Foliar Fertilizer Based on Calcined Bones, Boron and Molybdenum—A Study on the Development and Potential Effects on Maize Grain Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Process for the Solubilization of Minerals from Calcined Bones
2.3. Description of the Pot Experiments
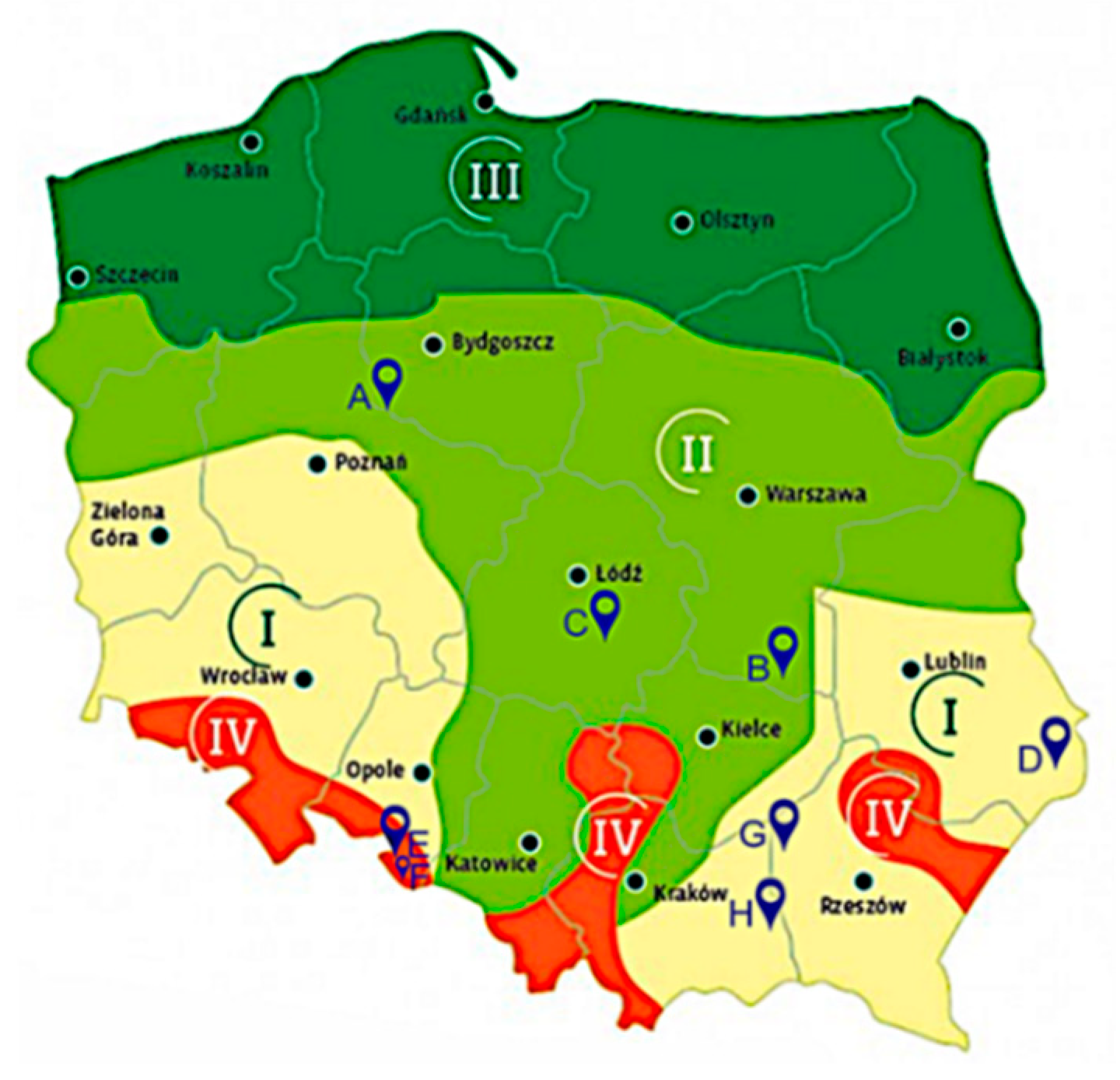
2.4. Description of the Field Experiments
2.5. Analysis of Raw Materials and Plants
2.6. Statistical Analysis
3. Results and Discussion
3.1. The Solubilization Procedure of Minerals from Calcined Bones
3.2. Pot Experiments
3.3. Field Experiments
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dawson, C.J.; Hilton, J. Fertilizer availability in a resource limited world: Production and recycling of nitrogen and phosphorus. Food Policy 2011, 36, 14–22. [Google Scholar] [CrossRef]
- Gorazda, K.; Tarko, B.; Wzorek, Z.; Kominko, H.; Nowak, A.K.; Kulczycka, J.; Henclik, A.; Smol, M. Fertilizers production from ashes after sewage sludge combustion—A strategy towards sustainable development. Environ. Res. 2017, 154, 171–180. [Google Scholar] [CrossRef] [PubMed]
- De Ridder, M.; De Jong, S.; Polchar, J.; Lingemann, S. Risks and Opportunities in the Global Phosphate Rock Market; The Hague Centre for Strategic Studies (HCSS): Hague, The Netherlands, 2012. [Google Scholar]
- Rittmann, B.E.; Mayer, B.; Westerhoff, P.; Edwards, M. Capturing the lost phosphorus. Chemosphere 2011, 84, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębska, M.; Saeid, A.; Kostrzewska, M.K.; Baśladyńska, S. New phosphorus biofertilizers from renewable raw materials in the aspect of cadmium and lead contents in soil and plants, P-biofertilizers from waste and Cd and Pb in soil and plant. Open Chem. 2018, 16. [Google Scholar] [CrossRef]
- Rolewicz, M.; Rusek, P.; Borowik, K. Obtaining of granular fertilizers based on ashes from combustion of waste residues and ground bones using phosphorus solubilization by bacteria Bacillus megaterium. J. Environ. Manag. 2017, 216, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Ehmann, A.; Bach, I.-M.; Laopeamthong, S.; Bilbao, J.; Lewandowski, I. Can phosphate salts recovered from manure replace conventional phosphate fertilizer? Agriculture 2017, 7, 1. [Google Scholar] [CrossRef]
- Smith, M.T.E.; Cade-Menun, B.J.; Tibbett, M. Soil phosphorus dynamics and phytoavailability from sewage sludge at different stages in a treatment stream. Biol. Fertil. Soils 2006, 42, 186–197. [Google Scholar] [CrossRef]
- Staroń, A.; Kowalski, Z.; Banach, M.; Wzorek, Z. Methods of thermal utilization of animal waste. Chem. Czas. Tech. Chem. Tech. Trans. 2010, 107, 323–332. [Google Scholar]
- Saeid, A.; Labuda, M.; Chojnacka, K.; Górecki, H. Valorization of Bones to Liquid Phosphorus Fertilizer by Microbial Solubilization. Waste Biomass Valor. 2014, 5, 265–272. [Google Scholar] [CrossRef]
- Bovine Spongiform Encephalopathy (BSE), World Health Organization. Available online: https://www.who.int/zoonoses/diseases/bse/en/ (accessed on 3 September 2019).
- Demirbas, A.; Abalı, Y.; Mert, E. Recovery of phosphate from calcined bone by dissolution in hydrochloric acid solutions. Res. Conserv. Rec. 1999, 26, 251–258. [Google Scholar] [CrossRef]
- Xiao, C.-Q.; Chi, R.-A.; Huang, X.-H.; Zhang, W.-X.; Qiu, G.-Z.; Wang, D.-Z. Optimization for rock phosphate solubilization by phosphate-solubilizing fungi isolated from phosphate. Ecol. Eng. 2008, 33, 187–193. [Google Scholar] [CrossRef]
- Vassileva, M.; Azcon, R.; Barea, J.-M.; Vassilev, N. Rock phosphate solubilization by free and encapsulated cells of Yarowia lipolytica. Process Biochem. 2000, 35, 693–697. [Google Scholar] [CrossRef]
- Vassilev, N.; Vassileva, M.; Fenice, M.; Federici, F. Immobilized cell technology applied in solubilization of insoluble inorganic (rock) phosphates and P plant acquisition. Bioresour. Technol. 2001, 79, 263–271. [Google Scholar] [CrossRef]
- Fazy rozwojowe podstawowych zbóż w skali BBCH z komentarzem agrotechnicznym. Wiadomości Rol. Pol. 2018, 151.
- Coste, S.; Baraloto, C.; Leroy, C.; Marcon, É.; Renaud, A.; Richardson, A.D.; Roggy, J.-C.; Schimann, H.; Uddling, J.; Hérault, B. Assessing foliar chlorophyll contents with the SPAD-502 chlorophyll meter: A calibration test with thirteen tree species of tropical rainforest in French Guiana. Ann. For. Sci. 2010, 67, 607. [Google Scholar] [CrossRef]
- Kukurydza—Informacje Ogólne, Instytut Hodowli i Aklimatyzacji Roślin—Państwowy Instytut Badawczy. Available online: http://www.zdhar.pl/kukurydza.htm (accessed on 5 August 2019).
- Weather, Institute of Meteorology and Water Management. Available online: http://www.imgw.pl/en/ (accessed on 5 August 2019).
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. Methods of Analysis and Assessment of Soil and Plant Properties; Environmental Protection Institute: Warszawa, Poland, 1991. [Google Scholar]
- Kruk, D.J.; Elektorowicz, M.; Oleszkiewicz, J.A. Struvite precipitation and phosphorus removal using magnesium sacrificial anode. Chemosphere 2014, 101, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Wzorek, Z. Alternative phosphorus raw materials. Przemysł Chem. 2006, 85, 880–882. [Google Scholar]
- Witek, G.; Gorzelany, J.; Matłok, N. The impact of Dr Green foliar fertilization on plant health during the growing period as well as on the potato yield and quality (Jelly variety). Agric. Eng. 2016, 20, 151–158. [Google Scholar] [CrossRef]
- Jaskulski, D. Comparsion of the effect of foliar fertilization fertilizers on economic and production effects of growing some field crops. Fragm. Agron. 2007, 1, 106–112. [Google Scholar]
- Michałojć, Z.; Szewczuk, C. Theoretical aspects of foliar nutrition of plants. Acta Agrophys. 2003, 85, 7–28. [Google Scholar]
- Sienkiewicz-Cholewa, U. The importance of microelements in rape fertilization. Zesz. Post. Nauk Rol. 2002, 5, 19–28. [Google Scholar]
- Bukvić, G.; Antunović, M.; Popović, S.; Rastija, M. Effect of P and Zn fertilisation on biomass yield and its uptake by maize lines (Zea mays L.) Plant. Soil Environ. 2003, 49, 505–510. Plant. Soil Environ. 2003, 49, 505–510. [Google Scholar]
- Barczak, B.; Nowak, K.; Kozera, W.; Majcherczak, E. The effect of foliar fertilization with microelements on the size of spring barley grain yield. Fragm. Agron. 2005, 4, 5–18. [Google Scholar]
- Jaskulski, D.; Jaskulska, I. Production effect of foliar application of magnesium and microelements fertilizer Sonata on the cultivation of winter wheat depending on the rainfall and soil richness. Zesz. Probl. Post. Nauk. Rol. 2009, 541, 157–164. [Google Scholar]
- Kulczycki, G.; Januszkiewicz, R.; Jachymczak, A. The effect of foliar applied fertilizer ekolist on the yield and chemical composition of spring wheat. Zesz. Nauk. UP Wrocław. 2009, 574, 19–28. (In Polish) [Google Scholar]
- Abdel-Aziz, H.M.M.; Hasaneen, M.N.A.; Omer, A.M. Nano chitosan-NPK fertilizer enhances the growth and productivity of wheat plants grown in sandy soil. Span. J. Agric. Res. 2016, 14. [Google Scholar] [CrossRef]
| Na | K | Mg | Ca | P | N | Fe | Mn | Zn | Cu |
|---|---|---|---|---|---|---|---|---|---|
| g kg−1 | mg kg−1 | ||||||||
| 4.74 ± 0.43 | 37.48 ± 3.50 | 16.77 ± 1.25 | 492.46 ± 9.51 | 161.13 ± 0.7 | 0.06 ±0.01 | 21.03 ± 0.80 | 80.76 ± 2.22 | 10.40 ± 0.04 | 40.44 ± 0.97 |
| Location | Climatic Region of Poland for Maize Cultivation (Figure 1) | Meteorological Conditions in the Vegetative Period 2018 [19] | Soil Conditions | Fore Crop | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sum of Rainfall | Average Temp. | Granulometric Composition (USDA) | pH KCl, H2O) | Mg | K | P | ||||
| Geographical Coordinates | mm | °C | mg kg−1 | |||||||
| A | 52°36′33.8′′ N 17°24′06.2′′ E | II (FAO 230–260) | 200 | 18.2 | sand | 6.80, 7.10 | 170 | 196 | 45.3 | colza |
| B | 50°53′09.4′′ N 21°29′35.6′′ E | II (FAO 230–260) | 275 | 18.4 | sandy loam | 5.78, 5.96 | 68 | 79 | 10.9 | carrot |
| C | 51°28′32.5′′ N 19°49′48.0′′ E | II (FAO 230–260) | 323 | 17.2 | loamy sand | 7.18, 7.42 | 55 | 118 | 92.0 | winter wheat |
| D | 50°27′25.5′′ N 23°46′33.4′′ E | I (FAO do 300) | 298 | 17.4 | sandy loam | 6.45, 6.80 | 93 | 171 | 20.5 | winter wheat/straw ploughed |
| E | 50°05′03.6′′ N 17°49′43.3′′ E | IV (FAO do 200) | 312 | 18.2 | sandy loam | 6.69, 7.04 | 118 | 273 | 42.7 | winter wheat/straw ploughed |
| F | 49°59′32.6′′ N 18°15′26.8′′ E | IV (FAO do 200) | 352 | 17.6 | loamy sand | 6.94, 7.25 | 90 | 323 | 46.7 | colza |
| G | 50°03′55.6′′ N 21°03′02.0′′ E | I (FAO do 300) | 405 | 17.8 | loamy sand | 5.50, 5.91 | 218 | 249 | 15.3 | winter wheat |
| H | 50°20′14.9′′ N 21°21′24.9′′ E | I (FAO do 300) | 261 | 17.7 | loamy sand | 5.42, 5.80 | 266 | 226 | 40.5 | maize for grain |
| Content of the Acquired Soluble Fraction | % | 75.62 ± 0.38 | |
|---|---|---|---|
| Na | K | Mg | Ca |
| g kg−1 | |||
| 6.50 ± 0.59 | 2.01 ± 0.05 | 8.45 ± 1.55 | 138.98 ± 7.30 |
| N | Cl | P | S |
| g kg−1 | |||
| 107.48 ± 3.25 | 0.45 ± 0.03 | 111.22 | 1.25 ± 0.02 |
| Fe | Mn | Zn | Cu |
| mg kg−1 | |||
| 12.60 ± 0.22 | 0.62 ± 0.13 | 9.59 ± 0.24 | 1.75 ± 0.13 |
| Mineral Composition of Foliar Fertilizer | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P (g kg−1) | N (g kg−1) | Na (g kg−1) | K (g kg−1) | Mg (g kg−1) | Ca (g kg−1) | B (g kg−1) | Mo (g kg−1) | Fe (mg kg−1) | Zn (mg kg−1) | Cu (mg kg−1) |
| 48.8 | 12.5 | 4.2 | 1.2 | 4.4 | 80.3 | 70 | 2 | 10 | 8 | 1.5 |
| Fertilizer Dose mg pot−1 | Biometric Features of Maize Plants | ||
|---|---|---|---|
| Relative Amount of Chlorophyll (SPAD) | Dry Mass of the Aboveground Parts of the Plant g Plant−1 | Dry Mass of the Underground Parts of the Plant g Plant−1 | |
| 0 | 37.20 a | 12.10 a | 0.63 a |
| 11.8 | 39.30 b | 12.39 a | 0.86 b |
| 23.6 | 39.63 c | 14.27 b | 0.93 c |
| 35.4 | 45.20 d | 14.47 c | 1.21 d |
| Fertilizer Dose mg pot−1 | P | Ca | K | Na | Mg | Fe | Mn | Zn | Cu |
|---|---|---|---|---|---|---|---|---|---|
| g kg−1 | mg kg−1 | ||||||||
| Underground Parts of the Plant | |||||||||
| 0 | 0.19 a | 1.66 a | 4.53 b | 0.98 b | 0.64 a | 167.16 c | 102.54 d | 25.88 a | 4.40 a |
| 11.8 | 0.22 b | 2.11 c | 3.97 a | 0.98 b | 0.71 c | 171.05 d | 74.01 b | 39.75 c | 6.61 c |
| 23.6 | 0.28 d | 1.83 b | 4.67 c | 0.88 a | 0.66 b | 171.54 a | 55.87 a | 33.33 b | 5.86 b |
| 35.4 | 0.25 c | 2.57 d | 4.85 d | 1.28 c | 0.88 d | 115.84 b | 81.68 c | 50.67 d | 7.32 d |
| Aboveground Parts of the Plant | |||||||||
| g kg−1 | mg kg−1 | ||||||||
| 0 | 3.19 c | 5.53 a | 32.2 a | 0.09 b | 1.90 a | 245.63 d | 58.65 d | 66.78 b | 10.71 c |
| 11.8 | 4.22 d | 7.26 c | 37.0 b | 0.17 d | 2.39 b | 198.77 c | 50.40 b | 59.56 a | 8.36 b |
| 23.6 | 4.16 a | 10.09 d | 43.8 d | 0.06 a | 3.00 d | 215.14 a | 37.16 a | 71.21 c | 8.29 a |
| 35.4 | 3.27 b | 6.40 b | 38.9 c | 0.11 c | 2.65 c | 192.79 b | 52.69 c | 80.81 d | 11.76 d |
| Fertilizer Dose kg ha−1 | Biometric Features of Maize Plant | Maize Graint ha−1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Plants Highcm Plant−1 | Dry Mass of the Aboveground Parts of the Plantg Plant−1 | Dry Mass of the Underground Parts of the Plantg Plant−1 | |||||||
| Location | 0 | 3.0 | 0 | 3.0 | 0 | 3.0 | 0 | 3.0 | |
| A | 184.0 a | 207.3 b | 80.1 a | 117.8 b | 62.3 a | 117.8 b | 13.4 a | 14.0 b | |
| B | 122.3 a | 211.4 b | 188.6 a | 479.2 b | 28.5 a | 39.4 b | 11.0 a | 11.8 b | |
| C | 153.0 | 167.3 | 247.0 a | 347.7 b | 38.4 a | 69.1 b | 12.0 a | 12.5 b | |
| D | 121.0 a | 183.7 b | 83.7 a | 170.2 b | 29.1 a | 41.9 b | 11.4 a | 11.9 b | |
| E | 95.3 | 110.0 | 107.4 a | 161.0 b | 29.0 a | 54.2 b | 10.1 a | 10.6 b | |
| F | 155.5 a | 172.3 b | 307.2 a | 419.5 b | 39.9 a | 84.6 b | 12.2 a | 12.8 b | |
| G | 101.7 a | 196.3 b | 218.0 a | 376.9 b | 41.2 a | 57.7 b | 10.5 a | 11.2 b | |
| H | 102.3 | 108.7 | 75.1 a | 148.1 b | 15.2 a | 39.1 b | 11.6 a | 12.1 b | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balawejder, M.; Matłok, N.; Gorzelany, J.; Pieniążek, M.; Antos, P.; Witek, G.; Szostek, M. Foliar Fertilizer Based on Calcined Bones, Boron and Molybdenum—A Study on the Development and Potential Effects on Maize Grain Production. Sustainability 2019, 11, 5287. https://doi.org/10.3390/su11195287
Balawejder M, Matłok N, Gorzelany J, Pieniążek M, Antos P, Witek G, Szostek M. Foliar Fertilizer Based on Calcined Bones, Boron and Molybdenum—A Study on the Development and Potential Effects on Maize Grain Production. Sustainability. 2019; 11(19):5287. https://doi.org/10.3390/su11195287
Chicago/Turabian StyleBalawejder, Maciej, Natalia Matłok, Józef Gorzelany, Marcin Pieniążek, Piotr Antos, Grzegorz Witek, and Małgorzata Szostek. 2019. "Foliar Fertilizer Based on Calcined Bones, Boron and Molybdenum—A Study on the Development and Potential Effects on Maize Grain Production" Sustainability 11, no. 19: 5287. https://doi.org/10.3390/su11195287
APA StyleBalawejder, M., Matłok, N., Gorzelany, J., Pieniążek, M., Antos, P., Witek, G., & Szostek, M. (2019). Foliar Fertilizer Based on Calcined Bones, Boron and Molybdenum—A Study on the Development and Potential Effects on Maize Grain Production. Sustainability, 11(19), 5287. https://doi.org/10.3390/su11195287







