Maize Open-Pollinated Populations Physiological Improvement: Validating Tools for Drought Response Participatory Selection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Setup
2.2.1. Field Conditions
2.2.2. Growth Chamber Conditions
2.3. Measured Parameters
2.3.1. Soil and Substrate Water Content
2.3.2. Individual Leaf Gas Exchange
2.3.3. Plant Water Status
2.3.4. Leaf Temperature
2.3.5. Chlorophyll a Fluorescence
2.3.6. Leaf Photosynthetic Pigments
2.3.7. Growth and Morphology
2.4. Data Analysis
3. Results
3.1. Field Trial
3.1.1. Soil Water Content
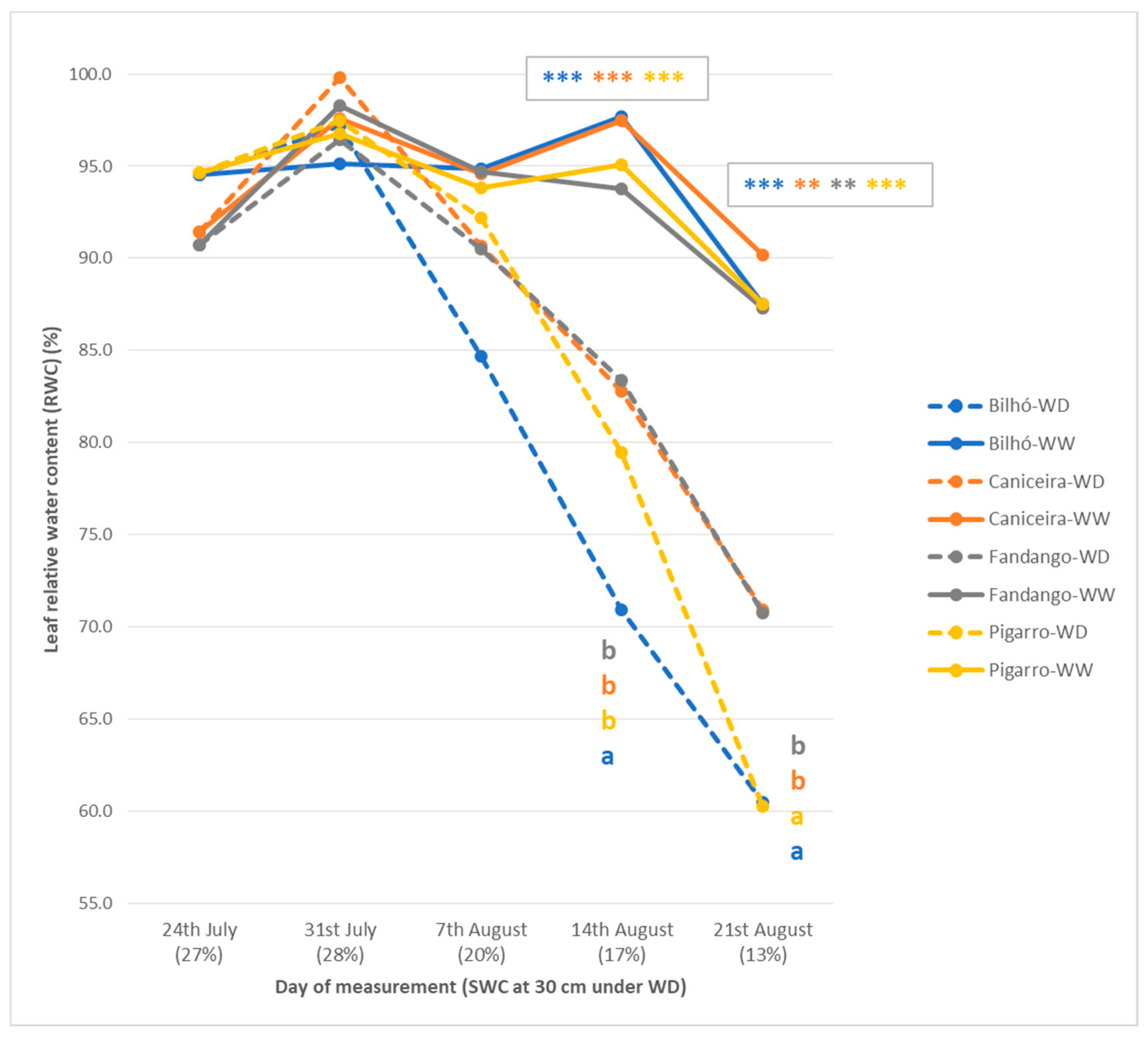
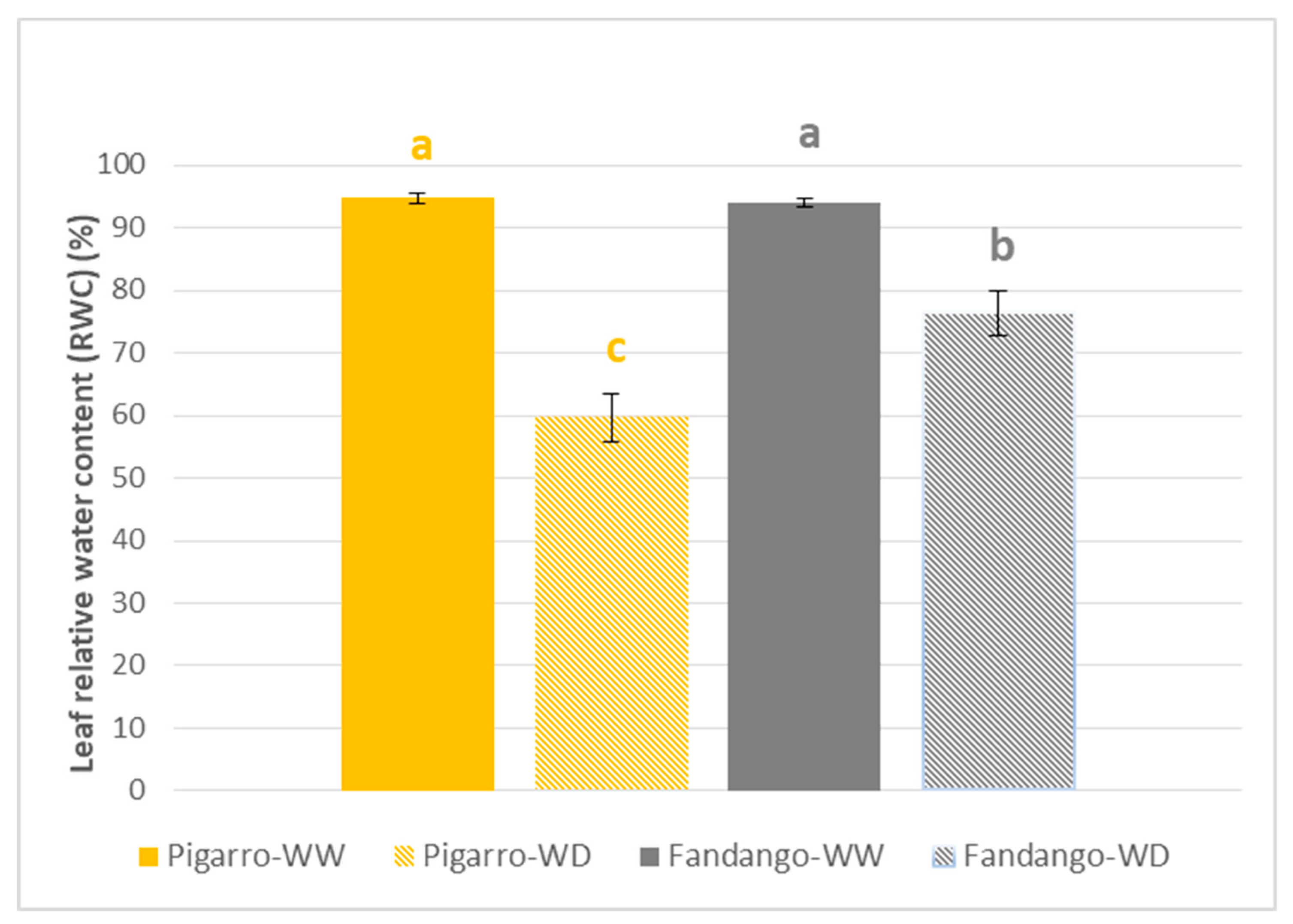
3.1.2. Plant Water Status, Leaf Temperature and Gas Exchange Performance
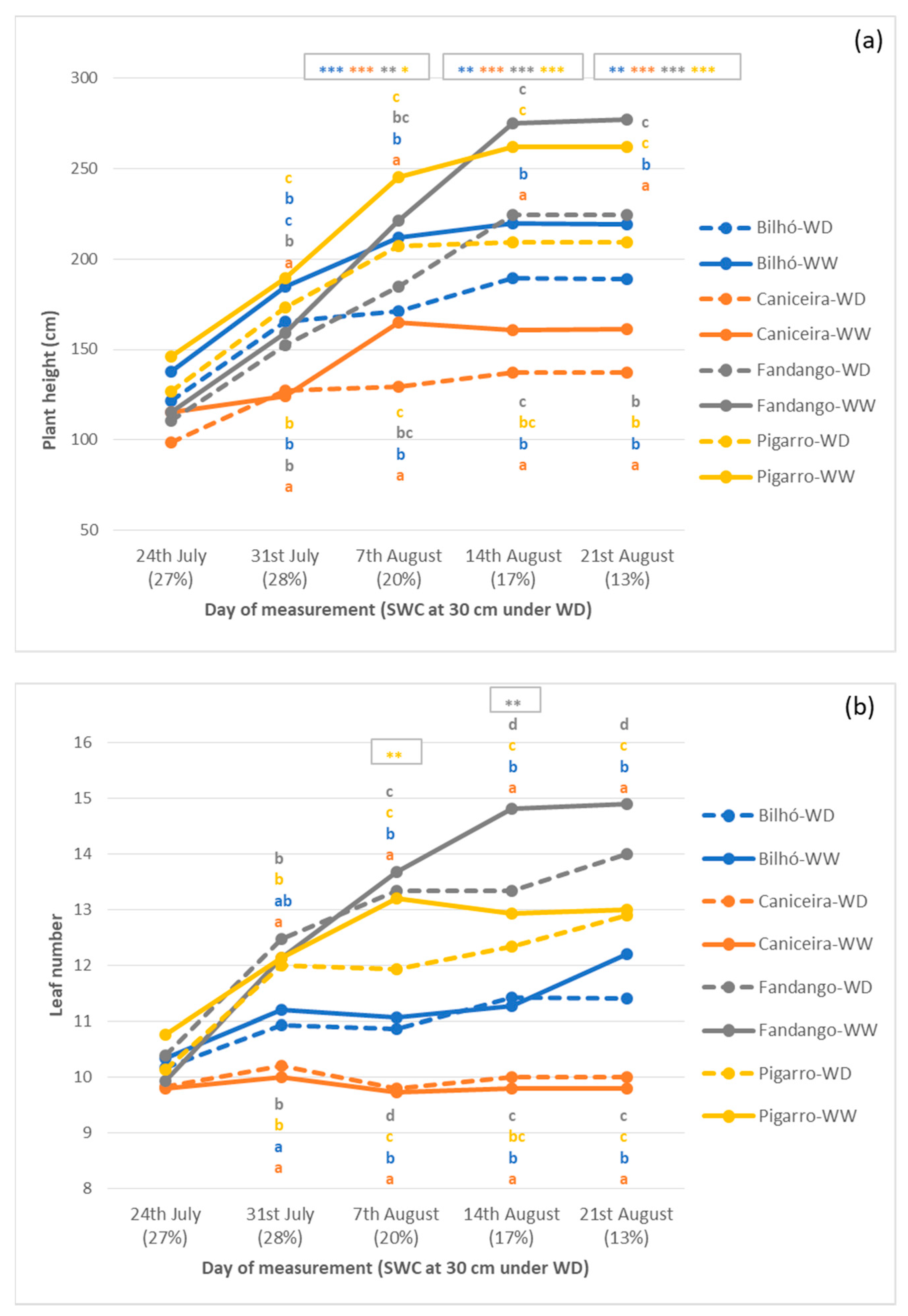
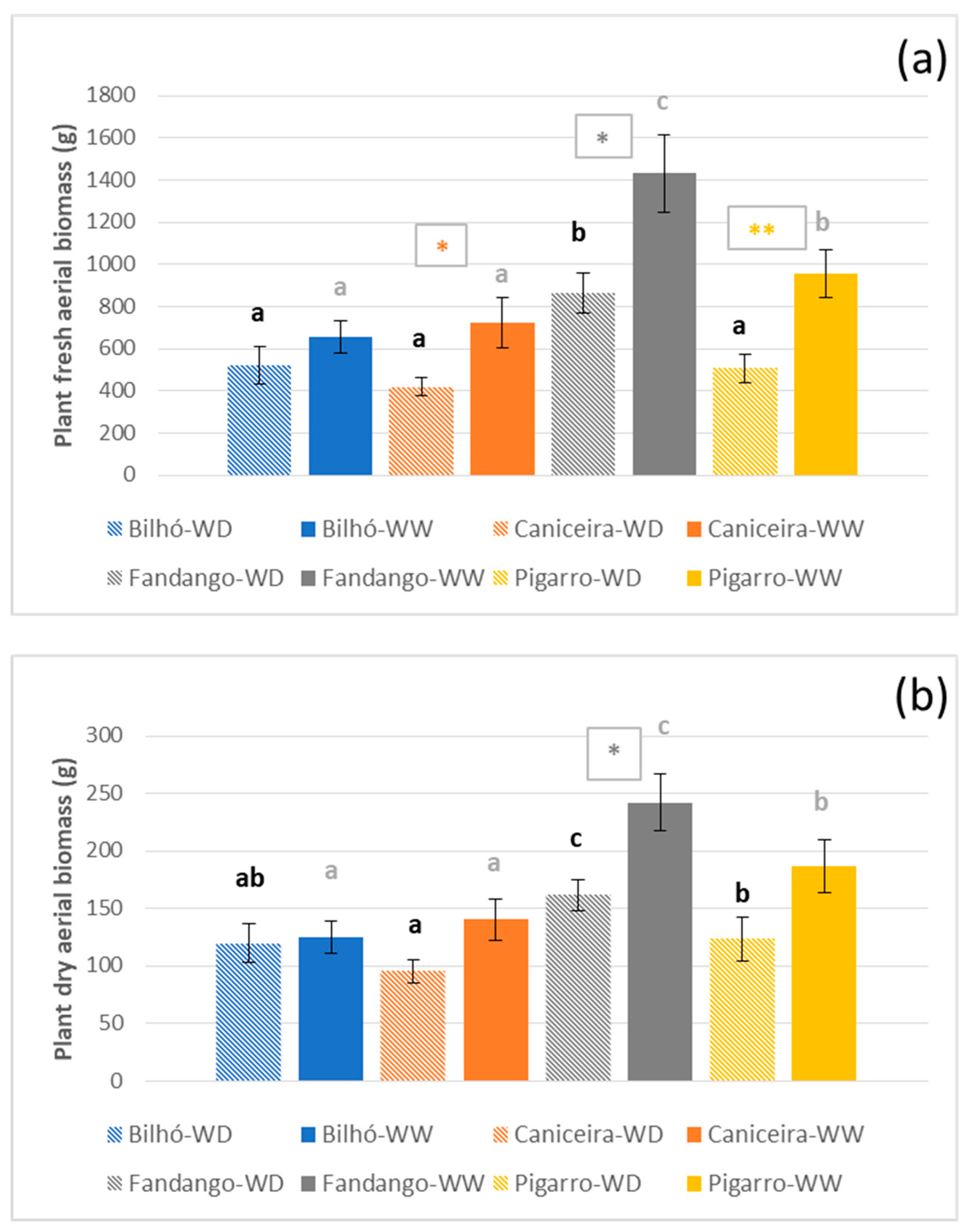
3.1.3. Growth, Morphology and Yield Components
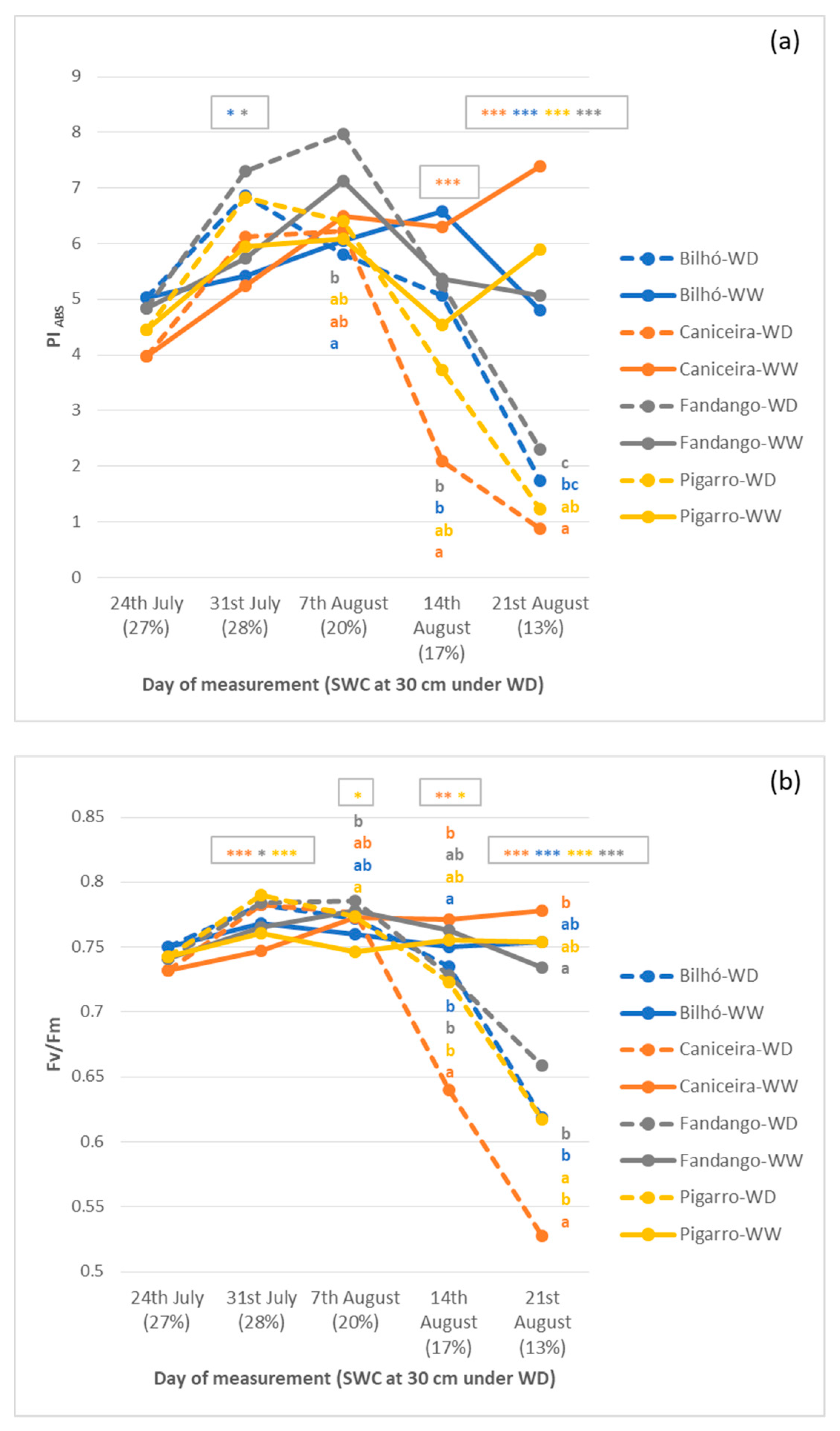
3.1.4. OJIP Parameters and Chl a Fluorescence
3.1.5. Correlation Analysis
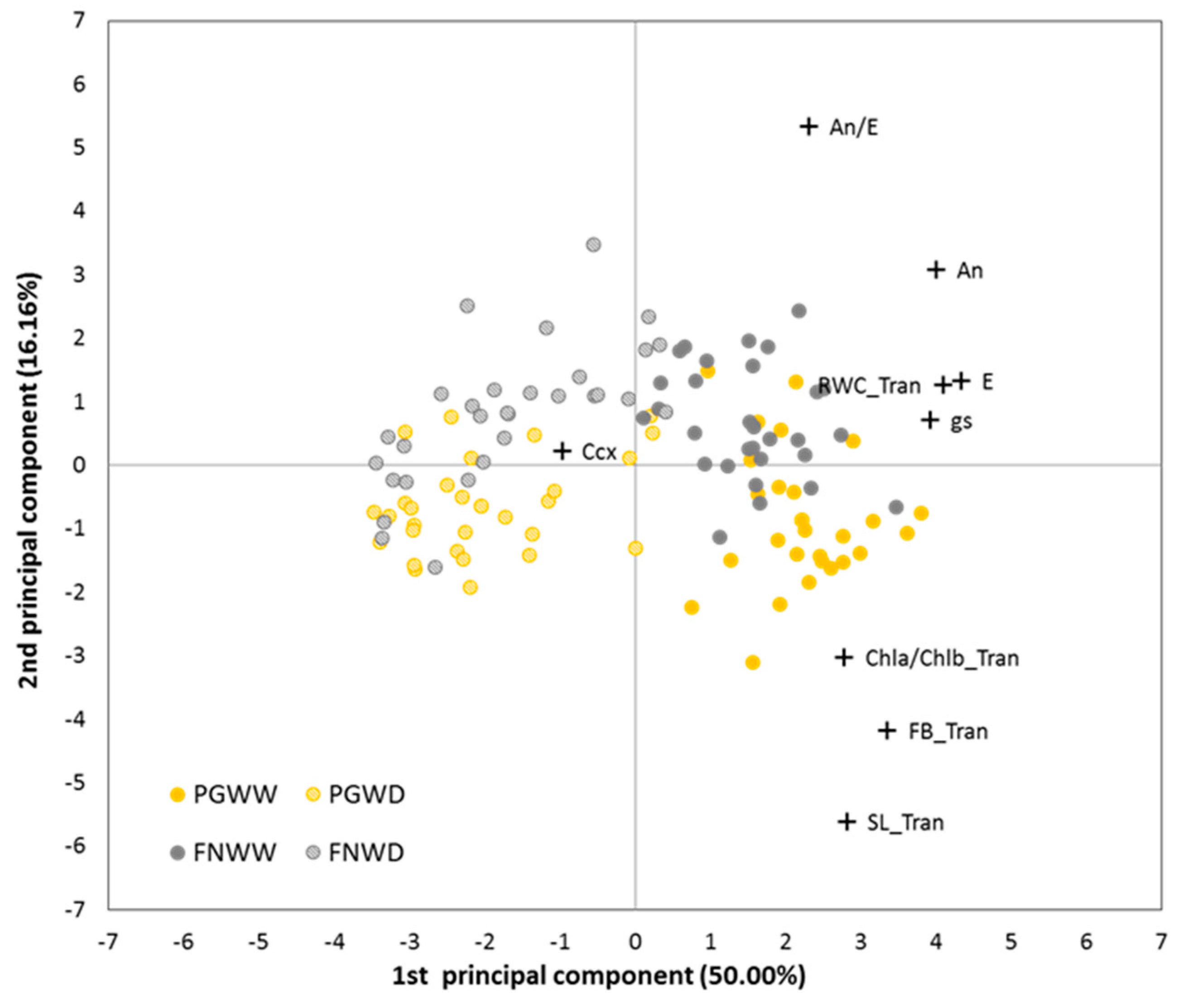
3.1.6. Multivariate Analysis
3.2. Growth Chamber Trial
3.2.1. Soil Water Content and Plant Water Status
3.2.2. Individual Leaf Gas Exchange and Leaf Temperature
3.2.3. Plant Growth and Morphology
3.2.4. Leaf Photosynthetic Pigments
3.2.5. Correlation Analysis
3.2.6. Multivariate Analysis
4. Discussion
4.1. Portuguese Maize Populations Depict Significant Variation in Response to Water Availability
4.2. Diversity of Responses under Moderate Stress, Similar Strategies under Severe Stress
4.3. High Chances to Identify Individual Maize Genotypes within OPVs Better Performing under WD
4.4. Remote Does Not Always Mean Efficient
4.5. Performance Index (PIABS), an Expedite, Low-Cost, and Efficient Field Phenotyping Approach for Drought Responses in Maize under Moderate Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prohens, J. Plant Breeding: A success story to be continued thanks to the advances in genomics. Front. Plant Sci. 2011, 2, 51. [Google Scholar] [CrossRef] [PubMed]
- Hallauer, A.R.; Carena, M.J. Maize breeding. In Handbook of Plant Breeding: Cereals; Carena, M.J., Ed.; Springer: New York, NY, USA, 2009; pp. 3–98. [Google Scholar]
- Vaz Patto, M.C.; Mendes-Moreira, P.M.; Alves, M.L.; Mecha, E.; Brites, C.; Bronze, R.; Pego, S. Participatory plant quality breeding: An ancient art revisited by knowledge sharing. The Portuguese experience. In Plant Breeding from Laboratories to Fields; Andersen, S.B., Ed.; InTech: London, UK, 2013; Chapter 10; pp. 255–288. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Grando, S. Participatory plant breeding: Who did it, who does it and where? Exp. Agric. 2019, 33, 335–344. [Google Scholar] [CrossRef]
- Weltzien, E.; Christinck, A. Participatory breeding: Developing improved and relevant crop varieties with farmers. In Agricultural Systems, 2nd ed.; Agroecology and Rural Innovation Development. Agricultural Systems; Academic Press: Cambridge, MA, USA, 2017; pp. 259–301. [Google Scholar]
- Vaz Patto, M.C.; Moreira, P.M.; Carvalho, V.; Pêgo, S. Collecting maize with technological ability for bread production in Portugal. Genet. Res. Crop Evol. 2007, 54, 1555–1563. [Google Scholar] [CrossRef]
- Alves, M.L.; Carbas, B.; Gaspar, D.; Paulo, M.; Brites, C.; Mendes-Moreira, P.; Brites, C.M.; Malosetti, M.; van Eeuwijk, F.; Vaz Patto, M.C. Genome-wide association study for kernel composition and flour pasting behavior in wholemeal maize flour. BMC Plant Biol. 2019, 19, 123. [Google Scholar] [CrossRef]
- Pêgo, S.; Martins, R. Resistance or tolerance? Philosophy may be the answer. In Proceedings of the XIX Conference of the International Working Group on Ostrinia Nubilabis and Other Maize Pests, Guimarães, Portugal, 30 August–5 September 1997; IOBC, IWGO: Guimarães, Portugal, 1997; pp. 303–341. [Google Scholar]
- Mendes-Moreira, P.M.M.; Vaz Patto, M.C.; Mota, M.; Mendes-Moreira, J.; Santos, J.P.N.; Santos, J.P.P.; Andrade, E.; Hallauer, A.R.; Pego, S.E. Fandango: Long term adaptation of exotic germplasm to a Portuguese on-Farm-conservation and breeding project. Maydica 2009, 54, 269–285. [Google Scholar]
- Mendes-Moreira, P.; Satovic, Z.; Mendes-Moreira, J.; Santos, J.P.; Nina Santos, J.P.; Pêgo, S.E.; Vaz Patto, M.C. Maize participatory breeding in Portugal. Comparison of farmer’s and breeder’s on-farm selection. Plant Breed. 2017, 136, 861–871. [Google Scholar] [CrossRef]
- Alves, M.L.; Belo, M.; Carbas, B.; Brites, C.; Paulo, M.; Mendes-Moreira, P.; Brites, C.; Bronze, M.R.; Šatović, Z.; Vaz Patto, M.C. Long term on-farm participatory maize breeding by stratified mass selection retains molecular diversity while improving agronomic performance. Evol. Applic. 2018, 11, 254–270. [Google Scholar] [CrossRef]
- Moreira, P.M. Participatory maize breeding in Portugal. A case study. Acta Agron. Hung. 2006, 54, 431–439. [Google Scholar] [CrossRef]
- Mitter, H.; Larcher, M.; Schönhart, M.; Stöttinger, M.; Schmid, E. Exploring farmers’ climate change perceptions and adaptation intentions: Empirical evidence from Austria. Environ. Manag. 2019, 63, 804–821. [Google Scholar] [CrossRef]
- Resenzweig, C.; Elliott, J.; Deryng, D.; Ruane, A.C.; Müller, C.; Arneth, A.; Boote, K.J.; Folberth, C.; Glotter, M.; Khabarov, N. Assessing agricultural risks of climate change in the 21st century in a global gridded crop model intercomparison. Proc. Natl. Acad. Sci. USA 2014, 111, 3268–3273. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, X.; Lian, F.; Gao, Y.; Lin, D.; Wang, J. Drought risk assessment based on vulnerability surfaces: A case study of maize. Sustainability 2016, 8, 813–835. [Google Scholar] [CrossRef]
- Bänziger, M.; Araus, J.L. Recent advances in breeding maize for drought and salinity stress tolerance. In Advances in Molecular Breeding Toward Drought and Salt Tolerant Crops; Jenks, M.A., Hasegawa, P.M., Jain, S.M., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 587–601. [Google Scholar]
- Witt, S.; Galicia, L.; Lisec, J.; Cairns, J.; Tiessen, A.; Araus, J.L.; Palacios-Rojas, N.; Fernie, A.R. Metabolic and phenotypic responses of greenhouse-grown maize hybrids to experimentally controlled drought stress. Mol. Plant 2012, 5, 401–417. [Google Scholar] [CrossRef] [PubMed]
- European Environment Agency (EEA). Water Resources in Europe in the Context of Vulnerability. In EEA 2012 State of Water Assessment. EEA Report No 11/2012ISSN 1725-9177. Available online: https://www.eea.europa.eu/publications/water-resources-and-vulnerability (accessed on 21 August 2019).
- Costa, J.M.; Egipto, R.; Sánchez-Virosta, A.; Lopes, C.M.; Chaves, M.M. Canopy and soil thermal patterns to support water and heat stress management in vineyards. Agric. Water Manag. 2019, 216, 484–496. [Google Scholar] [CrossRef]
- Fita, A.; Rodríguez-Burruezo, A.; Boscaiu, M.; Prohens, J.; Vicente, O. Breeding and domesticating crops adapted to drought and salinity: A new paradigm for increasing food production. Front. Plant Sci. 2015, 6, 978. [Google Scholar] [CrossRef]
- Araus, J.L.; Serret, M.D.; Edmeades, G.O. Phenotyping maize for adaptation to drought. Front. Physiol. 2012, 3, 305–325. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, M.S.; Baresel, J.P.; Desclaux, D.; Goldringer, I.; Hoad, S.; Kovacs, G.; Löschenberger, F.; Miedaner, T.; Østergård, H.; Lammerts van Bueren, E.T. Developments in breeding cereals for organic agriculture. Euphytica 2008, 163, 323–346. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, D.I.; Hodgkin, T.; Sthapit, B.R.; Fadda, C.; Lopez-Noriega, I. An heuristic framework for identifying multiple ways of supporting the conservation and use of traditional crop varieties within the agricultural production system. Crit. Rev. Plant Sci. 2011, 30, 125–176. [Google Scholar] [CrossRef]
- Vaz Patto, M.C.; Alves, M.L.; Almeida, N.F.; Santos, C.; Mendes-Moreira, P.; Satovic, Z.; Brites, C. Is the bread making technological ability of Portuguese traditional maize landraces associated with their genetic diversity? Maydica 2009, 54, 297–311. [Google Scholar]
- Alves, M.L.; Brites, C.; Paulo, M.; Carbas, B.; Belo, M.; Mendes-Moreira, P.; Brites, C.; Bronze, M.R.; Gunjaca, J.; Satovic, Z.; et al. Setting up decision-making tools towards a quality-oriented participatory maize breeding program. Front. Plant Sci. 2017, 8, 2203. [Google Scholar] [CrossRef]
- Cruz de Carvalho, R.; Cunha, A.; Marques da Silva, J. Photosynthesis by six Portuguese maize cultivars during drought stress and recovery. Acta Physiol. Plant 2011, 33, 359–374. [Google Scholar] [CrossRef]
- Garcia-Tejero, I.V.; Costa, J.M.; Nunes da Lima, R.S.; Duran-Zuago, V.H.; Chaves, M.M.; Vaz Patto, M.C. Thermal imaging to phenotype traditional maize landraces for drought tolerance. Com. Sci. 2015, 6, 334–343. [Google Scholar] [CrossRef] [Green Version]
- Moreira, P.M.R.M.; Pêgo, S.E.; Vaz Patto, M.C.; Hallauer, A.R. Comparison of selection methods on Pigarro, a Portuguese improved maize population with fasciation expression. Euphytica 2008, 163, 481–499. [Google Scholar] [CrossRef]
- Mendes-Moreira, P.; Mendes-Moreira, J.; Fernandes, A.; Andrade, E.; Hallauer, A.R.; Pêgo, S.E.; Vaz Patto, M.C. Is ear value an effective indicator for maize yield evaluation? Field Crop Res. 2014, 161, 75–86. [Google Scholar] [CrossRef]
- Araus, J.L.; Slafer, G.A.; Reynolds, M.P.; Royo, C. Plant breeding and drought in C3 cereals: What should we breed for? Ann. Bot. 2002, 89, 925–940. [Google Scholar] [CrossRef]
- Araus, J.L.; Slafer, G.A.; Royo, C.; Serret, M.D. Breeding for yield potential and stress adaptation in cereals. Crit. Rev. Plant Sci. 2008, 27, 1–36. [Google Scholar] [CrossRef]
- Monneveux, P.; Ribaut, J.M. Secondary traits for drought tolerance improvements in cereals. In Drought Adaptation in Cereals; Ribaut, J.M., Ed.; Haworth Press: Binhampton, UK, 2006; pp. 97–143. [Google Scholar]
- Lopes, M.S.; Araus, J.L.; van Heerden, P.D.R.; Foyer, C.H. Enhancing drought tolerance in C4 crops. J. Exp. Bot. 2011, 62, 3135–3153. [Google Scholar] [CrossRef]
- Prasanna, B.M.; Araus, J.L.; Crossa, J.; Cairns, J.E.; Palacios, N.; Mahuku, G.; Das, B.; Magorokosho, C. High-throughput and precision phenotyping in cereal breeding programs. In Cereal Genomics II, 3rd ed.; Kluwer Academic Publishers: New York, NY, USA, 2012; pp. 341–374. [Google Scholar]
- Fischer, K.S.; Fukai, S.; Kumar, A.; Leung, H.; Jongdee, B. Field phenotyping strategies and breeding for adaptation of rice to drought. Front. Physiol. 2012, 3, 282. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Long, S.P.; Bernacchi, C.J. Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J. Exp. Bot. 2003, 54, 2393–2401. [Google Scholar] [CrossRef] [Green Version]
- Jones, H.G. Plants and Microclimate, 2nd ed.; Cambridge University Press: Cambridge, UK, 1992; 423p. [Google Scholar]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Kummerlen, B.; Dauwe, S.; Schmundt, D.; Schurr, U. Thermography to measure water relations of plant leaves. In Handbook of Computer Vision and Applications: Systems and Applications; Jähne, B., Hauecker, H., Geiler, P., Eds.; Academic Press: Heidelberg, Germany, 1999; pp. 763–781. [Google Scholar]
- Jones, H.G.; Vaughan, R.A. Remote Sensing of Vegetation: Principles, Techniques and Applications; Oxford University Press: Oxford, UK, 2010; 284p. [Google Scholar]
- Costa, J.M.; Grant, O.; Chaves, M.M. Thermography to explore plant-environment interactions. J. Exp. Bot. 2013, 64, 3937–3949. [Google Scholar] [CrossRef] [PubMed]
- García-Tejero, I.F.; Hernández-Cotán, A.; Apolo, O.E.; Durán-Zuazo, V.H.; Aguilar Portero, M.; Rubio-Casal, A.E. Infrared thermography to select commercial varieties of maize in relation to drought adaptation. Quant. InfraRed Thermog. J. 2017, 14, 54–67. [Google Scholar] [CrossRef]
- Carrol, D.A., 2nd; Hansen, N.C.; Hopkins, B.G.; DeJonge, K.C. Leaf temperature of maize and Crop Water Stress Index with variable irrigation and nitrogen supply. Irrig. Sci. 2017, 35, 549–560. [Google Scholar] [CrossRef]
- Jones, H.G.; Serraj, R.; Loveys, B.R.; Xiong, L.; Wheaton, A.; Price, A.H. Thermal infrared image of crop canopies for the remote diagnosis and quantification of plant responses to water stress in the field. Funct. Plant Biol. 2009, 36, 978–989. [Google Scholar] [CrossRef]
- Thomas, H.; Ougham, H. The stay-green trait. J. Exp. Bot. 2014, 65, 3889–3900. [Google Scholar] [CrossRef] [PubMed]
- Bekavac, G.; Stojaković, M.; Ivanović, M.; Jocković, Đ.; Vasić, N.; Purar, B.; Boćanski, J.; Nastasić, A. Relationships of stay green trait in maize. Genetika 2002, 34, 33–40. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Ann. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Cendrero-Mateo, M.P.; Muller, O.; Albrecht, H.; Burkart, A.; Gatzke, S.; Janssen, B.; Keller, B.; Körber, N.; Kraska, T.; Matsubara, S.; et al. Field phenotyping: Concepts and examples to quantify dynamic plant traits across scales in the field. In Terrestrial Ecosystem Research Infrastructures; Chabbi, A., Loescher, H.W., Eds.; Taylor & Francis: London, UK, 2017; pp. 54–80. [Google Scholar]
- Araújo, S.S.; Duque, A.S.; Marques da Silva, J.; Santos, D.; Bernardes da Silva, A.; Fevereiro, P. Water deficit and recovery response of Medicago truncatula plants expressing the ELIP-like DSP22. Biol. Plant 2013, 57, 159–163. [Google Scholar] [CrossRef]
- Silvestre, S.; Araújo, S.S.; Vaz Patto, M.C.; Marques da Silva, J. Performance index: An expeditious tool to screen for improved drought resistance in the Lathyrus genus. J. Integ. Plant Biol. 2014, 56, 610–621. [Google Scholar] [CrossRef]
- Mendes-Moreira, P.; Alves, M.L.; Satovic, Z.; Santos, J.P.; Santos, J.N.; Souza, J.C.; Pego, S.E.; Hallauer, A.R.; Vaz Patto, M.C. Genetic architecture of ear fasciation in maize (Zea mays) under QTL scrutiny. PLoS ONE 2015, 10, e0124543. [Google Scholar] [CrossRef]
- Ritchie, S.W.; Hanway, J.J.; Benson, G.O. How a Corn Plant Develops; Special Report 48; Iowa State University of Science and Technology, Cooperative Extension Service: Ames, IA, USA, 1993; 21p. [Google Scholar]
- Topp, G.C.; Davis, J.L.; Annan, A.P. Electromagnetic determination of soil water content. Water Resour. Res. 1980, 16, 574–582. [Google Scholar] [CrossRef]
- Campbell, J.E. Dielectric properties and influence of conductivity in soils at one to fifty megahertz. Soil Sci. Soc. Am. J. 1990, 54, 332–341. [Google Scholar] [CrossRef]
- Whalley, W.R. Considerations on the use of time-domain reflectometry (TDR) for measuring soil moisture content. J. Soil Sci. 1993, 44, 1–9. [Google Scholar] [CrossRef]
- Whalley, W.R.; Cope, R.E.; Nicholl, C.J.; Whitmore, A.P. In-field calibration of a dielectric soil moisture meter designed for use in an access tube. Soil Use Manag. 2004, 20, 203–206. [Google Scholar] [CrossRef]
- Greco, R.; Guida, A. Field measurements of topsoil moisture profiles by vertical TDR probes. J. Hydrol. 2008, 348, 442–451. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S., 3rd; Pons, T.L. 2A-Photosynthesis, Respiration, and Long-Distance Transport. In Plant Physiological Ecology; Springer: New York, NY, USA, 2009; pp. 11–99. [Google Scholar] [CrossRef]
- Čatský, J. Determination of water deficit in disks cut out from leaf blades. Biol. Plant 1960, 2, 76. [Google Scholar] [CrossRef]
- Jones, H.G.; Stoll, M.; Santos, T.; de Souza, C.; Chaves, M.M.; Grant, O. Use of infrared thermography for monitoring stomatal closure in the field: Applications to grapevine. J. Exp. Bot. 2002, 53, 1–12. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanisms, Regulations and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor & Francis: London, UK, 2000; pp. 445–483. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M. Activity and heterogeneity of PSII probed in vivo by the chlorophyll a fluorescence rise O-(K)-J-I-P. In Photosynthesis: Mechanisms and Effects; Garab, G., Ed.; Kluwer Academic Publishers: Alphen aan den Rijn, The Netherlands, 1998; Volume V, pp. 4321–4324. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. In Current Protocols in Food Analytical Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001. [Google Scholar] [CrossRef]
- VSN. GenStat for Windows; VSN International: Hemel Hempstead, UK, 2017. [Google Scholar]
- Djemel, A.; Alvarez-Iglesias, L.; Pedrol, N.; Lopez-Malvar, A.; Ordás, A.; Revilla, P. Identification of drought tolerant populations at multi-stage growth phases in temperate maize germplasm. Euphytica 2018, 214, 138. [Google Scholar] [CrossRef]
- Djemel, A.; Cherchali, F.Z.; Benchikh-Le-Hocine, M.; Malvar, R.A.; Revilla, P. Assessment of drought tolerance among Algerian maize populations from oases of the Saharan. Euphytica 2018, 214, 149. [Google Scholar] [CrossRef]
- Ziygomo, C.; Bernardo, R. Drought tolerance in maize: Indirect selection through secondary traits versus genome-wide selection. Crop Sci. 2013, 52, 1269–1275. [Google Scholar] [CrossRef]
- Vadez, V.; Kholova, J.; Zaman-Allah, M.; Belko, N. Water: The most important ‘molecular’ component of water stress tolerance research. Funct. Plant Biol. 2013, 40. [Google Scholar] [CrossRef]
- Araus, J.L.; Sanchez, C.; Edmeades, G.O. Phenotyping maize for adaptation to drought. In Drought Phenotyping in Crops: From Theory to Practice; Monneveux, P., Ribaut, J.M., Eds.; CGIAR Generation Challenge Programme: Texcoco, Mexico, 2011; pp. 263–283. [Google Scholar]
- Gouesnard, B.; Zanetto, A.; Welcker, C. Identification of adaptation traits to drought in collections of maize landraces from southern Europe and temperate regions. Euphytica 2016, 209, 565–584. [Google Scholar] [CrossRef]
- Maiti, R.K.; Satya, P. Research advances in major cereal crops for adaptation to abiotic stresses. GM Crops Food 2014, 5, 259–279. [Google Scholar] [CrossRef] [Green Version]
- Tardieu, F.; Granier, C.; Muller, B. Water deficit and growth. Co-ordinating processes without an orchestrator? Curr. Opin. Plant Biol. 2011, 14, 283–289. [Google Scholar] [CrossRef]
- Aslam, M.; Muhammad, A.M.; Cengiz, R. Effects of drought on maize. In Drought Stress in Maize (Zea mays L.); Springer: Berlin, Germany, 2016; pp. 5–17. [Google Scholar]
- Chiuta, N.E.; Mutengwa, C.S. Response of yellow quality protein maize inbred lines to drought stress at seedling stage. Agronomy 2018, 8, 278. [Google Scholar] [CrossRef]
- Mangani, R.; Tesfamariam, E.H.; Bellocchi, G.; Hassen, A. Growth, development, leaf gaseous exchange, and grain yield response of maize cultivars to drought and flooding stress. Sustainability 2018, 10, 3492–3510. [Google Scholar] [CrossRef]
- Pelleschi, S.; Rocher, J.P.; Prioul, J.L. Effect of water restriction on carbohydrate metabolism and photosynthesis in mature maize leaves. Plant Cell Environ. 1997, 20, 493–503. [Google Scholar] [CrossRef]
- Voronin, P.Y.; Rakhmankulova, Z.F.; Maevskaya, S.N.; Nikolaeva, M.K.; Shuiskaya, E.V. Changes in photosynthesis caused by adaptation of maize seedlings to short-term drought. Russ. J. Plant Physiol. 2014, 61, 131–135. [Google Scholar] [CrossRef]
- Chaves, M.M. Effects of water deficits on carbon assimilation. J. Exp. Bot. 1991, 42, 1–16. [Google Scholar] [CrossRef]
- Farqujar, G.D.; Hubick, K.T.; Condon, A.G.; Richards, R.A. Carbon isotope fractionation and plant water-use efficiency. In Stable Isotopes in Ecological Research; Springer: Berlin, Germany, 2017; pp. 21–40. [Google Scholar]
- Tardieu, F. Plant response to environmental conditions: Assessing potential production, water demand, and negative effects of water deficit. Front. Phys. 2013, 18, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Ač, A.; Marek, M.V.; Kalina, J.; Urban, O. Differences in pigment composition, photosynthetic rates and chlorophyll fluorescence images of sun and shade leaves of four tree species. Plant Physiol. Biochem. 2007, 45, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Sapeta, H.; Lourenço, T.; Lorenz, S.; Grumaz, C.; Kirstahler, P.; Barros, P.M.; Costa, J.M.; Sohn, K.; Oliveira, M.M. Transcriptomics and physiological analyses reveal co-ordinated alteration of metabolic pathways in Jatropha curcas drought tolerance. J. Exp. Bot. 2016, 67, 845–860. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.; Langridge, P. Physiological breeding. Curr. Opin. Plant Biol. 2016, 31, 162–171. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, M.; Tattaris, M.; Cossani, C.M.; Ellis, M.; Yamaguchi-Shinozaki, K.; Pierre, C.S. Exploring genetic resources to increase adaptation of wheat to climate change. In Advances in Wheat Genetics: From Genome to Field; Ogihara, Y., Takumi, S., Handa, H., Eds.; Springer: Tokyo, Japan, 2015; pp. 355–368. [Google Scholar] [CrossRef]
- Araus, J.L.; Kefauver, S.C. Breeding to adapt agriculture to climate change: Affordable phenotyping solutions. Curr. Opin. Plant Biol. 2018, 45, 237–247. [Google Scholar] [CrossRef]
- Costa, J.M.; Marques da Silva, J.; Pinheiro, C.; Barón, M.; Mylona, P.; Centritto, M.; Haworth, M.; Loreto, F.; Uzilday, B.; Turkan, I.; et al. Opportunities and limitations of crop phenotyping in southern European countries. Front. Plant Sci. 2019, 10, 1125. [Google Scholar] [CrossRef]














| Name | Kernel Color | Endosperm Type | Place of Origin (Altitude) | Improvement Level |
|---|---|---|---|---|
| Bilhó | white | flint | Travassos, Portugal (800 m) | Farmer’s OPV |
| Caniceira | white | flint | Tocha, Portugal (65 m) | Farmer’s OPV |
| Fandango | yellow | dent | Mixed Portugal-USA | Synthetic OPV PPB |
| Pigarro | white | flint | Paredes, Portugal (189 m) | Farmer’s OPV PPB |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leitão, S.T.; Ferreira, E.; Bicho, M.C.; Alves, M.L.; Pintado, D.; Santos, D.; Mendes-Moreira, P.; Araújo, S.S.; Costa, J.M.; Vaz Patto, M.C. Maize Open-Pollinated Populations Physiological Improvement: Validating Tools for Drought Response Participatory Selection. Sustainability 2019, 11, 6081. https://doi.org/10.3390/su11216081
Leitão ST, Ferreira E, Bicho MC, Alves ML, Pintado D, Santos D, Mendes-Moreira P, Araújo SS, Costa JM, Vaz Patto MC. Maize Open-Pollinated Populations Physiological Improvement: Validating Tools for Drought Response Participatory Selection. Sustainability. 2019; 11(21):6081. https://doi.org/10.3390/su11216081
Chicago/Turabian StyleLeitão, Susana T., Emanuel Ferreira, M. Catarina Bicho, Mara L. Alves, Duarte Pintado, Daniela Santos, Pedro Mendes-Moreira, Susana S. Araújo, J. Miguel Costa, and Maria Carlota Vaz Patto. 2019. "Maize Open-Pollinated Populations Physiological Improvement: Validating Tools for Drought Response Participatory Selection" Sustainability 11, no. 21: 6081. https://doi.org/10.3390/su11216081
APA StyleLeitão, S. T., Ferreira, E., Bicho, M. C., Alves, M. L., Pintado, D., Santos, D., Mendes-Moreira, P., Araújo, S. S., Costa, J. M., & Vaz Patto, M. C. (2019). Maize Open-Pollinated Populations Physiological Improvement: Validating Tools for Drought Response Participatory Selection. Sustainability, 11(21), 6081. https://doi.org/10.3390/su11216081







