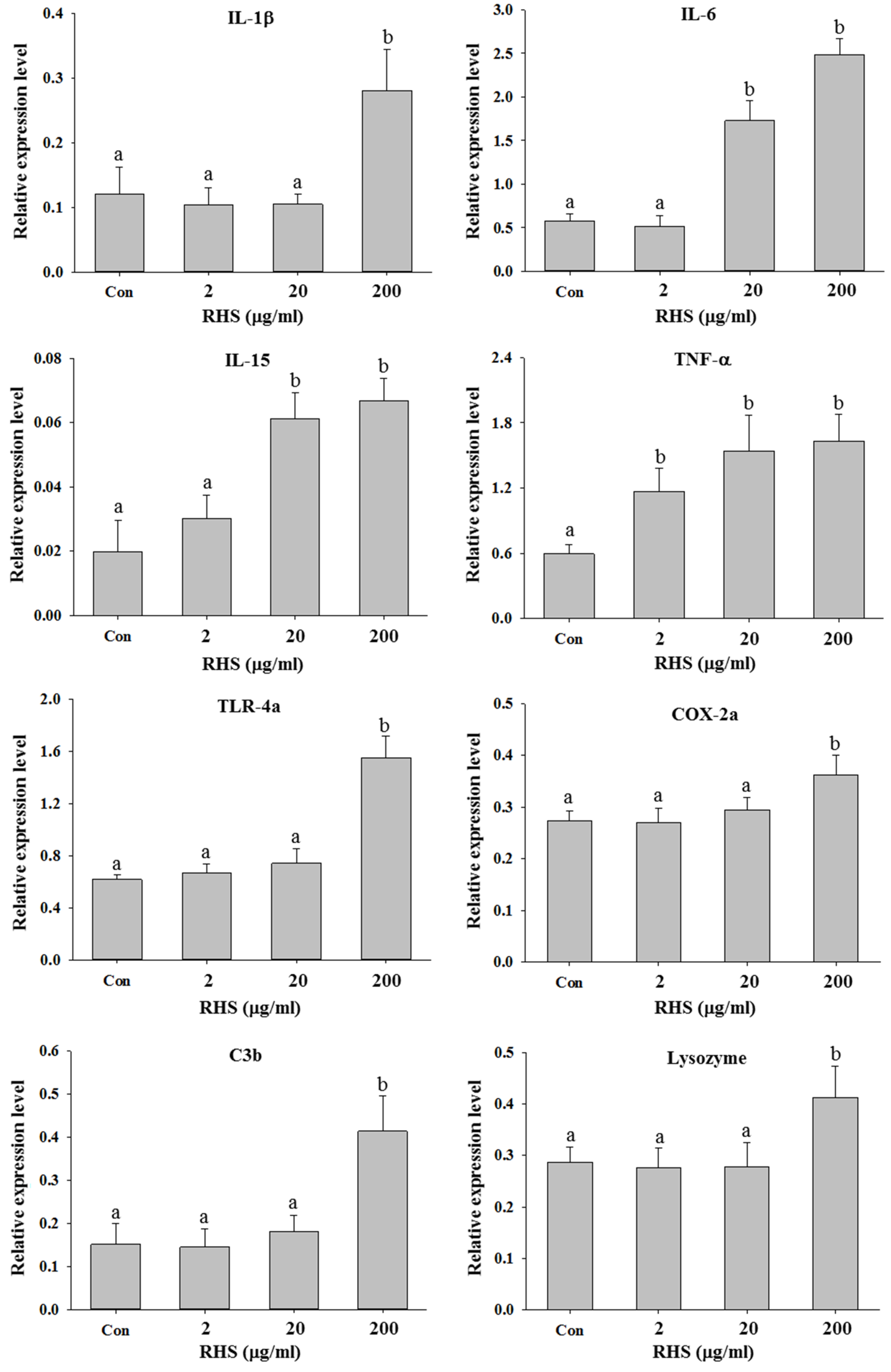
3.3. RHS Immersion Treatment Enhanced Innate Immunity in Zebrafish
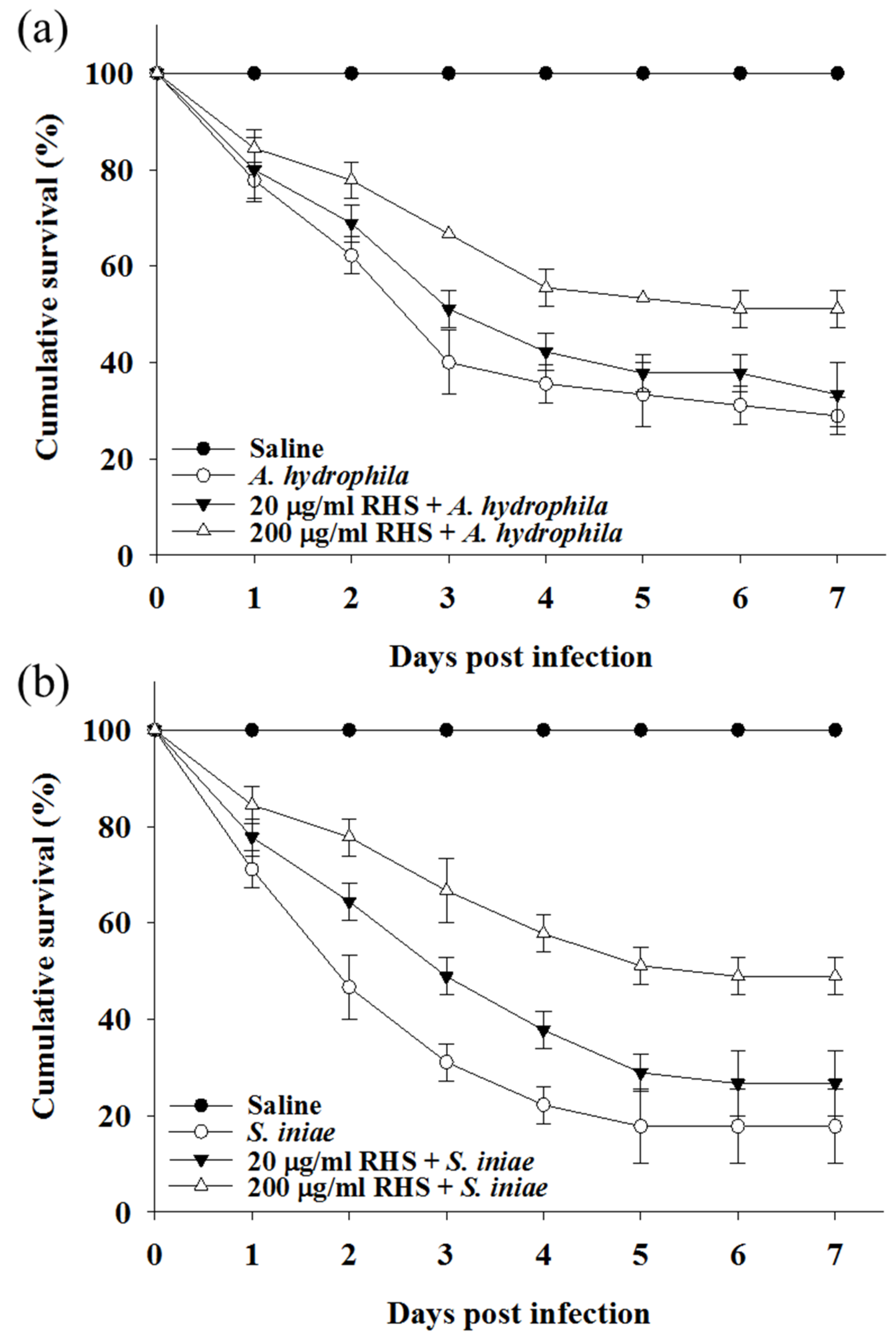
3.4. Treatment with RHS Enhanced Disease Resistance in Zebrafish
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, X.W. An Overview of Recently Published Global Aquaculture Statistics. Available online: https://search.proquest.com/openview/ab844da3e564fb8bcf1d27abf9a6b5af/1?pq-origsite=gscholar&cbl=237326 (accessed on 13 September 2019).
- Orozova, P.; Sirakov, I.; Austin, D.A.; Austin, B. Recovery of Bacillus mycoides, B. pseudomycoides and Aeromonas hydrophila from common carp (Cyprinus carpio) and rainbow trout (Oncorhynchus mykiss) with gill disease. J. Fish Dis. 2018, 41, 125–129. [Google Scholar] [CrossRef]
- Ortega, C.; Garcia, I.; Irgang, R.; Fajardo, R.; Tapia-Cammas, D.; Acosta, J.; Avendano-Herrera, R. First identification and characterization of Streptococcus iniae obtained from tilapia (Oreochromis aureus) farmed in Mexico. J. Fish Dis. 2018, 41, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Steele, J.C.; Meng, X.Z. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: A review. Environ. Pollut. 2017, 223, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Adams, A. Progress, challenges and opportunities in fish vaccine development. Fish Shellfish Immunol. 2019, 90, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; Sharma, A.; Jaiswal, S.; Fatma, S.; Arora, V.; Iquebal, M.A.; Nandi, S.; Sundaray, J.K.; Jayasankar, P.; Rai, A.; et al. Development of antimicrobial peptide prediction tool for aquaculture industries. Probiotics Antimicrob. Proteins 2016, 8, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.W.; Liu, C.H.; Hu, S.Y. Dietary administration of probiotic Paenibacillus ehimensis NPUST1 with bacteriocin-like activity improves growth performance and immunity against Aeromonas hydrophila and Streptococcus iniae in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2019, 84, 695–703. [Google Scholar] [CrossRef]
- Kuebutornye, F.K.A.; Abarike, E.D.; Lu, Y. A review on the application of Bacillus as probiotics in aquaculture. Fish Shellfish Immunol. 2019, 87, 820–828. [Google Scholar] [CrossRef]
- Faikoh, E.N.; Hong, Y.H.; Hu, S.Y. Liposome-encapsulated cinnamaldehyde enhances Zebrafish (Danio rerio) immunity and survival when challenged with Vibrio vulnificus and Streptococcus agalactiae. Fish Shellfish Immunol. 2014, 38, 15–24. [Google Scholar] [CrossRef]
- Vallejos-Vidal, E.; Reyes-Lopez, F.; Teles, M.; MacKenzie, S. The response of fish to immunostimulant diets. Fish Shellfish Immunol. 2016, 56, 34–69. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Rice Market Monito. Available online: http://www.fao.org/3/I9243EN/i9243en.pdf (accessed on 18 April 2018).
- Shen, Y. Rice husk silica-derived nanomaterials for battery applications: A literature review. J. Agric. Food Chem. 2017, 65, 995–1004. [Google Scholar] [CrossRef]
- Vaibhav, V.; Vijayalakshmi, U.; Roopan, S.M. Agricultural waste as a source for the production of silica nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 139, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, Y.; Zhu, Y.; An, D.; Gao, W.; Wang, Z.; Ma, Y.; Wang, Z. A sustainable route for the preparation of activated carbon and silica from rice husk ash. J. Hazard. Mater. 2011, 186, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, D.; Zhu, X. Reduction in time required for synthesis of high specific surface area silica from pyrolyzed rice husk by precipitation at low pH. Bioresour. Technol. 2011, 102, 7001–7003. [Google Scholar] [CrossRef] [PubMed]
- Zahin, N.; Anwar, R.; Tewari, D.; Kabir, M.T.; Sajid, A.; Mathew, B.; Uddin, M.S.; Aleya, L.; Abdel-Daim, M.M. Nanoparticles and its biomedical applications in health and diseases: Special focus on drug delivery. Environ. Sci. Pollut. Res. Int. 2019, 1–18. [Google Scholar] [CrossRef]
- Petrarca, C.; Clemente, E.; Amato, V.; Pedata, P.; Sabbioni, E.; Bernardini, G.; Iavicoli, I.; Cortese, S.; Niu, Q.; Otsuki, T.; et al. Engineered metal based nanoparticles and innate immunity. Clin. Mol. Allergy 2015, 13, 13. [Google Scholar] [CrossRef]
- Luo, Y.H.; Chang, L.W.; Lin, P. Metal-based nanoparticles and the immune system: Activation, inflammation, and potential applications. Biomed. Res. Int. 2015, 2015, 143720. [Google Scholar] [CrossRef]
- Smith, D.M.; Simon, J.K.; Baker, J.R. Applications of nanotechnology for immunology. Nat. Rev. Immunol. 2013, 13, 592–605. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, H.; Zhou, M.; Duan, Y.; Li, N.; Gong, X.; Hu, R.; Hong, M.; Hong, F. Signaling pathway of inflammatory responses in the mouse liver caused by TiO2 nanoparticles. J. Biomed. Mater. Res. A 2011, 96, 221–229. [Google Scholar] [CrossRef]
- Ochoa-Meza, A.R.; Alvarez-Sanchez, A.R.; Romo-Quinonez, C.R.; Barraza, A.; Magallon-Barajas, F.J.; Chavez-Sanchez, A.; Garcia-Ramos, J.C.; Toledano-Magana, Y.; Bogdanchikova, N.; Pestryakov, A.; et al. Silver nanoparticles enhance survival of white spot syndrome virus infected Penaeus vannamei shrimps by activation of its immunological system. Fish Shellfish Immunol. 2019, 84, 1083–1089. [Google Scholar] [CrossRef]
- Anjugam, M.; Vaseeharan, B.; Iswarya, A.; Gobi, N.; Divya, M.; Thangaraj, M.P.; Elumalai, P. Effect of beta-1, 3 glucan binding protein based zinc oxide nanoparticles supplemented diet on immune response and disease resistance in Oreochromis mossambicus against Aeromonas hydrophila. Fish Shellfish Immunol. 2018, 76, 247–259. [Google Scholar] [CrossRef]
- Lee-Estevez, M.; Figueroa, E.; Cosson, J.; Short, S.E.; Valdebenito, I.; Ulloa-Rodríguez, P.; Farías, J.G. Zebrafish as a useful model for immunological research with potential applications in aquaculture. Rev. Aquac. 2016, 10, 213–223. [Google Scholar] [CrossRef]
- Ulloa, P.E.; Medrano, J.F.; Feijoo, C.G. Zebrafish as animal model for aquaculture nutrition research. Front. Genet. 2014, 5, 313. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.N.; Pan, C.Y.; Wu, H.Y.; Chen, J.Y. Antimicrobial peptide Epinecidin-1 promotes complete skin regeneration of methicillin-resistant Staphylococcus aureus-infected burn wounds in a swine model. Oncotarget 2017, 8, 21067–21080. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.Y.; Wu, S.H.; Chen, C.Y.; Huang, C.W.; Lu, J.K.; Chou, H.Y. Complete genome sequence of Streptococcus iniae 89353, a virulent strain isolated from diseased tilapia in Taiwan. Genome Announc. 2017, 5, e01524-16. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.T.; Shiu, Y.L.; Wu, T.M.; Lin, Y.S.; Liu, C.H. Improvement in non-specific immunity and disease resistance of barramundi, Lates calcarifer (Bloch), by diets containing Daphnia similis meal. Fish Shellfish Immunol. 2015, 44, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Shiu, Y.L.; Yeh, S.P.; Liu, C.H. High mortality of broodstock of Chinese mitten crab, Eriocheir sinensis, infected by Vibrio parahaemolyticus in a reproductive period. J. Fish. Soc. Taiwan 2013, 40, 1–9. [Google Scholar]
- Liu, C.H.; Cheng, W.; Hsu, J.P.; Chen, J.C. Vibrio alginolyticus infection in the white shrimp Litopenaeus vannamei confirmed by polymerase chain reaction and 16S rDNA sequencing. Dis. Aquat. Organ. 2004, 61, 169–174. [Google Scholar] [CrossRef]
- Hsu, J.P.; Huang, C.; Liao, C.M.; Hsuan, S.L.; Hung, H.H.; Chien, M.S. Engulfed pathogen-induced apoptosis in haemocytes of giant freshwater prawn, Macrobrachium rosenbergii. J. Fish Dis. 2005, 28, 729–735. [Google Scholar] [CrossRef]
- Saputra, F.; Yen, C.H.; Hsieh, C.Y.; Ou, T.Y.; Risjani, Y.; Cheah, W.K.; Hu, S.Y. Toxicity effects of the environmental hormone 4-tert-octylphenol in zebrafish (Danio rerio). Int. J. Mar. Sci. 2016, 4, 1–12. [Google Scholar]
- Lin, Y.S.; Saputra, F.; Chen, Y.C.; Hu, S.Y. Dietary administration of Bacillus amyloliquefaciens R8 reduces hepatic oxidative stress and enhances nutrient metabolism and immunity against Aeromonas hydrophila and Streptococcus agalactiae in Zebrafish (Danio rerio). Fish Shellfish Immunol. 2019, 86, 410–419. [Google Scholar] [CrossRef]
- Yi, C.C.; Liu, C.H.; Chuang, K.P.; Chang, Y.T.; Hu, S.Y. A potential probiotic Chromobacterium aquaticum with bacteriocin-like activity enhances the expression of indicator genes associated with nutrient metabolism, growth performance and innate immunity against pathogen infections in Zebrafish (Danio rerio). Fish Shellfish Immunol. 2019, 93, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Yetisen, A.K.; Qu, H.; Manbachi, A.; Butt, H.; Dokmeci, M.R.; Hinestroza, J.P.; Skorobogatiy, M.; Khademhosseini, A.; Yun, S.H. Nanotechnology in textiles. ACS Nano 2016, 10, 3042–3068. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Rizwan, M.; Hussain, A.; Rehman, M.Z.U.; Ali, B.; Yousaf, B.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Silicon nanoparticles enhanced the growth and reduced the cadmium accumulation in grains of wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2019, 140, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jahangirian, H.; Kalantari, K.; Izadiyan, Z.; Rafiee-Moghaddam, R.; Shameli, K.; Webster, T.J. A review of small molecules and drug delivery applications using gold and iron nanoparticles. Int. J. Nanomedicine 2019, 14, 1633–1657. [Google Scholar] [CrossRef]
- Vranic, S.; Shimada, Y.; Ichihara, S.; Kimata, M.; Wu, W.; Tanaka, T.; Boland, S.; Tran, L.; Ichihara, G. Toxicological evaluation of SiO(2) nanoparticles by Zebrafish embryo toxicity test. Int. J. Mol. Sci. 2019, 20, 882. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Seil, J.T.; Webster, T.J. Antimicrobial applications of nanotechnology: Methods and literature. Int. J. Nanomedicine 2012, 7, 2767–2781. [Google Scholar]
- Pageni, P.; Yang, P.; Chen, Y.P.; Huang, Y.; Bam, M.; Zhu, T.; Nagarkatti, M.; Benicewicz, B.C.; Decho, A.W.; Tang, C. Charged metallopolymer-grafted silica nanoparticles for antimicrobial applications. Biomacromolecules 2018, 19, 417–425. [Google Scholar] [CrossRef]
- Na-Phatthalung, P.; Teles, M.; Tort, L.; Oliveira, M. Gold nanoparticles exposure modulates antioxidant and innate immune gene expression in the gills of Sparus aurata. Genomics 2018, 110, 430–434. [Google Scholar] [CrossRef]
- Pinsino, A.; Russo, R.; Bonaventura, R.; Brunelli, A.; Marcomini, A.; Matranga, V. Titanium dioxide nanoparticles stimulate sea urchin immune cell phagocytic activity involving TLR/p38 MAPK-mediated signalling pathway. Sci. Rep. 2015, 5, 14492. [Google Scholar] [CrossRef]
- Bhanja, S.K.; Hotowy, A.; Mehra, M.; Sawosz, E.; Pineda, L.; Vadalasetty, K.P.; Kurantowicz, N.; Chwalibog, A. In ovo administration of silver nanoparticles and/or amino acids influence metabolism and immune gene expression in chicken embryos. Int. J. Mol. Sci. 2015, 16, 9484–9503. [Google Scholar] [CrossRef] [PubMed]
- Secombes, C.J.; Wang, T.; Hong, S.; Peddie, S.; Crampe, M.; Laing, K.J.; Cunningham, C.; Zou, J. Cytokines and innate immunity of fish. Dev. Comp. Immunol. 2001, 25, 713–723. [Google Scholar] [CrossRef]
- Duan, J.; Liang, S.; Yu, Y.; Li, Y.; Wang, L.; Wu, Z.; Chen, Y.; Miller, M.R.; Sun, Z. Inflammation-coagulation response and thrombotic effects induced by silica nanoparticles in Zebrafish embryos. Nanotoxicology 2018, 12, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Liang, S.; Feng, L.; Yu, Y.; Sun, Z. Silica nanoparticles trigger hepatic lipid-metabolism disorder in vivo and in vitro. Int. J. Nanomedicine 2018, 13, 7303–7318. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Han, Y.; Zou, X.; Zhu, K.; Wang, Z.; Ye, X.; Liu, Y.; Dong, S.; Chen, X.; Liu, D.; et al. Silica nanoparticles as an enhancer in the IL-1beta-induced inflammation cycle of A549 cells. Immunopharmacol. Immunotoxicol. 2019, 41, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Ragland, S.A.; Criss, A.K. From bacterial killing to immune modulation: Recent insights into the functions of lysozyme. PLoS Pathog. 2017, 13, e1006512. [Google Scholar] [CrossRef]
- Rus, H.; Cudrici, C.; Niculescu, F. The role of the complement system in innate immunity. Immunol. Res. 2005, 33, 103–112. [Google Scholar] [CrossRef]
| Gene Name | Primer Sequence (5′→3′) | PCR Product Size (bp) | Accession Number |
|---|---|---|---|
| Interleukin-1β (IL-1β) | TGGACTTCGCAGCACAAAATG | 147 | AY340959 |
| CACTTCACGCTCTTGGATGA | |||
| Interleukin-6 (IL-6) | TCAACTTCTCCAGCGTGATG | 73 | JN698962 |
| TCTTTCCCTCTTTTCCTCCTG | |||
| Interleukin-15 (IL-15) | ATGTCATTGGAACTCAGAGGTTT | 100 | BC162843 |
| CTGTTCTGGATGTCCTGCTTGA | |||
| Tumor necrosis factor-α (TNF-α) | AAGGAGAGTTGCCTTTACCG | 152 | BC165066 |
| ATTGCCCTGGGTCTTATGC | |||
| Toll-like receptor-4a (TLR-4a) | TTTCAGATGCCACATCAGA | 150 | EU551724 |
| TCCACAAGAACAAGCCTTTG | |||
| Cyclooxygenase-2a (COX-2a) | GATCTCCCAAATGCCAAGCA | 100 | NM_153657 |
| GGGCGAAGAAAGCAAACATG | |||
| Complement component C3b | CGTCTCCGTACACCATCCATT | 100 | NM_131243 |
| GGCGTCTCATCAGGATTTGTTAC | |||
| Lysozyme | CGTGGATGTCCTCGTGTGAAG | 100 | NM_139180 |
| CCAATGGAGAATCCCTCAAA | |||
| Elongation factor-1α (EF-1α) | AACAGCTGATCGTTGGAGTCAA | 100 | AY422992 |
| TTGATGTATGCGCTGACTTCCT |
| Concentration of RHS Solution (μg/mL) | 3 dpf | 4 dpf | ||||
|---|---|---|---|---|---|---|
| Survival Rate (%) | Hatching Rate (%) | Malformation Rate (%) | Survival Rate (%) | Hatching Rate (%) | Malformation Rate (%) | |
| Control | 89 ± 4% (n = 134/150) a | 100 ± 0% (n = 134/134) a | 2 ± 3% (n = 2/134) a | 89 ± 4% (n = 134/150) a | 100 ± 0% (n = 134/134) a | 2 ± 3% (n = 2/134) a |
| 2 | 84 ± 3% (n = 126/150) a | 98 ± 3% (n = 123/126) a | 1 ± 2% (n = 1/123) a | 83 ± 4% (n = 125/150) a | 100 ± 0% (n = 123/123) a | 2 ± 2% (n = 2/123) a |
| 20 | 89 ± 5% (n = 133/150) a | 100 ± 0% (n = 134/134) a | 0 ± 0% (n = 0/134) a | 88 ± 5% (n = 132/150) a | 100 ± 0% (n = 132/132) a | 0 ± 0% (n = 0/132) a |
| 200 | 84 ± 3% (n = 126/150) a | 98 ± 4% (n = 123/126) a | 1 ± 1% (n = 2/123) a | 84 ± 3% (n = 126/150) a | 100 ± 0% (n = 123/123) a | 1 ± 1% (n = 2/123) a |
| 2000 | 30 ± 13% (n = 50/150) b | 39 ± 8% (n = 20/50) b | 66 ± 4% (n = 33/50) b | 19 ± 5% (n = 28/150) b | 43 ± 1% (n = 12/28) b | 69 ± 10% (n = 19/28) b |
| Concentration of RHS Solution (μg/mL) | Survival Rate (%) | ||||||
|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | |
| Control | 100 ± 0% (n = 90/90) a | 97 ± 6% (n = 87/90) a | 97 ± 6% (n = 87/90) a | 97 ± 6% (n = 87/90) a | 97 ± 6% (n = 87/90) a | 97 ± 6% (n = 87/90) a | 97 ± 6% (n = 87/90) a |
| 0.2 | 100 ± 0% (n = 90/90) a | 100 ± 0% (n = 90/90) a | 97 ± 6% (n = 87/90) a | 97 ± 6% (n = 87/90) a | 97 ± 6% (n = 87/90) a | 97 ± 6% (n = 87/90) a | 97 ± 6% (n = 87/90) a |
| 2 | 97 ± 6% (n = 87/90) a | 97 ± 6% (n = 87/90) a | 97 ± 6% (n = 87/90) a | 97 ± 6% (n = 87/90) a | 97 ± 6% (n = 87/90) a | 97 ± 6% (n = 87/90) a | 97 ± 6% (n = 87/90) a |
| 20 | 100 ± 0% (n = 87/90) a | 97 ± 6% (n = 87/30) a | 97 ± 6% (n = 87/30) a | 93 ± 6% (n = 84/90) a | 90 ± 0% (n = 81/90) a | 90 ± 0% (n = 81/90) a | 90 ± 0% (n = 81/90) a |
| 200 | 93 ± 6% (n = 84/90) a | 93 ± 6% (n = 84/90) a | 93 ± 6% (n = 84/90) a | 90 ± 6% (n = 81/90) a | 90 ± 10% (n = 81/90) a | 90 ± 10% (n = 81/90) a | 90 ± 10% (n = 81/90) a |
| 2000 | 70 ± 10% (n = 63/90) b | 60 ± 17% (n = 54/90) b | 47 ± 15% (n = 42/90) b | 43 ± 12% (n = 39/90) b | 40 ± 10% (n = 36/90) b | 40 ± 10% (n = 36/90) b | 40 ± 10% (n = 36/90) b |
| Pathogens and Sources | RHS (μg/mL) | Kanamycin (μg/mL) | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| Aeromonas hydrophila | 120 | 1000 | 0.5 | 2 |
| Streptococcus iniae | 140 | 2000 | 3 | 6 |
| Vibrio parahaemolyticusa | 140 | 2000 | NE c | NE c |
| Vibrio alginolyticusa | 200 | NE c | NE c | NE c |
| Vibrio vulnificus | 180 | 20,000 | 3 | 6 |
| Debaryomyces hansenii | 100 | 1000 | NE c | NE c |
| Escherichia coli BCRC11634 b | 180 | 20,000 | 1 | 2 |
| Staphylococcus aureus BCRC12991 b | 160 | NE c | 0.5 | 2 |
| Salmonella typhimurium BCRC12947 b | 180 | NE c | 1 | 2 |
| Listeria monocytogenes BCRC14932 b | 200 | NE c | 1 | 3 |
| Methicillin-resistant S. aureus (MRSA) a | 240 | NE c | NE c | NE c |
| Pseudomonas aeruginosa BCRC12902 b | 160 | NE c | 2 | 4 |
| Burkholderia gladioli BCRC13899 b | 180 | NE c | 3 | 4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Y.-H.; Tseng, C.-C.; Setyoningrum, D.; Yang, Z.-P.; Maftuch; Hu, S.-Y. Rice Husk Silica Enhances Innate Immune in Zebrafish (Danio rerio) and Improves Resistance to Aeromonas hydrophila and Streptococcus iniae Infection. Sustainability 2019, 11, 6504. https://doi.org/10.3390/su11226504
Hong Y-H, Tseng C-C, Setyoningrum D, Yang Z-P, Maftuch, Hu S-Y. Rice Husk Silica Enhances Innate Immune in Zebrafish (Danio rerio) and Improves Resistance to Aeromonas hydrophila and Streptococcus iniae Infection. Sustainability. 2019; 11(22):6504. https://doi.org/10.3390/su11226504
Chicago/Turabian StyleHong, Yong-Han, Chung-Chih Tseng, Desy Setyoningrum, Zu-Po Yang, Maftuch, and Shao-Yang Hu. 2019. "Rice Husk Silica Enhances Innate Immune in Zebrafish (Danio rerio) and Improves Resistance to Aeromonas hydrophila and Streptococcus iniae Infection" Sustainability 11, no. 22: 6504. https://doi.org/10.3390/su11226504
APA StyleHong, Y.-H., Tseng, C.-C., Setyoningrum, D., Yang, Z.-P., Maftuch, & Hu, S.-Y. (2019). Rice Husk Silica Enhances Innate Immune in Zebrafish (Danio rerio) and Improves Resistance to Aeromonas hydrophila and Streptococcus iniae Infection. Sustainability, 11(22), 6504. https://doi.org/10.3390/su11226504






