Pleurotus spp. Cultivation on Different Agri-Food By-Products: Example of Biotechnological Application
Abstract
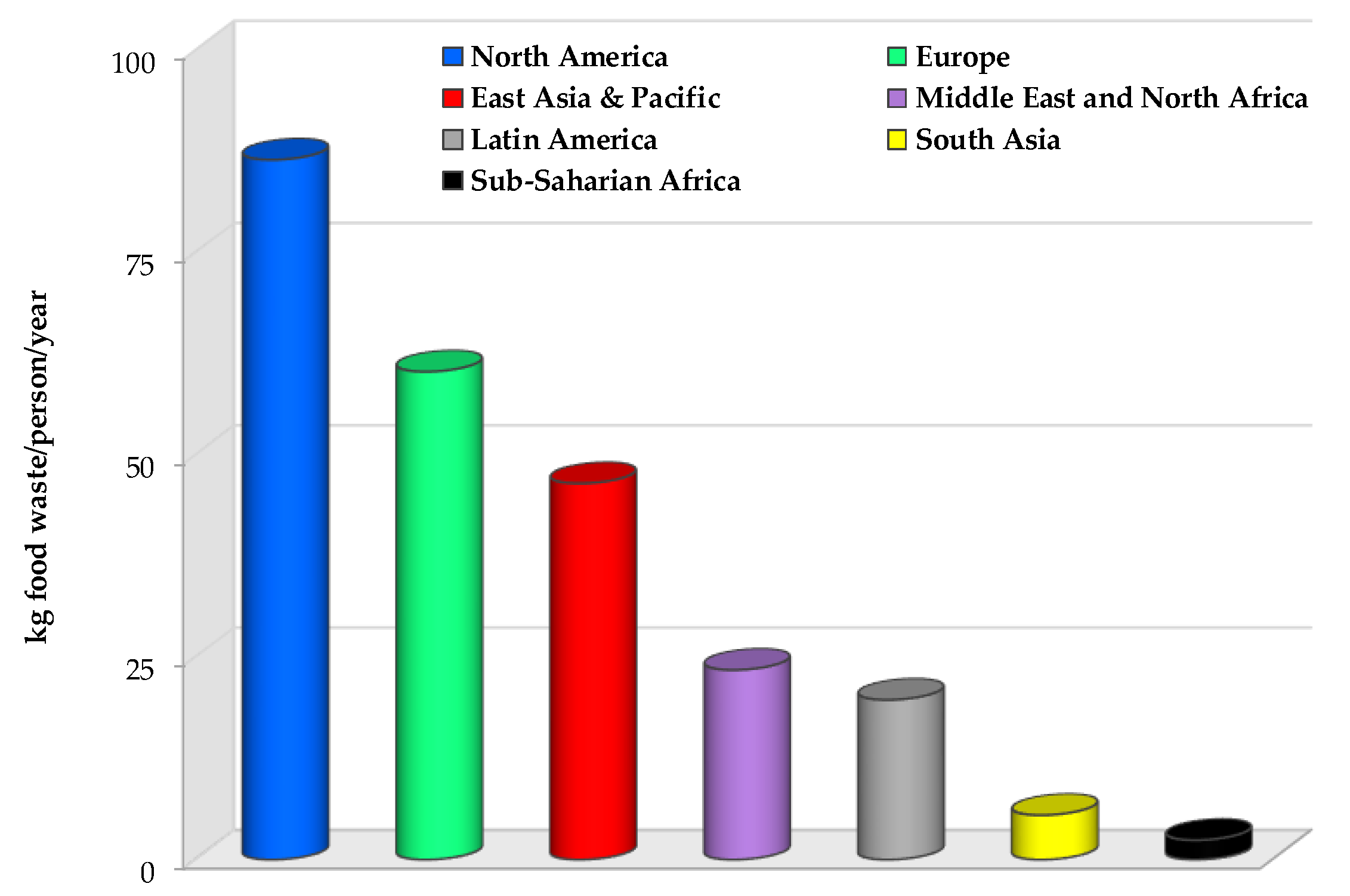
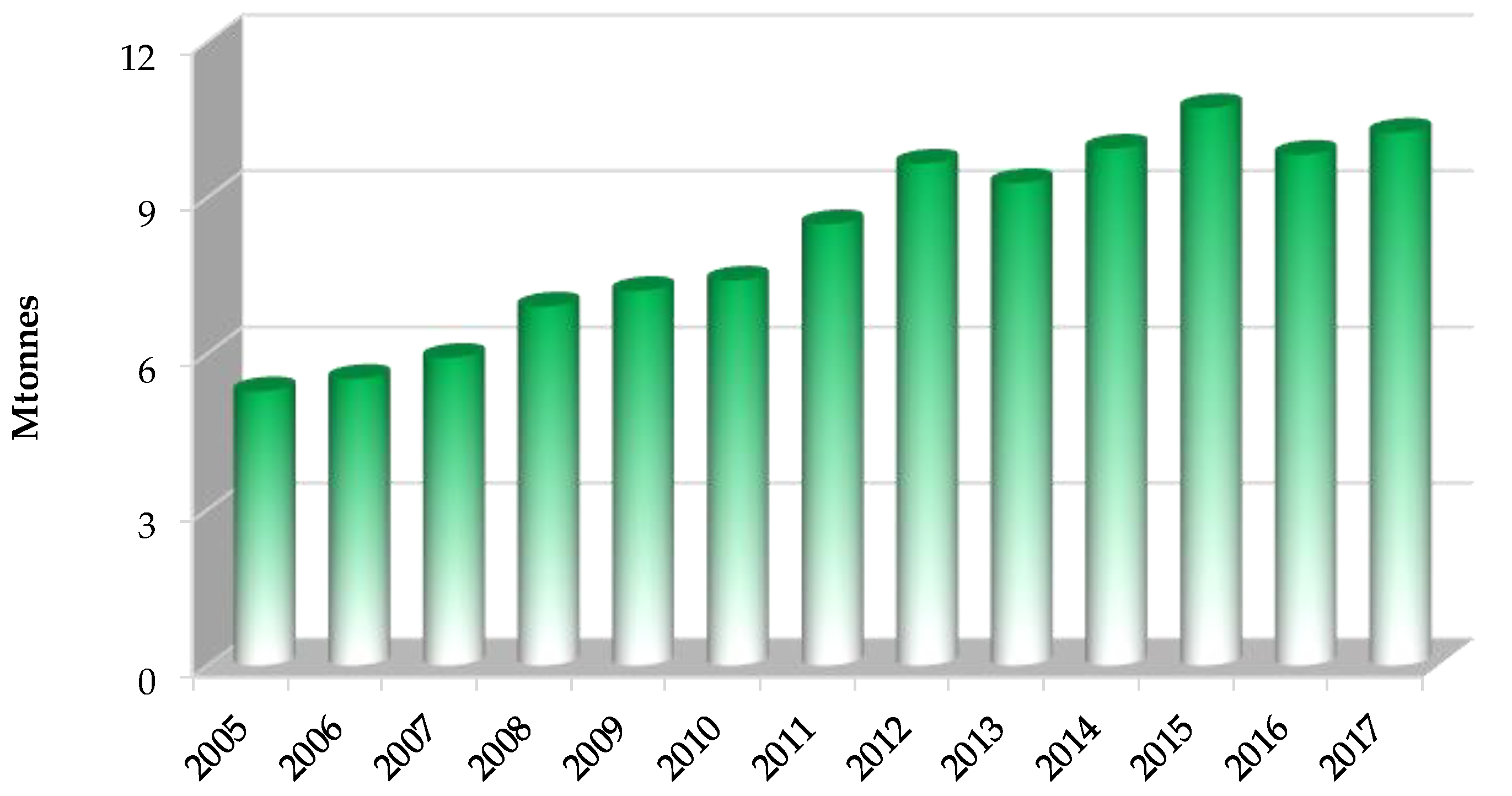
1. Introduction
2. Pleurotus spp. Cultivation on Field-Based Residues
3. Pleurotus spp. Cultivation on Processing-Based Residues
3.1. Olive Mill Wastes
3.2. Grape Marc
3.3. Brewers Grain
3.4. Coconut Husk/Coir
3.5. Coffee Pulp/Husk
3.6. Corncob
3.7. Sugarcane Bagasse
3.8. Rice Husk
3.9. Hazelnut Husk
4. Aspects Related to Pleurotus spp. Cultivation on Agri-Food by-Products
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Garg, V.K.; Suthar, S.; Yadav, A. Management of food industry waste employing vermicomposting technology. Bioresour. Technol. 2012, 126, 437–443. [Google Scholar] [CrossRef] [PubMed]
- The Economist Intelligence Unit, Barilla Centre For Food and Nutrition. Food Sustainability Index. Available online: http://foodsustainability.eiu.com/ (accessed on 11 September 2019).
- Stenmarck, Å.; Jensen, C.; Quested, T.; Moates, G. FUSIONS-Estimates of European Food Waste Levels; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2016. [Google Scholar]
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. Exploitation of food industry waste for high-value products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Morales-Polo, C.; del Mar Cledera-Castro, M.; Moratilla Soria, B.Y. Reviewing the Anaerobic Digestion of Food Waste: From Waste Generation and Anaerobic Process to Its Perspectives. Appl. Sci. 2018, 8, 1804. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Ballesteros, L.F.; Martins, S.; Teixeira, J.A. Use of agro-industrial wastes in solid-state fermentation processes. In Industrial Waste; Guo, K.-Y.S.a.X., Ed.; InTech Open Access Publisher: London, UK, 2012; pp. 121–140. [Google Scholar]
- Pandey, A. Solid-state fermentation. Biochem. Eng. J. 2003, 13, 81–84. [Google Scholar] [CrossRef]
- Soccol, C.R.; Costa, E.S.F.d.; Letti, L.A.J.; Karp, S.G.; Woiciechowski, A.L.; Vandenberghe, L.P.d.S. Recent developments and innovations in solid state fermentation. Biotechnol. Res. Innov. 2017, 1, 52–71. [Google Scholar] [CrossRef]
- Philippoussis, A. Production of Mushrooms Using Agro-Industrial Residues as Substrates. In Biotechnology for Agro-Industrial Residues Utilisation: Utilisation of Agro-Residues; Singh nee’ Nigam, P., Pandey, A., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 163–196. [Google Scholar] [CrossRef]
- Baldrian, P. Fungal laccases-occurrence and properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef]
- Hatakka, A. Lignin-modifying enzymes from selected white-rot fungi: Production and role from in lignin degradation. FEMS Microbiol. Rev. 1994, 13, 125–135. [Google Scholar] [CrossRef]
- Pérez, J.; Muñoz-Dorado, J.; de la Rubia, T.; Martínez, J. Biodegradation and biological treatments of cellulose, hemicellulose and lignin: An overview. Int. Microbiol. 2002, 5, 53–63. [Google Scholar] [CrossRef]
- Tengerdy, R.P.; Szakacs, G. Bioconversion of lignocellulose in solid substrate fermentation. Biochem. Eng. J. 2003, 13, 169–179. [Google Scholar] [CrossRef]
- Sánchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnology Advances 2009, 27, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Persky, L.; Hadar, Y. Biotechnological applications and potential of wood-degrading mushrooms of the genus Pleurotus. Appl. Microbiol. Biotechnol. 2002, 58, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Lankinen, P. Ligninolytic Enzymes of the Basidiomycetous Fungi Agaricus bisporus and Phlebia radiata on Lignocellulosecontaining Media. Ph.D. Dissertation, University of Helsinki, Helsinki, Finland, 2004. [Google Scholar]
- Grimm, D.; Wösten, H.A.B. Mushroom cultivation in the circular economy. Appl. Microbiol. Biotechnol. 2018, 102, 7795–7803. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Food and Agriculture Data. Available online: http://www.fao.org/faostat/en/#home (accessed on 11 September 2019).
- Manzi, P.; Aguzzi, A.; Vivanti, V.; Paci, M.; Pizzoferrato, L. Mushrooms as a source of functional ingredients. In Proceedings of the Euro Food Chem X European Conference on: Functional Foods. A New Challange for the Food Chemist, Budapest, Hungary, 22–24 September 1999; pp. 86–93. [Google Scholar]
- Manzi, P.; Gambelli, L.; Marconi, S.; Vivanti, V.; Pizzoferrato, L. Nutrients in edible mushrooms: An inter-species comparative study. Food Chem. 1999, 65, 477–482. [Google Scholar] [CrossRef]
- Kalač, P. Chemical composition and nutritional value of European species of wild growing mushrooms: A review. Food Chem. 2009, 113, 9–16. [Google Scholar] [CrossRef]
- Kalač, P. Chemical composition and nutritional value of European species of wild growing mushrooms. In Mushrooms: Types, Properties and Nutrition; Andres, S., Baumann, N., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2012; pp. 129–152. [Google Scholar]
- Kalač, P. A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef]
- Manzi, P.; Pizzoferrato, L. Beta-glucans in edible mushrooms. Food Chem. 2000, 68, 315–318. [Google Scholar] [CrossRef]
- Ruthes, A.C.; Smiderle, F.R.; Iacomini, M. Mushroom heteropolysaccharides: A review on their sources, structure and biological effects. Carbohydr. Polym. 2016, 136, 358–375. [Google Scholar] [CrossRef]
- Gargano, M.L.; Griensven, L.J.L.D.; Isikhuemhen, O.S.; Lindequist, U.; Venturella, G.; Wasser, S.P.; Zervakis, G.I. Medicinal mushrooms: Valuable biological resources of high exploitation potential. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2017, 151, 548–565. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Kalogeropoulos, N.; Stathopoulos, P.; Kaliora, A.C.; Zervakis, G.I. Bioactive compounds and antioxidant activity exhibit high intraspecific variability in Pleurotus ostreatus mushrooms and correlate well with cultivation performance parameters. World J. Microbiol. Biotechnol. 2017, 33, 1–14. [Google Scholar] [CrossRef]
- Wang, S.; Bao, L.; Zhao, F.; Wang, Q.; Li, S.; Ren, J.; Li, L.; Wen, H.; Guo, L.; Liu, H. Isolation, Identification, and Bioactivity of Monoterpenoids and Sesquiterpenoids from the Mycelia of Edible Mushroom Pleurotus cornucopiae. J. Agric. Food. Chem. 2013, 61, 5122–5129. [Google Scholar] [CrossRef] [PubMed]
- Kanagasabapathy, G.; Malek, S.N.A.; Kuppusamy, U.R.; Vikineswary, S. Chemical Composition and Antioxidant Properties of Extracts of Fresh Fruiting Bodies of Pleurotus sajor-caju (Fr.) Singer. J. Agric. Food. Chem. 2011, 59, 2618–2626. [Google Scholar] [CrossRef] [PubMed]
- Parola, S.; Chiodaroli, L.; Orlandi, V.; Vannini, C.; Panno, L. Lentinula edodes and Pleurotus ostreatus: Functional food with antioxidant-antimicrobial activity and an important source of vitamin D and medicinal compounds. Funct. Foods Health Dis. 2017, 7, 773–794. [Google Scholar] [CrossRef]
- Sanchez, C. Reactive oxygen species and antioxidant properties from mushrooms. Synth. Syst. Biotechnol. 2017, 2, 13–22. [Google Scholar] [CrossRef]
- Manzi, P.; Aguzzi, A.; Pizzoferrato, L. Nutritional value of mushrooms widely consumed in Italy. Food Chem. 2001, 73, 321–325. [Google Scholar] [CrossRef]
- Lal, R. World crop residues production and implications of its use as a biofuel. Environ. Int. 2005, 31, 575–584. [Google Scholar] [CrossRef]
- Cherubin, M.R.; Silva Oliveira, D.M.; Feigl, B.J.; Pimentel, L.G.; Lisboa, I.P.; Gmach, M.R.; Varanda, L.L.; Morais, M.C.; Satiro, L.S.; Popin, G.V.; et al. Crop residue harvest for bioenergy production and its implications on soil functioning and plant growth: A review. Sci. Agric. 2018, 75, 255–272. [Google Scholar] [CrossRef]
- Hoa, H.T.; Wang, C.L.; Wang, C.H. The effects of different substrates on the growth, yield, and nutritional composition of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 2015, 43, 423–434. [Google Scholar] [CrossRef]
- Dou, Z.; Toth, J.D.; Westendorf, M.L. Food waste for livestock feeding: Feasibility, safety, and sustainability implications. Glob. Food Secur. 2018, 17, 154–161. [Google Scholar] [CrossRef]
- Issaka, J.; Alemawor, F.; Dzogbefia, V.P. Bioconversion impact of Pleurotus ostreatus on the value of rice and groundnut by-products as feed resources. Res. Biotechnol. 2013, 4, 24–30. [Google Scholar]
- Nasehi, M.; Torbatinejad, N.M.; Zerehdaran, S.; Safaie, A.R. Effect of solid-state fermentation by oyster mushroom (Pleurotus florida) on nutritive value of some agro by-products. J. Appl. Anim. Res. 2017, 45, 221–226. [Google Scholar] [CrossRef]
- Telang, S.M.; Patil, S.S.; Baig, M.M.V. Biological efficiency and nutritional value of Pleurotus sapidus cultivated on different substrates. Food Sci. Res. J. 2010, 1, 127–129. [Google Scholar]
- Koutrotsios, G.; Mountzouris, K.C.; Chatzipavlidis, I.; Zervakis, G.I. Bioconversion of lignocellulosic residues by Agrocybe cylindracea and Pleurotus ostreatus mushroom fungi—Assessment of their effect on the final product and spent substrate properties. Food Chem. 2014, 161, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Rivera, N.; De Jesús-Merales, J. Edible mushroom Pleurotus ostreatus production on cellulosic biomass of sugar cane. Sugar Tech 2010, 12, 176–178. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, S.; Saha, S.; Walia, S. Yield and nutritional content of Pleurotus sajor caju on wheat straw supplemented with raw and detoxified mahua cake. Food Chem. 2013, 141, 4231–4239. [Google Scholar] [CrossRef] [PubMed]
- Telang, S.M.; Patil, S.S.; Baig, M.M.V. Comparative study on yield and nutritional aspect of Pleurotus eous mushroom cultivated on different substrate. Food Sci. Res. J. 2010, 1, 60–63. [Google Scholar]
- Maynard, A.J. Methods in Food Analysis, 2nd ed.; Maynard, A.J., Ed.; Academic Press: New York, NY, USA, 1970. [Google Scholar]
- Wankhede, D.B.; Tharanathan, R.N. Sesame (Sesamum indicum) carbohydrates. J. Agric. Food. Chem. 1976, 24, 655–659. [Google Scholar] [CrossRef]
- Shevale, S.B.; Deshmukh, H.V. Yield performance and nutritional analysis of Pleurotus species on different agro wastes and vegetable wastes. Int. J. Plant Prot. 2016, 9, 162–167. [Google Scholar] [CrossRef]
- Zervakis, G.I.; Koutrotsios, G.; Katsaris, P. Composted versus raw olive mill waste as substrates for the production of medicinal mushrooms: An assessment of selected cultivation and quality parameters. BioMed Res. Int. 2013, 2013, 546830. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Kalogeropoulos, N.; Kaliora, A.C.; Zervakis, G.I. Toward an Increased Functionality in Oyster (Pleurotus) Mushrooms Produced on Grape Marc or Olive Mill Wastes Serving as Sources of Bioactive Compounds. J. Agric. Food. Chem. 2018, 66, 5971–5983. [Google Scholar] [CrossRef] [PubMed]
- Sardar, H.; Ali, M.A.; Anjum, M.A.; Nawaz, F.; Hussain, S.; Naz, S.; Karimi, S.M. Agro-industrial residues influence mineral elements accumulation and nutritional composition of king oyster mushroom (Pleurotus eryngii). Sci. Hort. 2017, 225, 327–334. [Google Scholar] [CrossRef]
- Raja, S.; Mallesha, B.C.; Gowda, P.A. Effect of micronutrients on growth and yield of mushrooms. Mysore J. Agric. Sci. 2013, 47, 66–69. [Google Scholar]
- Pekşen, A.; Küçükomuzlu, B. Yield potential and quality of some Pleurotus species grown in substrates containing hazelnut husk. Pak. J. Biol. Sci. 2004, 7, 768–771. [Google Scholar]
- Hossain, M.M. Effect of different substrates on yield of Pleurotus ostreatus mushroom. Environ. Ecol. 2018, 36, 312–315. [Google Scholar]
- Chitamba, J.; Dube, F.; Chiota, W.M.; Handiseni, M. Evaluation of substrate productivity and market quality of oyster mushroom (Pleurotus ostreatus) grown on different substrates. Int. J. Agric. Res. 2012, 7, 100–106. [Google Scholar] [CrossRef]
- Mane, V.P.; Patil, S.S.; Syed, A.A.; Baig, M.M. Bioconversion of low quality lignocellulosic agricultural waste into edible protein by Pleurotus sajor-caju (Fr.) Singer. J. Zhejiang Univ. Sci. B 2007, 8, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Jeznabadi, E.K.; Jafarpour, M.; Eghbalsaied, S. King oyster mushroom production using various sources of agricultural wastes in Iran. Int. J. Recycl. Org. Waste Agric. 2016, 5, 17–24. [Google Scholar] [CrossRef]
- Kadam, K.L.; McMillan, J.D. Availability of corn stover as a sustainable feedstock for bioethanol production. Bioresour. Technol. 2003, 88, 17–25. [Google Scholar] [CrossRef]
- Khan, S.M.; Nawaz, A.; Ali, M.A.; Ahmad, T.; Khan, N.A.; Rehman, A.U. Response of oyster mushroom on different agricultural wastes of Southern Punjab. Pak. J. Agric. Sci. 2012, 49, 127–130. [Google Scholar]
- Mumtaz, M.S.; Khan, N.A.; Abdul, R.; Abdul, J. Production of oyster mushroom (Pleurotus pulmonarius) on different agriculture wastes combination with lemon grass (Cymbopogon citratus). Pak. J. Phytopathol. 2016, 28, 71–75. [Google Scholar]
- Vieira, F.R.; Andrade, M.C.N. Optimization of substrate preparation for oyster mushroom (Pleurotus ostreatus) cultivation by studying different raw materials and substrate preparation conditions (composting: Phases I and II). World J. Microbiol. Biotechnol. 2016, 32, 190. [Google Scholar] [CrossRef] [PubMed]
- Batish, D.R.; Tung, P.; Singh, H.P.; Kohli, R.K. Phytotoxicity of sunflower residues against some summer season crops. J. Agron. Crop Sci. 2002, 188, 19–24. [Google Scholar] [CrossRef]
- Nanjappa, H.; Poonguzhalan, R.; Ramachandrappa, B. Influence of sunflower on subsequent crops. Allelopath. J. 1999, 6, 69–74. [Google Scholar]
- Buah, J.N.; Van der Puije, G.C.; Bediako, E.A.; Abole, E.A.; Showemimo, F. The growth and yield performance of oyster mushroom (Pleurotus ostreatus) on different substrates. Biotechnology 2010, 9, 338–342. [Google Scholar] [CrossRef]
- Mansour-Benamar, M.; Savoie, J.M.; Chavant, L. Valorization of solid olive mill wastes by cultivation of a local strain of edible mushrooms. Comptes Rendus Biol. 2013, 336, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Roig, A.; Cayuela, M.L.; Sanchez-Monedero, M.A. An overview on olive mill wastes and their valorisation methods. Waste Manag. 2006, 26, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Ntougias, S.; Bourtzis, K.; Tsiamis, G. The microbiology of olive mill wastes. BioMed Res. Int. 2013, 2013, 784591. [Google Scholar] [CrossRef] [PubMed]
- Brozzoli, V.; Bartocci, S.; Terramoccia, S.; Contò, G.; Federici, F.; D’Annibale, A.; Petruccioli, M. Stoned olive pomace fermentation with Pleurotus species and its evaluation as a possible animal feed. Enzyme Microb. Technol. 2010, 46, 223–228. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Zervakis, G.I. Comparative examination of the olive mill wastewater biodegradation process by various wood-rot macrofungi. BioMed Res. Int. 2014, 2014, 482937. [Google Scholar] [CrossRef]
- Ntougias, S.; Baldrian, P.; Ehaliotis, C.; Nerud, F.; Antoniou, T.; Merhautova, V.; Zervakis, G.I. Biodegradation and detoxification of olive mill wastewater by selected strains of the mushroom genera Ganoderma and Pleurotus. Chemosphere 2012, 88, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Avni, S.; Ezove, N.; Hanani, H.; Yadid, I.; Karpovsky, M.; Hayby, H.; Gover, O.; Hadar, Y.; Schwartz, B.; Danay, O. Olive mill waste enhances alpha-glucan content in the edible mushroom Pleurotus eryngii. Int. J. Mol. Sci. 2017, 18, 1564. [Google Scholar] [CrossRef] [PubMed]
- Reverberi, M.; Di Mario, F.; Tomati, U. b-Glucan synthase induction in mushrooms grown on olive mill wastewaters. Appl. Microbiol. Biotechnol. 2004, 66, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Rodriguez, A.; Soler-Rivas, C.; Polonia, I.; Wichers, H.J. Effect of olive mill waste (OMW) supplementation to Oyster mushrooms substrates on the cultivation parameters and fruiting bodies quality. Int. Biodeterior. Biodegrad. 2010, 64, 638–645. [Google Scholar] [CrossRef]
- Zacharof, M.-P. Grape Winery Waste as Feedstock for Bioconversions: Applying the Biorefinery Concept. Waste Biomass Valorization 2017, 8, 1011–1025. [Google Scholar] [CrossRef]
- Nerantzis, E.T.; Tataridis, P. Integrated enology-utilization of winery by-products into high added value products. J. Sci. Technol. 2006, 1, 79–89. [Google Scholar]
- Pérez-Bibbins, B.; Torrado-Agrasar, A.; Pérez-Rodríguez, N.; Aguilar-Uscanga, M.G.; Domínguez, J.M. Evaluation of the liquid, solid and total fractions of beer, cider and wine lees as economic nutrient for xylitol production. J. Chem. Technol. Biotechnol. 2015, 90, 1027–1039. [Google Scholar] [CrossRef]
- Petre, M.; Teodorescu, A.; Giosanu, D.; Bejan, C. Enhanced cultivation of mushrooms on organic wastes from wine-making industry. J. Environ. Prot. Ecol. 2012, 13, 1488–1492. [Google Scholar]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ spent grain: Generation, characteristics and potential applications. J. Cerral Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Wang, D.; Sakoda, A.; Suzuki, M. Biological efficiency and nutritional value of Pleurotus ostreatus cultivated on spent beer grain. Bioresour. Technol. 2001, 78, 293–300. [Google Scholar] [CrossRef]
- Gregori, A.; Svagelj, M.; Pahor, B.; Berovic, M.; Pohleven, F. The use of spent brewery grains for Pleurotus ostreatus cultivation and enzyme production. New Biotechnol. 2008, 25, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.R.; Thomas, G.V. Biological conversion of coir pith into a value-added organic resource and its application in Agri-Horticulture: Current status, prospect and perspective. J. Plant. Crops 2002, 30, 1–17. [Google Scholar]
- Pandey, A.; Soccol, C.R.; Nigam, P.; Brand, D.; Mohan, R.; Roussos, S. Biotechnological potential of coffee pulp and coffee husk for bioprocesses. Biochem. Eng. J. 2000, 6, 153–162. [Google Scholar] [CrossRef]
- Parani, K.; Eyini, M. Biodegradation of coffee pulp waste by different fungal associations. Bio. Disc. 2012, 3, 222–228. [Google Scholar]
- García, O.; Bermúdez, S.; Gaime, P.; Rodríguez, P.; Aguilera, R.; Morris, Q. Production of Pleurotus’s ligninolityc enzymes on coffee pulp by solid state fermentation. In Proceedings of the 7th International Conference on Mushroom Biology and Mushroom Products, Arcachon, France, 4–7 October 2011; pp. 144–149. [Google Scholar]
- Da Luz, J.M.R.; Nunes, M.D.; Paes, S.A.; Torres, D.P.; Silva, M.C.S.; Kasuya, M.C.M. Lignocellulolytic enzyme production of Pleurotus ostreatus growth in agroindustrial wastes. Braz. J. Microbiol. 2012, 43, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Hoa, H.T.; Wang, C.H.; Tam, N.V.; Wang, C.L. Effects of substrates and drying methods on antioxidant compound and antioxidant activity of fruiting body extracts of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Int. Food Res. J. 2017, 24, 1998–2008. [Google Scholar]
- Wang, C.Q.; Wu, S.J.; Wei, S.Y.; Qin, X.J.; Chen, L.X.; Yang, C.; Chen, X.F.; Wu, X.J. Screening for cultivation medium formula of Pleurotus pulmonarius with eucalyptus sawdust as main material. J. South. Agric. 2016, 47, 624–628. [Google Scholar]
- Li, Z.; Pan, H.; Li, Y.; Yu, H.; Wang, R.; Zhou, F.; Tan, Q.; Guo, Q. Effects of corncob particle size on the mycelium growth and yield of Pleurotus eryngii in factory production. Acta Agric. Shanghai 2011, 27, 46–48. [Google Scholar]
- Satyanarayana, K.G.; Arizaga, G.G.C.; Wypych, F. Biodegradable composites based on lignocellulosic fibers—An overview. Prog. Polym. Sci. 2009, 34, 982–1021. [Google Scholar] [CrossRef]
- Saha, B.C. Hemicellulose bioconversion. J. Ind. Microbiol. Biotechnol. 2003, 30, 279–291. [Google Scholar] [CrossRef]
- Hofsetz, K.; Silva, M.A. Brazilian sugarcane bagasse: Energy and non-energy consumption. Biomass Bioenergy 2012, 46, 564–573. [Google Scholar] [CrossRef]
- Cardona, C.A.; Quintero, J.A.; Paz, I.C. Production of bioethanol from sugarcane bagasse: Status and perspectives. Bioresour. Technol. 2010, 101, 4754–4766. [Google Scholar] [CrossRef] [PubMed]
- Camassola, M.; Dillon, A.J.P. Biological pretreatment of sugar cane bagasse for the production of cellulases and xylanases by Penicillium echinulatum. Ind. Crops Prod. 2009, 29, 642–647. [Google Scholar] [CrossRef]
- Dong, X.Q.; Yang, J.S.; Zhu, N.; Wang, E.T.; Yuan, H.L. Sugarcane bagasse degradation and characterization of three white-rot fungi. Bioresour. Technol. 2013, 131, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Anyakorah, C.I.; Dike, E.N. Comparison of sawdust and rice husk as casing materials for Pleurotus pulmonarius propagation on cassava peel substrate. Agric. Biol. J. N. Am. 2012, 4, 552–554. [Google Scholar] [CrossRef]
- Dairo, A.; Ogunlade, S.W.; Oluwasola, T.A. Proximate composition and amino acid profile of rice husk biodegraded with Pleurotus ostreatus for different periods. Afr. J. Food Agric. Nutr. Dev. 2017, 17, 12243–12255. [Google Scholar] [CrossRef]
- Uzuner, S.; Sharma-Shivappa, R.R.; Cekmecelioglu, D. Bioconversion of Alkali Pretreated Hazelnut Shells to Fermentable Sugars for Generation of High Value Products. Waste Biomass Valorization 2017, 8, 407–416. [Google Scholar] [CrossRef]
- Çöpür, Y.; Güler, C.; Akgül, M.; Taşçıoğlu, C. Some chemical properties of hazelnut husk and its suitability for particleboard production. Build. Environ. 2007, 42, 2568–2572. [Google Scholar] [CrossRef]
- Esposito, T.; Sansone, F.; Franceschelli, S.; Del Gaudio, P.; Picerno, P.; Aquino, R.P.; Mencherini, T. Hazelnut (Corylus avellana L.) shells extract: Phenolic composition, antioxidant effect and cytotoxic activity on human cancer cell lines. Int. J. Mol. Sci. 2017, 18, 392. [Google Scholar] [CrossRef]
- Yuan, B.; Lu, M.; Eskridge, K.M.; Isom, L.D.; Hanna, M.A. Extraction, identification, and quantification of antioxidant phenolics from hazelnut (Corylus avellana L.) shells. Food Chem. 2018, 244, 7–15. [Google Scholar] [CrossRef]
- Zervakis, G.I.; Koutrotsios, G. Solid-State Fermentation of Plant Residues and Agro-industrial Wastes for the Production of Medicinal Mushrooms. In Medicinal Plants and Fungi: Recent Advances in Research and Development; Agrawal, D.C., Tsay, H.-S., Shyur, L.-F., Wu, Y.-C., Wang, S.-Y., Eds.; Springer: Singapore, 2017; pp. 365–396. [Google Scholar] [CrossRef]
- Yildiz, S.; Yildiz, Ü.C.; Gezer, E.D.; Temiz, A. Some lignocellulosic wastes used as raw material in cultivation of the Pleurotus ostreatus culture mushroom. Process Biochem. 2002, 38, 301–306. [Google Scholar] [CrossRef]
- Mandeel, Q.A.; Al-Laith, A.A.; Mohamed, S.A. Cultivation of oyster mushrooms (Pleurotus spp.) on various lignocellulosic wastes. World J. Microbiol. Biotechnol. 2005, 21, 601–607. [Google Scholar] [CrossRef]
- Lavelli, V.; Proserpio, C.; Gallotti, F.; Laureati, M.; Pagliarini, E. Circular reuse of bio-resources: The role of Pleurotus spp. in the development of functional foods. Food Funct. 2018, 9, 1353–1372. [Google Scholar] [CrossRef]
- Crisan, E.V.; Sands, A. Nutrtional value. In The Biology and Cultivation of Edible Mushrooms; Chang, S.T., Hayes, W.A., Eds.; Academic Press: New York, NY, USA, 1978. [Google Scholar]
- Bonatti, M.; Karnopp, P.; Soares, H.M.; Furlan, S.A. Evaluation of Pleurotus ostreatus and Pleurotus sajor-caju nutritional characteristics when cultivated in different lignocellulosic wastes. Food Chem. 2004, 88, 425–428. [Google Scholar] [CrossRef]
- Shashirekha, M.N.; Rajarathnam, S.; Bano, Z. Effects of supplementing rice straw growth substrate with cotton seeds on the analytical characteristics of the mushroom, Pleurotus florida (Block & Tsao). Food Chem. 2005, 92, 255–259. [Google Scholar]
- Pomeranz, Y.; Meloan, C.E. Food Analysis Theory and Practice; Springer: Medford, MA, USA, 2000; pp. XIV, 778. [Google Scholar]
- Nikkarinen, M.; Mertanen, E. Impact of geological origin on trace element composition of edible mushrooms. J. Food Compost. Anal. 2004, 17, 301–310. [Google Scholar] [CrossRef]
- Mikiashvili, N.; Wasser, S.P.; Nevo, E.; Elisashvili, V. Effects of carbon and nitrogen sources on Pleurotus ostreatus ligninolytic enzyme activity. World J. Microbiol. Biotechnol. 2006, 22, 999–1002. [Google Scholar] [CrossRef]
- Nunes, M.D.; Luz, J.M.R.d.; Paes, S.A.; Ribeiro, J.J.O.; Silva, M.d.C.S.d.; Kasuya, M.C.M. Nitrogen supplementation on the productivity and the chemical composition of oyster mushroom. J. Food Res. 2012, 1, 113–119. [Google Scholar] [CrossRef]
- Kurt, S.; Buyukalaca, S. Yield performances and changes in enzyme activities of Pleurotus spp. (P. ostreatus and P. sajor-caju) cultivated on different agricultural wastes. Bioresour. Technol. 2010, 101, 3164–3169. [Google Scholar] [CrossRef]
- Obodai, M.; Cleland-Okine, J.; Vowotor, K.A. Comparative study on the growth and yield of Pleurotus ostreatus mushroom on different lignocellulosic by-products. J. Ind. Microbiol. Biotechnol. 2003, 30, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Claye, S.S.; Idouraine, A.; Weber, C.W. Extraction and fractionation of insoluble fiber from five fiber sources. Food Chem. 1996, 57, 305–310. [Google Scholar] [CrossRef]
- Khan, T.S.; Umarah, M. Wheat straw: A pragmatic overview. Curr. Res. J. Biol. Sci. 2012, 4, 673–675. [Google Scholar]
- Knop, D.; Yarden, O.; Hadar, Y. The ligninolytic peroxidases in the genus Pleurotus: Divergence in activities, expression, and potential applications. Appl. Microbiol. Biotechnol. 2015, 99, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Pani, B.K. Evaluation of banana leaf as a new alternative substrate to paddy straw for oyster mushroom cultivation. J. Phytol. Res. 1995, 8, 145–148. [Google Scholar]
- Royse, D.J. Specialty mushrooms. In Mushroom Fact Sheet; Laboratory, M.S., Ed.; Penn State University: State College, PA, USA, 2004. [Google Scholar]

| Species | Substrate | Protein a | Fat a | Crude Fiber | Carbohydrates | Ash a | References |
|---|---|---|---|---|---|---|---|
| P. eous | Soybean straw | 21.37 | 2.60 | 9.00 x | 50.50 y | 6.50 | [41] § |
| P. eous | Paddy straw | 20.56 | 2.40 | 8.00 x | 48.00 y | 6.25 | [41] § |
| P. eous | Sunflower stalk | 19.19 | 2.20 | 7.92 x | 52.00 y | 5.25 | [41] § |
| P. eous | Wheat straw | 19.48 | 2.62 | 7.75 x | 50.20 y | 6.00 | [41] § |
| P. eous | Jowar straw | 19.17 | 2.10 | 7.45 x | 49.00 y | 6.40 | [41] § |
| P. eous | Bajra straw | 18.66 | 2.10 | 7.45 x | 49.00 y | 6.40 | [41] § |
| P. eous | Tur stalk straw | 18.66 | 2.30 | 7.30 x | 51.40 y | 5.50 | [41] § |
| P. ostreatus | Pine needles | 22.74 | 2.44 | 13.68 a | 75.88 z | 7.50 | [42] |
| P. ostreatus | Wheat straw | 14.64 | 2.56 | 19.07 a | 74.41 z | 8.56 | [42] |
| P. ostreatus | Wheat straw | 14.41 | 2.20 | 12.08 a | 40.96 a | 8.30 | [43] § |
| P. sajor-caju | Wheat straw + 20% mahua cake detoxified with methanol | 34.76 | 1.76 | n.r. | 30.66 ¥ | 8.23 | [44] |
| P. sajor-caju | Wheat straw + 10% mahua cake detoxified with methanol | 34.16 | 1.76 | n.r. | 30.50 ¥ | 8.09 | [44] |
| P. sajor-caju | Wheat straw + 10% mahua cake detoxified with cold water | 33.46 | 1.78 | n.r. | 31.43 ¥ | 7.98 | [44] |
| P. sajor-caju | Wheat straw + 10% mahua cake | 33.00 | 1.76 | n.r. | 31.86 ¥ | 8.19 | [44] |
| P. sajor-caju | Wheat straw + 30% mahua cake detoxified with methanol | 32.43 | 1.77 | n.r. | 29.33 ¥ | 8.00 | [44] |
| P. sajor-caju | Wheat straw + 20% mahua cake detoxified with cold water | 31.23 | 1.75 | n.r. | 29.66 ¥ | 7.92 | [44] |
| P. sajor-caju | Wheat straw + 20% mahua cake | 31.00 | 1.80 | n.r. | 31.90 ¥ | 8.04 | [44] |
| P. sajor-caju | Wheat straw + 30% mahua cake detoxified with cold water | 30.83 | 1.78 | n.r. | 28.60 ¥ | 8.03 | [44] |
| P. sajor-caju | Wheat straw + 40% mahua cake detoxified with methanol | 30.33 | 1.75 | n.r. | 28.83 ¥ | 7.99 | [44] |
| P. sajor-caju | Wheat straw | 29.36 | 2.07 | n.r. | 32.16 ¥ | 8.05 | [44] |
| P. sajor-caju | Wheat straw + 40% mahua cake detoxified with cold water | 29.16 | 1.76 | n.r. | 28.83 ¥ | 8.10 | [44] |
| P. sajor-caju | Wheat straw + 40% mahua cake | 28.16 | 1.81 | n.r. | 31.03 ¥ | 8.02 | [44] |
| P. sajor-caju | Wheat straw + 30% mahua cake | 27,43 | 1.83 | n.r. | 31.93 ¥ | 8.14 | [44] |
| P. sapidus | Soybean straw | 18,75 | 2.10 | 7.50 x | 24.95 y | 7.00 | [45] § |
| P. sapidus | Paddy straw | 16,40 | 1.60 | 8.00 x | 45.65 y | 6.35 | [45] § |
| P. sapidus | Jowar straw | 15.98 | 2.00 | 6.75 x | 50.60 y | 5.85 | [45] § |
| P. sapidus | Bajra straw | 15.49 | 1.80 | 6.00 x | 47.00 y | 5.40 | [45] § |
| P. sapidus | Tur stalk straw | 15.17 | 1.50 | 7.65 x | 44.25 y | 6.50 | [45] § |
| P. sapidus | Wheat straw | 14.96 | 2.60 | 6.80 x | 52.00 y | 5.63 | [45] § |
| P. sapidus | Sunflower stalk | 14.10 | 2.40 | 7.32 x | 48.50 y | 6.20 | [45] § |
| Species | Substrate | BE | References |
|---|---|---|---|
| P. citrinopileatus | Pea pod:paddy straw (30:70) | 94.3 | [48] |
| P. citrinopileatus | Brassica straw:paddy straw (30:70) | 92.3 | [48] |
| P. citrinopileatus | Cauliflower leaves:paddy straw (30:70) | 91.0 | [48] |
| P. citrinopileatus | Brassica straw:paddy straw (20:80) | 90.8 | [48] |
| P. citrinopileatus | Cauliflower leaves:paddy straw (20:80) | 90.8 | [48] |
| P. citrinopileatus | Paddy straw | 90.0 | [48] |
| P. citrinopileatus | Pea pod:paddy straw (20:80) | 90.0 | [48] |
| P. citrinopileatus | Peapod | 0 | [48] |
| P. citrinopileatus | Cauliflower leaves | 0 | [48] |
| P. eous | Soybean straw | 82.0 | [41] |
| P. eous | Paddy straw | 79.8 | [41] |
| P. eous | Wheat straw | 75.1 | [41] |
| P. eous | Jowar straw | 73.2 | [41] |
| P. eous | Bajra straw | 71.6 | [41] |
| P. eous | Tur stalk straw | 68.0 | [41] |
| P. eous | Sunflower stalk | 61.5 | [41] |
| P. eryngii | Wheat straw | 87.5 | [49] |
| P. eryngii | Wheat straw | 57.3 | [50] |
| P. eryngii | Wheat straw | 48.2 | [51] |
| P. eryngii | Wheat straw | 38.4 | [50] |
| P. eryngii | Rice straw | 46.0 | [51] |
| P. florida | Paddy straw + 4% CaSO4 | 95.5 | [52] |
| P. florida | Paddy straw + 6% MgSO4 | 85.2 | [52] |
| P. florida | Paddy straw + 0.4% FeSO4 | 51.5 | [52] |
| P. florida | Paddy straw + 0.2% ZnSO4 | 37.8 | [52] |
| P. ostreatus | Wheat straw | 114.9 | [43] |
| P. ostreatus | Wheat straw | 106.7 | [50] |
| P. ostreatus | Wheat straw + 5% wheat bran | 92.5 | [53] |
| P. ostreatus | Paddy straw | 82.7 | [54] |
| P. ostreatus | Wheat straw | 79.7 | [50] |
| P. ostreatus | Wheat straw | 70.2 | [49] |
| P. ostreatus | Wheat straw | 67.8 | [50] |
| P. ostreatus | Wheat straw | 60.3 | [54] |
| P. ostreatus | Deenanath grass | 59.6 | [54] |
| P. ostreatus | Banana leaves | 58.6 | [54] |
| P. ostreatus | Wheat straw | 57.4 | [55] |
| P. ostreatus | Wheat straw | 52.6 | [42] |
| P. ostreatus | Maize stover | 41.8 | [55] |
| P. ostreatus | Maize stalks and leaves | 27.0 | [54] |
| P. pulmonarius | Wheat straw | 99.7 | [49] |
| P. sajor-caju | Wheat straw + 5% wheat bran | 96.5 | [53] |
| P. sajor-caju | Cotton stalks + wheat straw | 79.6 | [56] ˜ |
| P. sajor-caju | Cotton stalks+groundnut haulms+soybean straw+pigeon pea stalks and leaves+wheat straw | 75.0 | [56] ˜ |
| P. sajor-caju | Pigeon pea stalks and leaves+wheat straw | 73.6 | [56] ˜ |
| P. sajor-caju | Cotton stalks+pigeon pea stalks and leaves | 71.3 | [56] ˜ |
| P. sajor-caju | Cotton stalks+pigeon pea stalks and leaves+wheat straw | 70.3 | [56] ˜ |
| P. sajor-caju | Soybean straw+wheat straw | 66.8 | [56] ˜ |
| P. sajor-caju | Cotton stalks+soybean straw | 66.1 | [56] ˜ |
| P. sajor-caju | Cotton stalks+groundnut haulms+wheat straw | 65.7 | [56] ˜ |
| P. sajor-caju | Cotton stalks+soybean straw+wheat straw | 64.1 | [56] ˜ |
| P. sajor-caju | Groundnut haulms+wheat straw | 63.6 | [56] ˜ |
| P. sajor-caju | Cotton stalks+soybean straw+pigeon pea stalks and leaves | 63.4 | [56] ˜ |
| P. sajor-caju | Groundnut haulms+soybean straw+pigeon pea stalks and leaves + wheat straw | 61.5 | [56] ˜ |
| P. sajor-caju | Cotton stalks+groundnut haulms | 59.7 | [56] ˜ |
| P. sajor-caju | Cotton stalks+groundnut haulms+soybean straw+wheat straw | 59.1 | [56] ˜ |
| P. sajor-caju | Cotton stalks+groundnut haulms+soybean straw+pigeon pea stalks and leaves | 58.3 | [56] ˜ |
| P. sajor-caju | Groundnut haulms+pigeon pea stalks and leaves | 55.7 | [56] ˜ |
| P. sajor-caju | Cotton stalks+groundnut haulms+pigeon pea stalks and leaves | 53.3 | [56] ˜ |
| P. sajor-caju | Groundnut haulms+soybean straw+wheat straw | 51.3 | [56] ˜ |
| P. sajor-caju | Cotton stalks | 51.1 | [56] ˜ |
| P. sajor-caju | Cotton stalks+groundnut haulms+soybean straw | 48.7 | [56] ˜ |
| P. sajor-caju | Pigeon pea stalks and leaves | 46.3 | [56] ˜ |
| P. sajor-caju | Soybean straw+pigeon pea stalks and leaves | 45.7 | [56] ˜ |
| P. sajor-caju | Soybean straw+pigeon pea stalks and leaves+wheat straw | 45.1 | [56] ˜ |
| P. sajor-caju | Wheat straw | 44.7 | [56] ˜ |
| P. sajor-caju | Groundnut haulms+soybean straw+pigeon pea stalks and leaves | 39.5 | [56] ˜ |
| P. sajor-caju | Groundnut haulms | 36.5 | [56] ˜ |
| P. sajor-caju | Groundnut haulms+soybean straw | 33.3 | [56] ˜ |
| P. sajor-caju | Soybean straw | 25.8 | [56] ˜ |
| P. sapidus | Soybean straw | 72.9 | [45] |
| P. sapidus | Paddy straw | 64.7 | [45] |
| P. sapidus | Wheat straw | 62.2 | [45] |
| P. sapidus | Jowar straw | 50.3 | [45] |
| P. sapidus | Tur stalk straw | 48.4 | [45] |
| P. sapidus | Wheat straw + 5% wheat bran | 47.9 | [53] |
| P. sapidus | Bajra straw | 46.7 | [45] |
| P. sapidus | Sunflower stalk | 45.9 | [45] |
| Species | Substrate | Protein a | Fat a | Crude Fiber | Carbohydrates | Ash a | References |
|---|---|---|---|---|---|---|---|
| P. ostreatus | Almond and walnut shells (1:1) | 31.36 | 2.49 | 13.00 a | 56.64 z | 9.86 | [42] |
| P. ostreatus | Extracted olive-press cake | 21.41 | 1.64 | 13.68 a | 70.21 z | 6.98 | [42] |
| P. ostreatus | Corncob | 20.81 | 2.67 | 29.75 a | 30.78 z | 7.10 | [37] § |
| P. ostreatus | Olive mill by-products (leaves and two-phase olive mill waste 1:1) | 19.74 | 2.72 | 12.50 a | 68.28 z | 9.48 | [42] |
| P. ostreatus | Sugarcane bagasse | 19.01 | 2.00 | 29.25 a | 34.94 z | 6.68 | [37] § |
| P. ostreatus | Sawdust:corncob (50:50) | 17.98 | 1.80 | 28.25 a | 37.50 z | 6.80 | [37] § |
| P. ostreatus | Sawdust:sugarcane bagasse (50:50) | 16.94 | 2.50 | 28.75 a | 37.88 z | 6.70 | [37] § |
| P. ostreatus | Date palm tree leaves | 16.13 | 3.41 | 19.89 a | 72.77 z | 7.83 | [42] |
| P. ostreatus | Beech sawdust | 16.06 | 3.46 | 15.78 a | 73.56 z | 6.21 | [42] |
| P. ostreatus | Grape marc + cotton gin trash (1:1) | 15.99 | 2.81 | 24.26 a | 72.95 z | 8.43 | [42] |
| P. ostreatus | Corncobs | 15.41 | 3.37 | 13.76 a | 73.37 z | 8.02 | [42] |
| P. ostreatus | Sawdust:sugarcane bagasse (80:20) | 15.33 | 2.78 | 24.02 a | 44.97 z | 6.35 | [37] § |
| P. ostreatus | Trash/bagasse mixture | 14.89 | 1.85 | 12.65 a | 40.41 a | 6.64 | [43] § |
| P. ostreatus | Sawdust:corncob (80:20) | 14.64 | 2.08 | 23.04 a | 47.62 z | 6.37 | [37] § |
| P. ostreatus | Sugar cane trash | 13.79 | 1.79 | 12.39 a | 41.20 a | 7.82 | [43] § |
| P. ostreatus | Sawdust | 13.68 | 1.32 | 22.00 a | 51.26 z | 5.90 | [37] § |
| P. ostreatus | Depithed Bagasse | 12.04 | 1.48 | 12.83 a | 44.78 a | 6.28 | [43] § |
| P. ostreatus | Bagasse from mills | 11.08 | 1.94 | 12.89 a | 45.14 a | 5.81 | [43] § |
| P. cystidiosus | Corncob | 17.20 | 3.00 | 24.25 a | 40.64 z | 7.57 | [37] § |
| P. cystidiosus | Sugarcane bagasse | 15.54 | 2.30 | 22.79 a | 45.25 z | 7.48 | [37] § |
| P. cystidiosus | Sawdust:corncob (50:50) | 15.05 | 2.80 | 23.58 a | 44.85 z | 7.30 | [37] § |
| P. cystidiosus | Sawdust:sugarcane bagasse (50:50) | 13.08 | 3.28 | 24.50 a | 46.86 z | 6.70 | [37] § |
| P. cystidiosus | Sawdust:sugarcane bagasse (80:20) | 12.58 | 3.33 | 25.05 a | 47.27 z | 6.40 | [37] § |
| P. cystidiosus | Sawdust:corncob (80:20) | 11.84 | 2.33 | 22.45 a | 51.93 z | 6.39 | [37] § |
| P. cystidiosus | Sawdust | 10.99 | 2.05 | 20.05 a | 55.92 z | 6.30 | [37] § |
| Species | Substrate | BE | References |
|---|---|---|---|
| P. cystidiosus | Corncob | 50.1 | [37] |
| P. cystidiosus | Sugarcane bagasse | 49.5 | [37] |
| P. cystidiosus | Sawdust:sugarcane bagasse (50:50) | 44.1 | [37] |
| P. cystidiosus | Sawdust (acacia):corncob (50:50) | 43.6 | [37] |
| P. cystidiosus | Sawdust:sugarcane bagasse (80:20) | 41.2 | [37] |
| P. cystidiosus | Sawdust (acacia):corncob (80:20) | 38.4 | [37] |
| P. cystidiosus | Sawdust (acacia) | 36.3 | [37] |
| P. eryngii | Composted two-phase olive mill wastes: wheat straw (40:60) | 120.0 | [49] |
| P. eryngii | Composted two-phase olive mill wastes: wheat straw (20:80) | 119.8 | [49] |
| P. eryngii | Two-phase olive mill wastes: wheat straw (20:80) | 96.1 | [49] |
| P. eryngii | Grape marc:wheat straw (1:1) | 87.2 | [50] |
| P. eryngii | Composted two-phase olive mill wastes: wheat straw (60:40) | 86.8 | [49] |
| P. eryngii | Olive leaves: Two-Phase Olive Mill Waste (1:1) | 73.3 | [50] |
| P. eryngii | Cotton waste | 71.6 | [51] |
| P. eryngii | Two-phase olive mill wastes: wheat straw (40:60) | 68.9 | [49] |
| P. eryngii | Corncob | 51.8 | [51] |
| P. eryngii | Grape marc:wheat straw (1:1) | 48.2 | [50] |
| P. eryngii | Olive leaves: Two-Phase Olive Mill Waste (1:1) | 42.3 | [50] |
| P. eryngii | Sugarcane bagasse | 41.3 | [51] |
| P. eryngii | Sawdust (acacia) | 35.5 | [51] |
| P. eryngii | Two-phase olive mill wastes: wheat straw (60:40) | 30.9 | [49] |
| P. florida | Coir pith + 4% CaSO4 | 74.2 | [52] |
| P. florida | Coir pith + 6% MgSO4 | 52.2 | [52] |
| P. florida | Coir pith + 0.4% FeSO4 | 27.5 | [52] |
| P. florida | Coir pith + 0.2% ZnSO4 | 22.7 | [52] |
| P. ostreatus | Grape marc + cotton gin trash (1:1) | 137.2 | [42] |
| P. ostreatus | Composted two-phase olive mill wastes: wheat straw (20:80) | 135.3 | [49] |
| P. ostreatus | Composted two-phase olive mill wastes: wheat straw (40:60) | 125.5 | [49] |
| P. ostreatus | Two-phase olive mill wastes: wheat straw (20:80) | 107.6 | [49] |
| P. ostreatus | Sugar cane Trash | 106.6 | [43] |
| P. ostreatus | Trash/bagasse mixture | 103.5 | [43] |
| P. ostreatus | Grape marc:wheat straw (1:1) | 98.0 | [50] |
| P. ostreatus | Grounded corncob | 91.2 | [64] |
| P. ostreatus | Composted two-phase olive mill wastes: wheat straw (60:40) | 88.3 | [49] |
| P. ostreatus | Sawdust | 85.7 | [64] |
| P. ostreatus | Grape marc:wheat straw (1:1) | 84.1 | [50] |
| P. ostreatus | Two-phase olive mill wastes: wheat straw (40:60) | 84.1 | [49] |
| P. ostreatus | Sawdust:grounded corncob (40:60) | 77.2 | [64] |
| P. ostreatus | Leaves and two-phase olive mill waste (1:1) | 71.6 | [42] |
| P. ostreatus | Sugarcane leaves | 71.0 | [54] |
| P. ostreatus | Bagasse from mills | 70.1 | [43] |
| P. ostreatus | Sawdust:grounded corncob (60:40) | 68.4 | [64] |
| P. ostreatus | Hazelnut husk: wheat straw: wheat bran (1:2:1) | 67.4 | [53] |
| P. ostreatus | Depithed Bagasse | 66.6 | [43] |
| P. ostreatus | Hazelnut husk:wheat straw:wheat bran (1.5:2:0.5) | 66.3 | [53] |
| P. ostreatus | Corncob | 66.1 | [37] |
| P. ostreatus | Sugarcane bagasse | 65.7 | [37] |
| P. ostreatus | Cotton waste | 59.4 | [55] |
| P. ostreatus | Sawdust:sugarcane bagasse (50:50) | 58.9 | [37] |
| P. ostreatus | Sawdust (acacia):corncob (50:50) | 58.8 | [37] |
| P. ostreatus | Pine needles | 55.7 | [42] |
| P. ostreatus | Olive leaves: Two-Phase Olive Mill Waste (1:1) | 55.4 | [50] |
| P. ostreatus | Sawdust:sugarcane bagasse (80:20) | 52.3 | [37] |
| P. ostreatus | Sugarcane bagasse | 50.4 | [54] |
| P. ostreatus | Sawdust (acacia):corncob (80:20) | 49.0 | [37] |
| P. ostreatus | Sawdust (acacia) | 46.4 | [37] |
| P. ostreatus | Two-phase olive mill wastes: wheat straw (60:40) | 45.4 | [49] |
| P. ostreatus | Grape marc:wheat straw (1:1) | 45.1 | [50] |
| P. ostreatus | Beech sawdust | 33.5 | [42] |
| P. ostreatus (local) | Solid olive mill wastes:CaCO3:wheat straw (88:2:10) | 33.1 | [65] |
| P. ostreatus | Corncob | 31.7 | [42] |
| P. ostreatus (commercial) | Solid olive mill wastes:CaCO3:wheat straw (88:2:10) | 29.8 | [65] |
| P. ostreatus | Corncobs | 28.3 | [55] |
| P. ostreatus | Olive leaves: Two-Phase Olive Mill Waste (1:1) | 26.9 | [50] |
| P. ostreatus | Date palm tree leaves | 24.7 | [42] |
| P. ostreatus | Extracted olive-press cake | 18.8 | [42] |
| P. ostreatus | Olive leaves: Two-Phase Olive Mill Waste (1:1) | 17.9 | [50] |
| P. ostreatus (local) | Solid olive mill wastes | 10.3 | [65] |
| P. ostreatus (commercial) | Solid olive mill wastes | 11.5 | [65] |
| P. ostreatus | Jatropha cake | 0 | [55] |
| P. pulmonarius | Composted two-phase olive mill wastes: wheat straw (20:80) | 125.8 | [49] |
| P. pulmonarius | Composted two-phase olive mill wastes: wheat straw (40:60) | 116.5 | [49] |
| P. pulmonarius | Two-phase olive mill wastes: wheat straw (20:80) | 116.3 | [49] |
| P. pulmonarius | Two-phase olive mill wastes: wheat straw (40:60) | 86.4 | [49] |
| P. pulmonarius | Composted two-phase olive mill wastes: wheat straw (60:40) | 81.3 | [49] |
| P. pulmonarius | Two-phase olive mill wastes: wheat straw (60:40) | 42.0 | [49] |
| P. sajor-caju | Hazelnut husk: wheat straw: wheat bran (1.5:2:0.5) | 71.0 | [53] |
| P. sajor-caju | Hazelnut husk: wheat straw: wheat bran (1:2:1) | 66.3 | [53] |
| P. sapidus | Hazelnut husk: wheat straw: wheat bran (1.5:2:0.5) | 44.2 | [53] |
| P. sapidus | Hazelnut husk: wheat straw: wheat bran (1:2:1) | 41.1 | [53] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ritota, M.; Manzi, P. Pleurotus spp. Cultivation on Different Agri-Food By-Products: Example of Biotechnological Application. Sustainability 2019, 11, 5049. https://doi.org/10.3390/su11185049
Ritota M, Manzi P. Pleurotus spp. Cultivation on Different Agri-Food By-Products: Example of Biotechnological Application. Sustainability. 2019; 11(18):5049. https://doi.org/10.3390/su11185049
Chicago/Turabian StyleRitota, Mena, and Pamela Manzi. 2019. "Pleurotus spp. Cultivation on Different Agri-Food By-Products: Example of Biotechnological Application" Sustainability 11, no. 18: 5049. https://doi.org/10.3390/su11185049
APA StyleRitota, M., & Manzi, P. (2019). Pleurotus spp. Cultivation on Different Agri-Food By-Products: Example of Biotechnological Application. Sustainability, 11(18), 5049. https://doi.org/10.3390/su11185049






