Study of a Li-Ion Cell Kinetics in Five Regions to Predict Li Plating Using a Pseudo Two-Dimensional Model
Abstract
1. Introduction
2. Theory
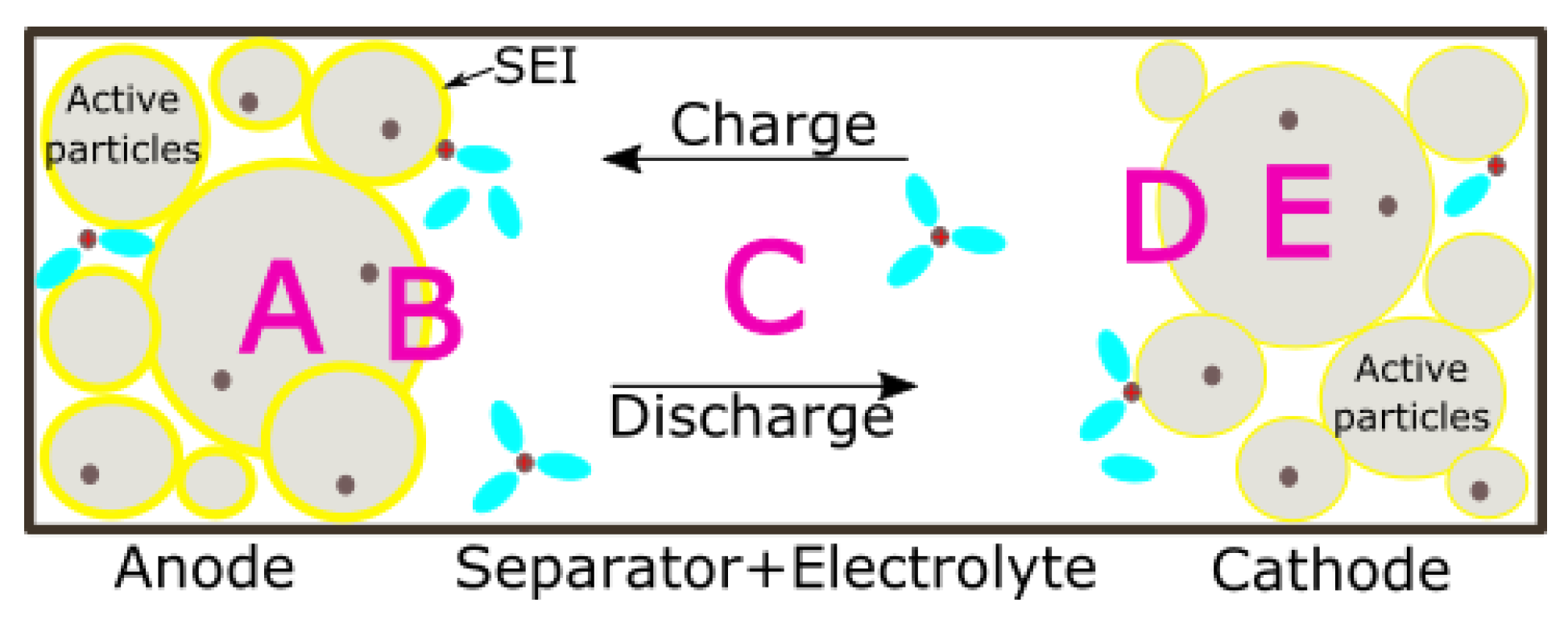
2.1. Transport Regions
- Regions A and E representing the inner part of solid active material particles in the anode and cathode respectively;
- Regions B and D that are the solid particles/electrolyte interfaces at the anode and cathode side respectively;
- Region C which indicates the electrolyte which can be in the anode, the separator, or the cathode.
2.2. Model Description
3. Results
3.1. Validation
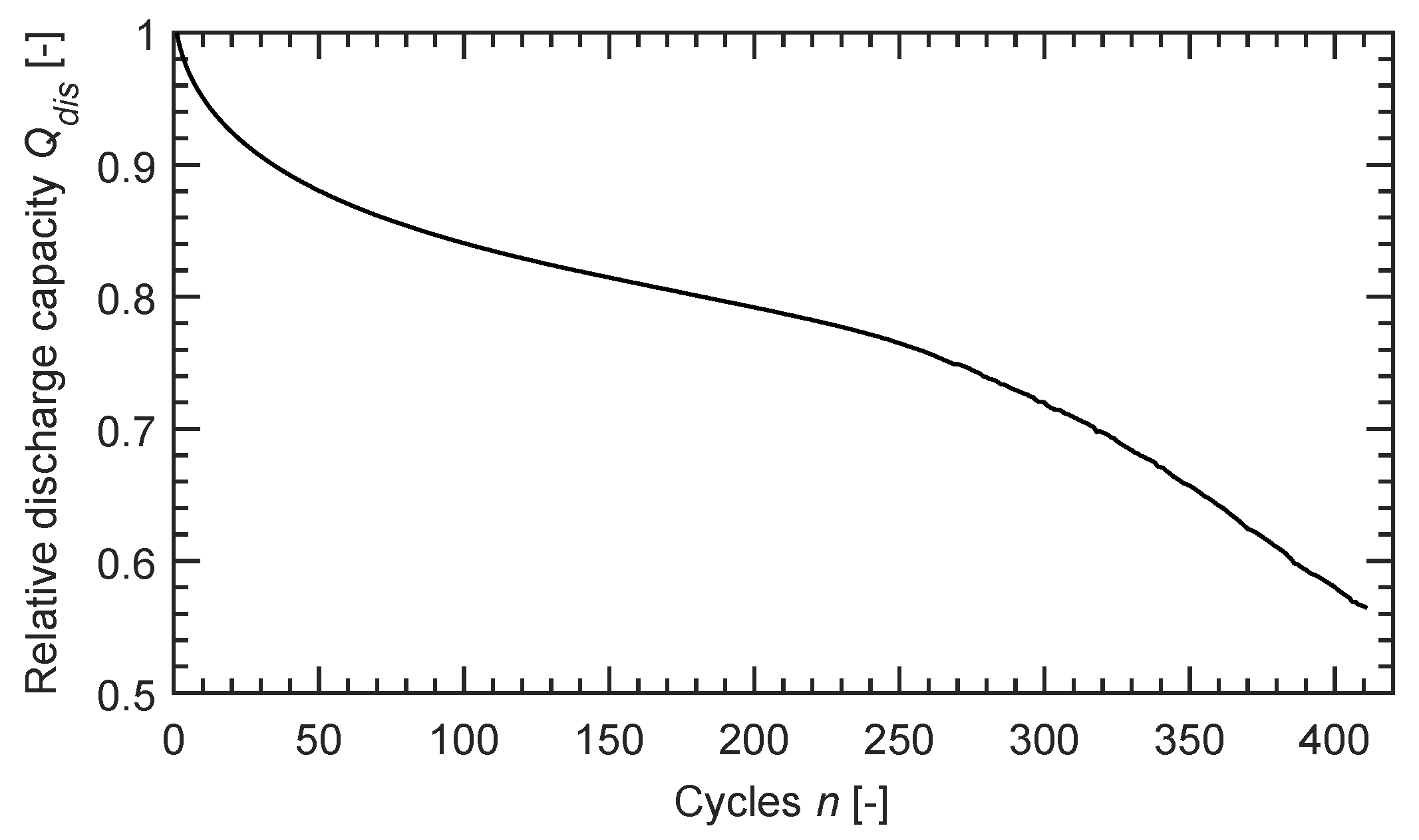
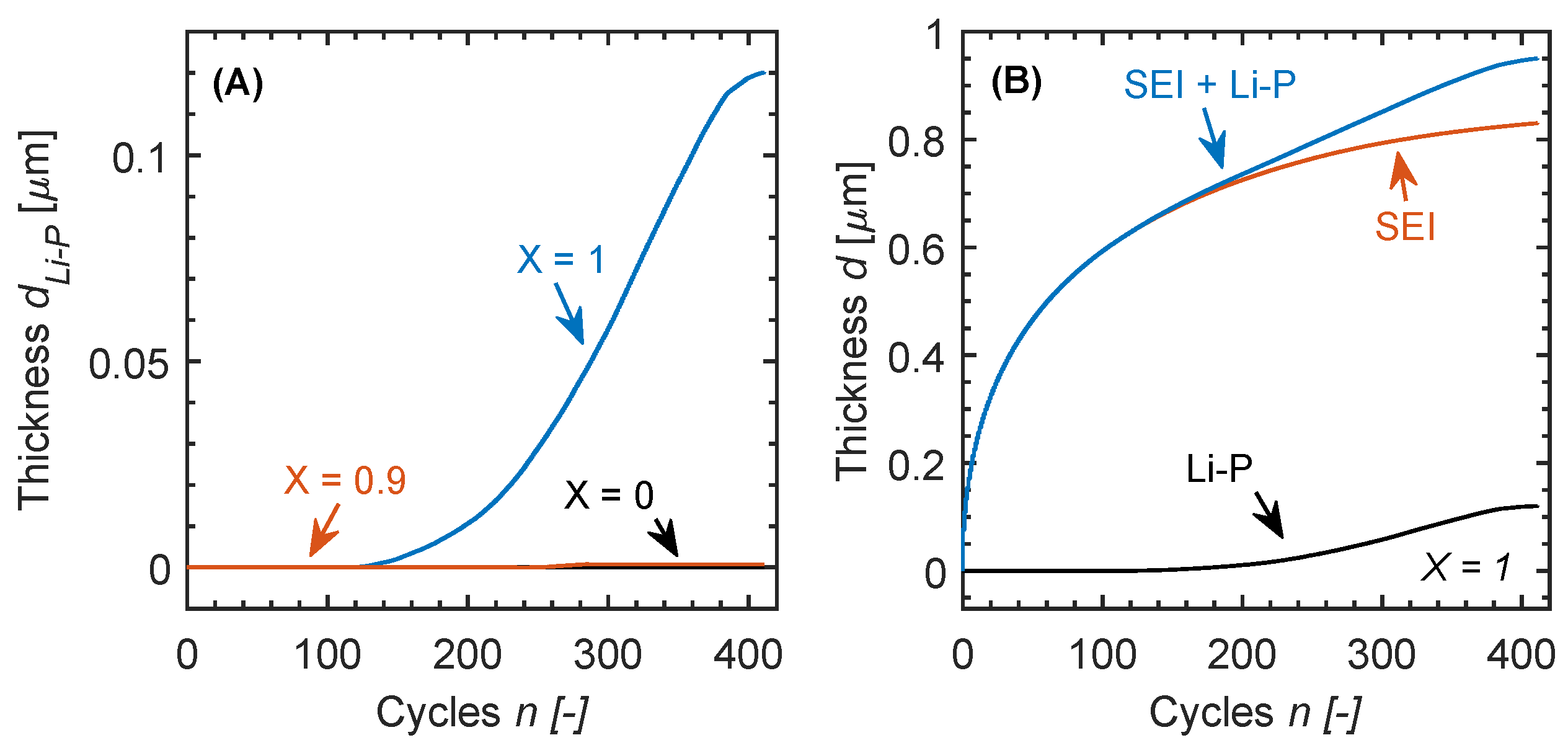
3.2. Simulation Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Abbreviations | |
| P2D | pseudo two-dimensional |
| SEI | solid electrolyte interface |
| Li-P | lithium plating |
| Li-ion | lithium ion |
| CTP | charge transfer process |
| SPD | solid diffusion process |
| SOC | state of charge |
| EC | ethylene carbonate |
| CC | constant current |
| CV | constant voltage |
| An. | anode |
| Ca. | cathode |
| Lit. | literature |
| Cal. | calculated |
| Symbols | |
| j | ionic current density |
| exchange current density | |
| v | drift velocity |
| u | electrical mobility of ions |
| Z | valence number |
| q | charge |
| K | Boltzamn constant |
| R | ideal gas constant |
| F | Faraday constant |
| D | diffusion coefficient |
| potential | |
| i | current density |
| conductivity | |
| electric field | |
| transfer coefficient | |
| surface overpotential | |
| E | potential |
| T | temperature |
| C | concentration |
| T | temperature |
| volume fraction | |
| Bruggeman exponent | |
| t | characteristic time |
| L | thickness |
| separator thickness | |
| Subscripts and Superscripts | |
| ion species | |
| total | |
| intercalation | |
| solid electrolyte interface | |
| lithium plating | |
| a | anodic, anode |
| c | cathodic, cathode |
| s | solid |
| anode, cathode, separator | |
| l | liquid |
| i | interface |
| equilibrium | |
| effective | |
| maximum | |
| average | |
| reference | |
References
- Brandt, K. Historical development of secondary lithium batteries. Solid State Ionics 1994, 69, 173–183. [Google Scholar] [CrossRef]
- Besenhard, J. The electrochemical preparation and properties of ionic alkali metal-and NR4-graphite intercalation compounds in organic electrolytes. Carbon 1976, 14, 111–115. [Google Scholar] [CrossRef]
- Whittingham, M.S. Electrical energy storage and intercalation chemistry. Science 1976, 192, 1126–1127. [Google Scholar] [CrossRef] [PubMed]
- Slane, S.M.; Foster, D.L. Lithium Ion Rechargeable Intercallation Cell. US Patent No. 07/625,181, 7 July 1992. [Google Scholar]
- Yoshino, A. Development of the lithium-ion battery and recent technological trends. In Lithium-Ion Batteries; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–20. [Google Scholar]
- Almeida, A.; Sousa, N.; Coutinho-Rodrigues, J. Quest for Sustainability: Life-Cycle Emissions Assessment of Electric Vehicles Considering Newer Li-Ion Batteries. Sustainability 2019, 11, 2366. [Google Scholar] [CrossRef]
- Onat, N.; Kucukvar, M.; Tatari, O. Towards life cycle sustainability assessment of alternative passenger vehicles. Sustainability 2014, 6, 9305–9342. [Google Scholar] [CrossRef]
- Burns, J.; Stevens, D.; Dahn, J. In-situ detection of lithium plating using high precision coulometry. J. Electrochem. Soc. 2015, 162, A959–A964. [Google Scholar] [CrossRef]
- Fleischhammer, M.; Waldmann, T.; Bisle, G.; Hogg, B.I.; Wohlfahrt-Mehrens, M. Interaction of cyclic ageing at high-rate and low temperatures and safety in lithium-ion batteries. J. Power Sources 2015, 274, 432–439. [Google Scholar] [CrossRef]
- Waldmann, T.; Hogg, B.I.; Wohlfahrt-Mehrens, M. Li plating as unwanted side reaction in commercial Li-ion cells—A review. J. Power Sources 2018, 384, 107–124. [Google Scholar] [CrossRef]
- Uhlmann, C.; Illig, J.; Ender, M.; Schuster, R.; Ivers-Tiffée, E. In situ detection of lithium metal plating on graphite in experimental cells. J. Power Sources 2015, 279, 428–438. [Google Scholar] [CrossRef]
- Doh, C.H.; Han, B.C.; Jin, B.S.; Gu, H.B. Structures and formation energies of LixC6 (x = 1-3) and its homologues for lithium rechargeable batteries. Bull. Korean Chem. Soc 2011, 32, 2045–2050. [Google Scholar] [CrossRef]
- Legrand, N.; Knosp, B.; Desprez, P.; Lapicque, F.; Raël, S. Physical characterization of the charging process of a Li-ion battery and prediction of Li plating by electrochemical modelling. J. Power Sources 2014, 245, 208–216. [Google Scholar] [CrossRef]
- Atkins, P.W.; De Paula, J.; Keeler, J. Atkins’ Physical Chemistry; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Hein, S.; Latz, A. Influence of local lithium metal deposition in 3D microstructures on local and global behavior of Lithium-ion batteries. Electrochim. Acta 2016, 201, 354–365. [Google Scholar] [CrossRef]
- Wu, M.S.; Chiang, P.C.J.; Lin, J.C. Electrochemical investigations on advanced lithium-ion batteries by three-electrode measurements. J. Electrochem. Soc. 2005, 152, A47–A52. [Google Scholar] [CrossRef]
- Lin, H.P.; Chua, D.; Salomon, M.; Shiao, H.; Hendrickson, M.; Plichta, E.; Slane, S. Low-temperature behavior of Li-ion cells. Electrochem. Solid-State Lett. 2001, 4, A71–A73. [Google Scholar] [CrossRef]
- Waldmann, T.; Hogg, B.I.; Kasper, M.; Grolleau, S.; Couceiro, C.G.; Trad, K.; Matadi, B.P.; Wohlfahrt-Mehrens, M. Interplay of operational parameters on lithium deposition in lithium-ion cells: systematic measurements with reconstructed 3-electrode pouch full cells. J. Electrochem. Soc. 2016, 163, A1232–A1238. [Google Scholar] [CrossRef]
- Persson, K.; Sethuraman, V.A.; Hardwick, L.J.; Hinuma, Y.; Meng, Y.S.; Van Der Ven, A.; Srinivasan, V.; Kostecki, R.; Ceder, G. Lithium diffusion in graphitic carbon. J. Phys. Chem. Lett. 2010, 1, 1176–1180. [Google Scholar] [CrossRef]
- Persson, K.; Hinuma, Y.; Meng, Y.S.; Van der Ven, A.; Ceder, G. Thermodynamic and kinetic properties of the Li-graphite system from first-principles calculations. Phys. Rev. B 2010, 82, 125416. [Google Scholar] [CrossRef]
- Smart, M.; Ratnakumar, B. Effects of electrolyte composition on lithium plating in lithium-ion cells. J. Electrochem. Soc. 2011, 158, A379–A389. [Google Scholar] [CrossRef]
- Ishihara, Y.; Miyazaki, K.; Fukutsuka, T.; Abe, T. Kinetics of lithium-ion transfer at the interface between Li4Ti5O12 thin films and organic electrolytes. ECS Electrochem. Lett. 2014, 3, A83–A86. [Google Scholar] [CrossRef]
- Abe, T.; Sagane, F.; Ohtsuka, M.; Iriyama, Y.; Ogumi, Z. Lithium-ion transfer at the interface between lithium-ion conductive ceramic electrolyte and liquid electrolyte—A key to enhancing the rate capability of lithium-ion batteries. J. Electrochem. Soc. 2005, 152, A2151–A2154. [Google Scholar] [CrossRef]
- Jow, T.R.; Delp, S.A.; Allen, J.L.; Jones, J.P.; Smart, M.C. Factors Limiting Li+ Charge Transfer Kinetics in Li-Ion Batteries. J. Electrochem. Soc. 2018, 165, A361–A367. [Google Scholar] [CrossRef]
- Arora, P.; Doyle, M.; White, R.E. Mathematical modeling of the lithium deposition overcharge reaction in lithium-ion batteries using carbon-based negative electrodes. J. Electrochem. Soc. 1999, 146, 3543–3553. [Google Scholar] [CrossRef]
- Tang, M.; Albertus, P.; Newman, J. Two-dimensional modeling of lithium deposition during cell charging. J. Electrochem. Soc. 2009, 156, A390–A399. [Google Scholar] [CrossRef]
- Jiang, F.; Peng, P. Elucidating the performance limitations of lithium-ion batteries due to species and charge transport through five characteristic parameters. Sci. Rep. 2016, 6, 32639. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.; Fuller, T.F.; Newman, J. Modeling of galvanostatic charge and discharge of the lithium/polymer/insertion cell. J. Electrochem. Soc. 1993, 140, 1526–1533. [Google Scholar] [CrossRef]
- Fuller, T.F.; Doyle, M.; Newman, J. Simulation and optimization of the dual lithium ion insertion cell. J. Electrochem. Soc. 1994, 141, 1–10. [Google Scholar] [CrossRef]
- Doyle, C.M. Design and Simulation of Lithium Rechargeable Batteries. Ph.D. Thesis, University of California, Berkeley, CA, USA, 1995. [Google Scholar]
- Doyle, M.; Newman, J.; Gozdz, A.S.; Schmutz, C.N.; Tarascon, J.M. Comparison of modeling predictions with experimental data from plastic lithium ion cells. J. Electrochem. Soc. 1996, 143, 1890–1903. [Google Scholar] [CrossRef]
- Müller, D.; Dufaux, T.; Birke, K.P. Model-Based Investigation of Porosity Profiles in Graphite Anodes Regarding Sudden-Death and Second-Life of Lithium Ion Cells. Batteries 2019, 5, 49. [Google Scholar] [CrossRef]
- Yang, X.G.; Leng, Y.; Zhang, G.; Ge, S.; Wang, C.Y. Modeling of lithium plating induced aging of lithium-ion batteries: Transition from linear to nonlinear aging. J. Power Sources 2017, 360, 28–40. [Google Scholar] [CrossRef]
- Darling, R.; Newman, J. Modeling side reactions in composite li y mn2 o 4 electrodes. J. Electrochem. Soc. 1998, 145, 990–998. [Google Scholar] [CrossRef]
- Cabañero, M.A.; Altmann, J.; Gold, L.; Boaretto, N.; Müller, J.; Hein, S.; Zausch, J.; Kallo, J.; Latz, A. Investigation of the temperature dependence of lithium plating onset conditions in commercial Li-ion batteries. Energy 2019, 171, 1217–1228. [Google Scholar] [CrossRef]
- Eddahech, A.; Briat, O.; Vinassa, J.M. Thermal characterization of a high-power lithium-ion battery: Potentiometric and calorimetric measurement of entropy changes. Energy 2013, 61, 432–439. [Google Scholar] [CrossRef]
- Safari, M.; Morcrette, M.; Teyssot, A.; Delacourt, C. Multimodal physics-based aging model for life prediction of Li-ion batteries. J. Electrochem. Soc. 2009, 156, A145–A153. [Google Scholar] [CrossRef]
- Kindermann, F.M.; Keil, J.; Frank, A.; Jossen, A. A SEI modeling approach distinguishing between capacity and power fade. J. Electrochem. Soc. 2017, 164, E287–E294. [Google Scholar] [CrossRef]
- Petzl, M.; Kasper, M.; Danzer, M.A. Lithium plating in a commercial lithium-ion battery—A low-temperature aging study. J. Power Sources 2015, 275, 799–807. [Google Scholar] [CrossRef]


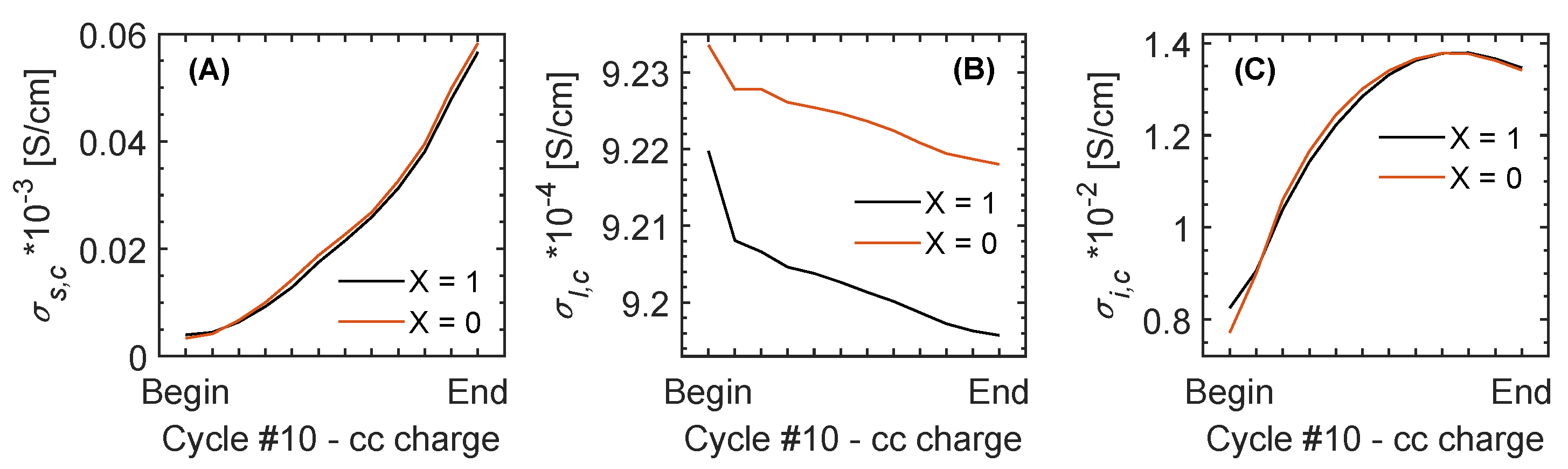
| Parameters | Anode | Separator | Cathode |
|---|---|---|---|
| Thickness L () | 116 | 16 | 88.7 |
| Particle radius () | 8.8 | - | 6.5 |
| Porosity (%) | 0.26 | 0.5 | 0.24 |
| Bruggeman exponent (-) | 1.8 | 1.5 | 1.56 |
| Initial electrolyte concentration (mol/m) | 1200 | ||
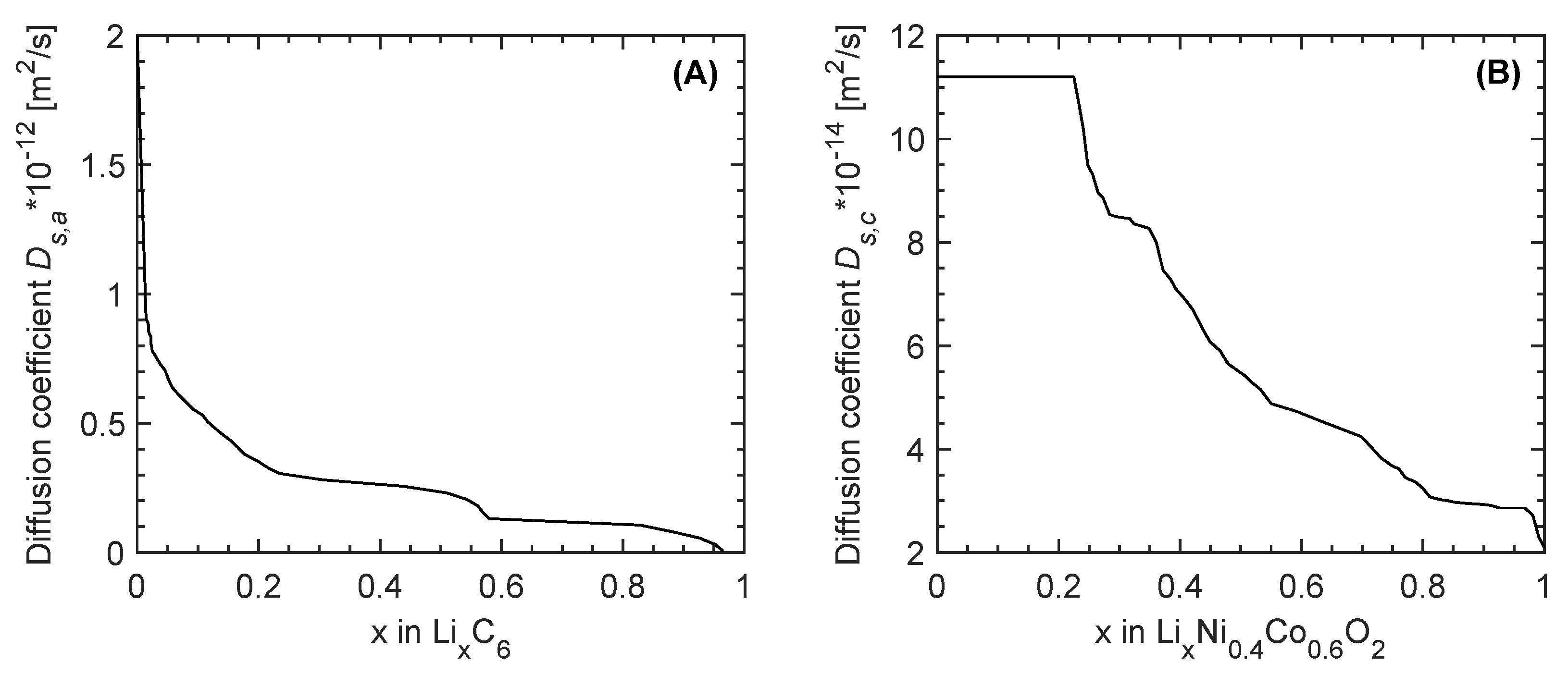
| Diffusion coefficient in solid (m/s) | Figure 2A [35] | - | Figure 2B [35] |
| Diffusion coefficient in liquid (m/s) | 3.7 × 10 * | ||
| Ionic conductivity in liquid (S/m) | 8.735 × 10 * | ||
| Maximum Li concentration in solid (mol/m) | 27,880 | - | 48,580 |
| Anodic/Cathodic transfer coefficient , (-) | 0.5 | 0.5 | |
| Transference number (-) | 0.577 | ||
| Simulation conditions | Values | ||
| Temperature T (K) | 298.15 | ||
| Lower and upper voltage boundary (V) | 2.7–4.2 | ||
| Charge and discharge rate | 0.5 | ||
| Equilibrium potential SEI formation (V) | 0.4 [37] | ||
| Equilibrium potential lithium plating (V) | 0 | ||
| Cathodic charge transfer coefficients for side reactions (/) | 0.5 [34] | ||
| [s] | [S/cm] | [s] | [S/cm] | [s] | [S/cm] | ||||
|---|---|---|---|---|---|---|---|---|---|
| Lit. | Cal. | Cal. | Lit. | Cal. | Cal. | Lit. | Cal. | Cal. | |
| Anode | 180 | 174 | 1.12 × 10 | 3.2 × 10 | 2.2 × 10 | (3.19-2.98) × 10 | 147.7 | 130 | 2.88 × 10 |
| Cathode | 103.2 | 1.21 × 10 | 71.1 | 71.1 | 1.28 × 10 | 101.3 | 92.3 | 1.16 × 10 | |
| Separator | 8.8 | 2.15 × 10 | - | - | - | - | - | - | |
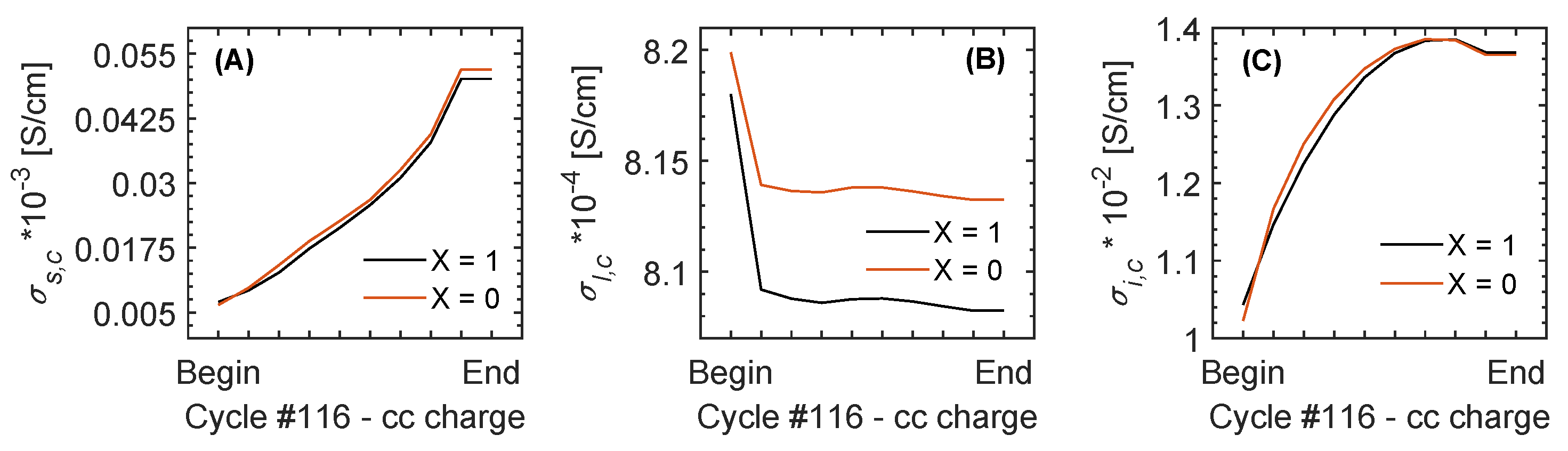
| Cycle | Charge – CC | [S/cm] | [S/cm] | [S/cm] |
|---|---|---|---|---|
| An. | An. | An. | ||
| 10 | Begin | 1.0 × 10 | (4.8-4.7) × 10 | 1.3 × 10 |
| End | (7.3-3.5) × 10 | (4.7-4.6) × 10 | (4.5-4.0) × 10 | |
| 50 | Begin | (9.9-10.3) × 10 | (2.5-2.4) × 10 | 0.7 × 10 |
| End | (14.7-3.7) × 10 | (2.5-2.4) × 10 | (2.6-2.2) × 10 | |
| 100 | Begin | (9.2-10.4) × 10 | 1.5 × 10 | (0.5-0.4) × 10 |
| End | (18.3-3.3) × 10 | (1.5-1.4) × 10 | (1.6-1.4) × 10 | |
| 116 | Begin | (9.1-10.4) × 10 | 1.3 × 10 | (0.5-0.4) × 10 |
| End | (19.1-3.1) × 10 | 1.3 × 10 | (1.4-1.2) × 10 | |
| 150 | Begin | (8.8-10.5) × 10 | 1.0 × 10 | (0.4-0.3) × 10 |
| End | (20.1-2.8) × 10 | (1.1-1.0) × 10 | (1.1-0.9) × 10 | |
| 230 | Begin | (7.2-10.5) × 10 | (0.6-0.5) × 10 | (0.3-0.2) × 10 |
| End | (21.7-0.2) × 10 | (0.6-0.5) × 10 | (0.7-0.3) × 10 | |
| Ca. | Ca. | Ca. | ||
| 10 | Begin | (3.4-4.0) × 10 | 9.2 × 10 | 0.8 × 10 |
| End | (5.8-5.7) × 10 | 9.2 × 10 | 1.3 × 10 | |
| 50 | Begin | (5.1-5.7) × 10 | (8.8-8.7) × 10 | (0.9-1.0) × 10 |
| End | (5.6-5.4) × 10 | 8.7 × 10 | 1.4 × 10 | |
| 100 | Begin | (6.2-6.7) × 10 | 8.3 × 10 | 1.0 × 10 |
| End | (5.3-5.1) × 10 | (8.3-8.2) × 10 | 1.4 × 10 | |
| 116 | Begin | (6.5-7.0) × 10 | 8.2 × 10 | 1 × 10 |
| End | (5.2-5.0) × 10 | 8.1 × 10 | 1.4 × 10 | |
| 150 | Begin | (6.9-7.5) × 10 | 8 × 10 | (1.0-1.1) × 10 |
| End | (5.0-4.7) × 10 | (7.9-7.8) × 10 | 1.4 × 10 | |
| 230 | Begin | (8.1-9.2) × 10 | 7.6 × 10 | 1.1 × 10 |
| End | (5.7-5.6) × 10 | 7.4 × 10 | 1.3 × 10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Momeni Boroujeni, S.; Birke, K.P. Study of a Li-Ion Cell Kinetics in Five Regions to Predict Li Plating Using a Pseudo Two-Dimensional Model. Sustainability 2019, 11, 6392. https://doi.org/10.3390/su11226392
Momeni Boroujeni S, Birke KP. Study of a Li-Ion Cell Kinetics in Five Regions to Predict Li Plating Using a Pseudo Two-Dimensional Model. Sustainability. 2019; 11(22):6392. https://doi.org/10.3390/su11226392
Chicago/Turabian StyleMomeni Boroujeni, Sanaz, and Kai Peter Birke. 2019. "Study of a Li-Ion Cell Kinetics in Five Regions to Predict Li Plating Using a Pseudo Two-Dimensional Model" Sustainability 11, no. 22: 6392. https://doi.org/10.3390/su11226392
APA StyleMomeni Boroujeni, S., & Birke, K. P. (2019). Study of a Li-Ion Cell Kinetics in Five Regions to Predict Li Plating Using a Pseudo Two-Dimensional Model. Sustainability, 11(22), 6392. https://doi.org/10.3390/su11226392






