Do We Know Enough to Save European Riverine Fish?—A Systematic Review on Autecological Requirements During Critical Life Stages of 10 Rheophilic Species at Risk
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Review
2.2. Data Analyses
3. Results
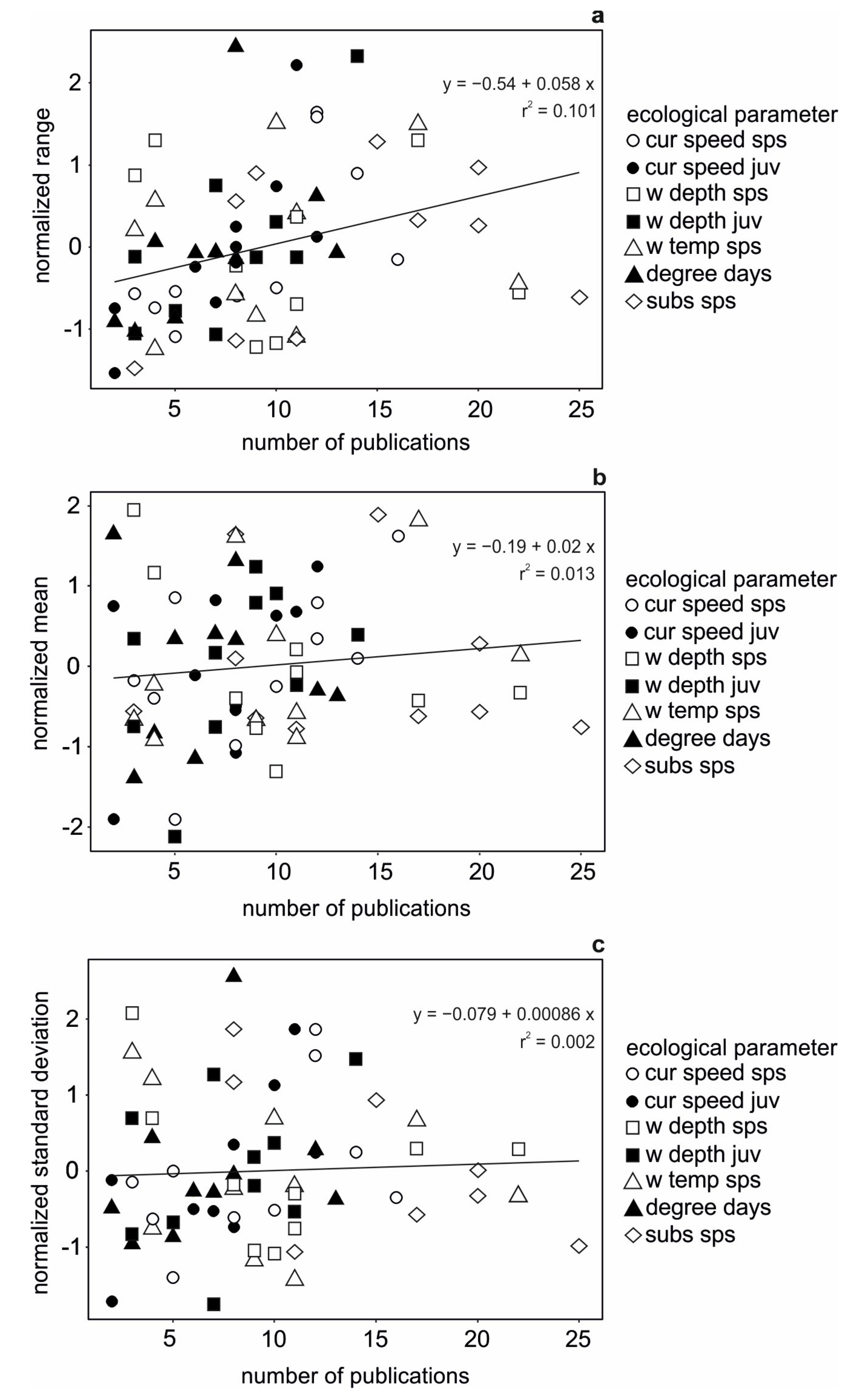
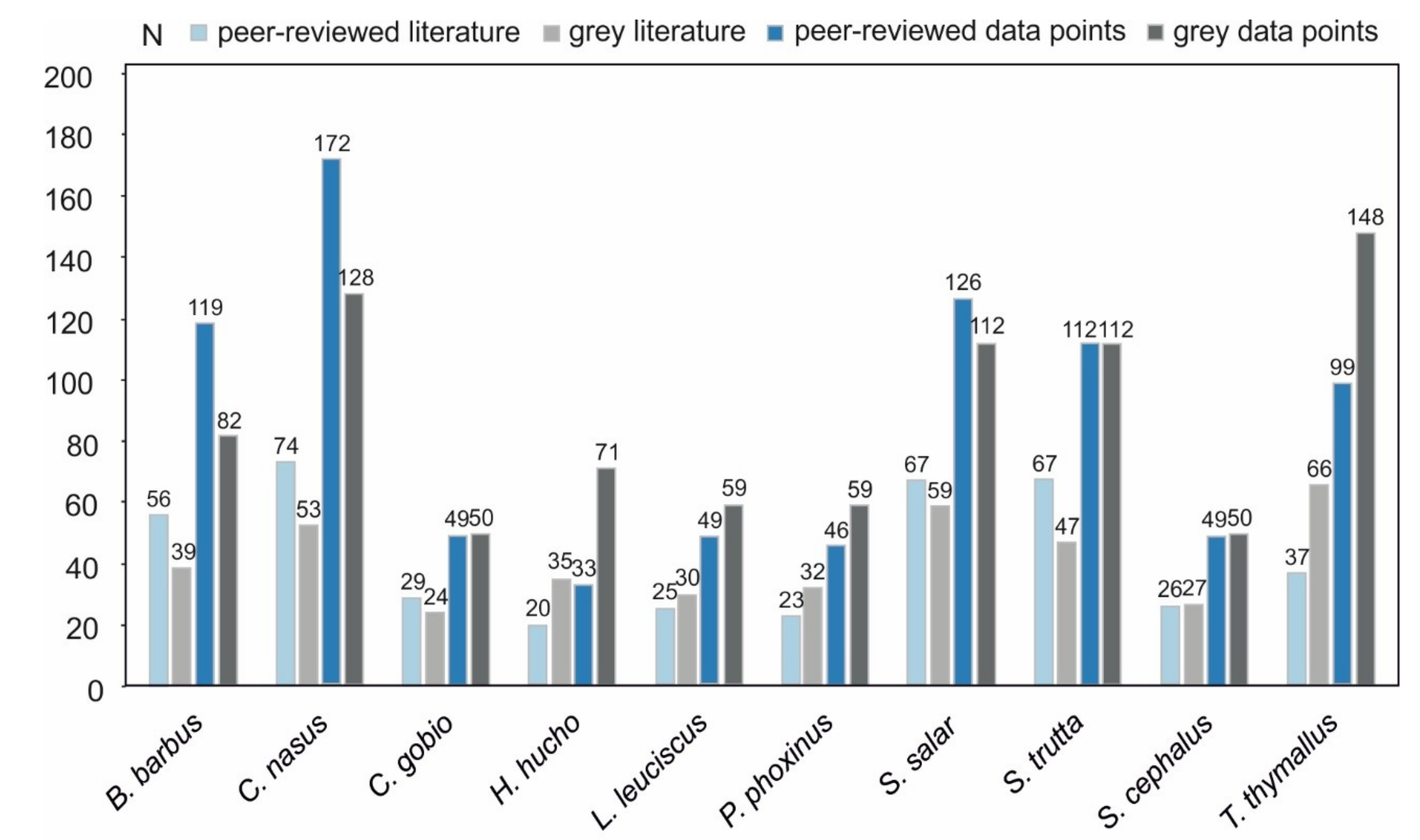
3.1. Data Availability
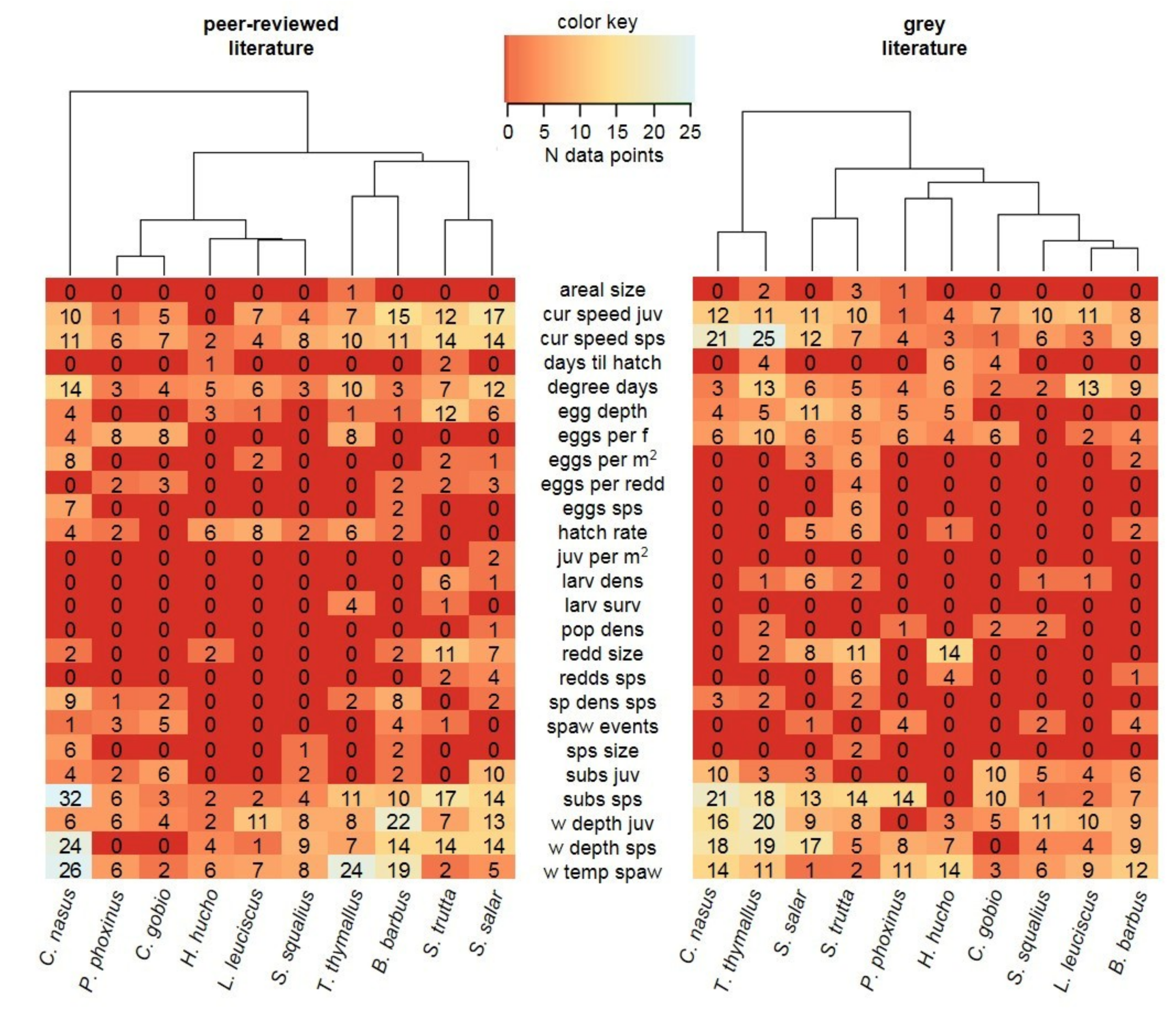
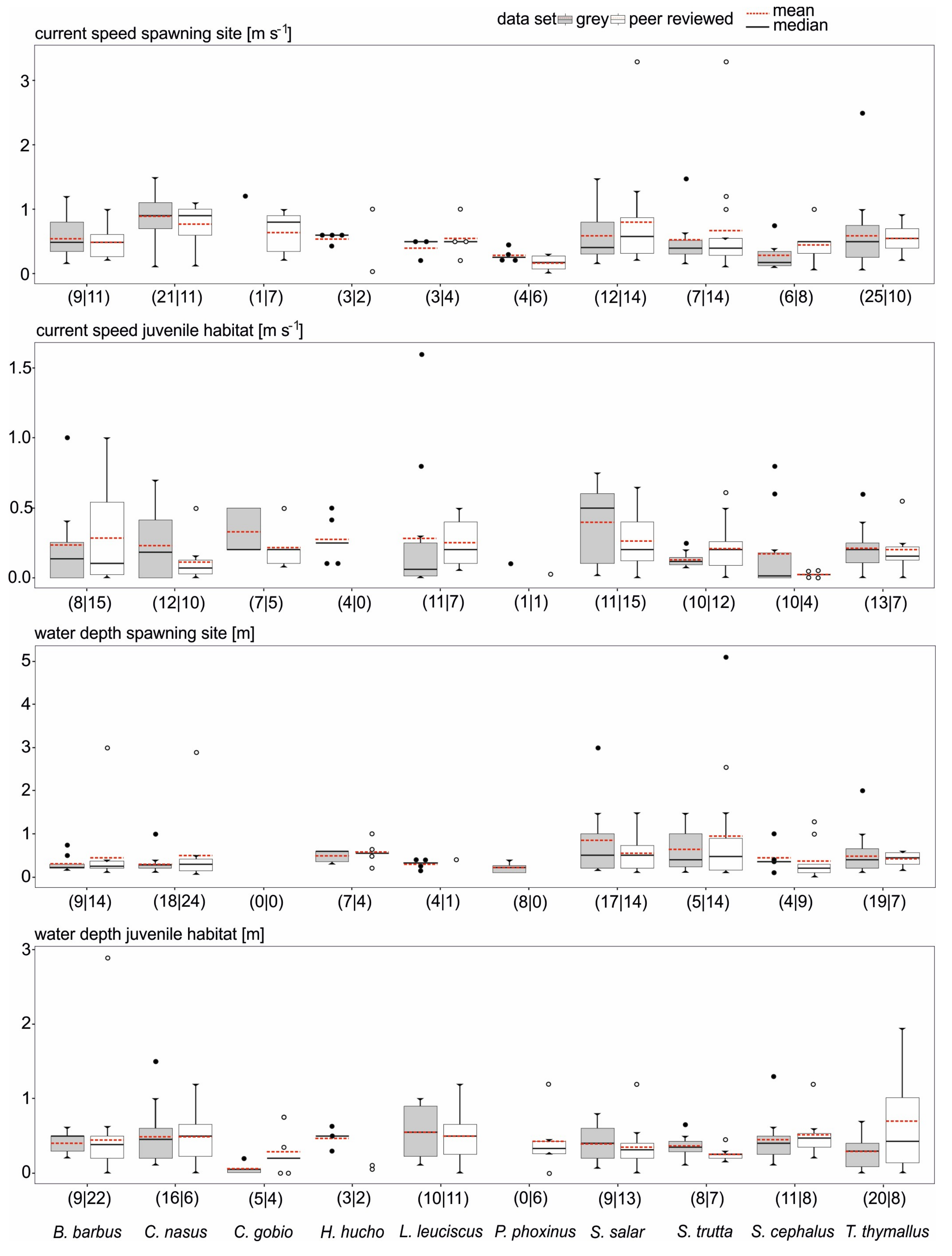
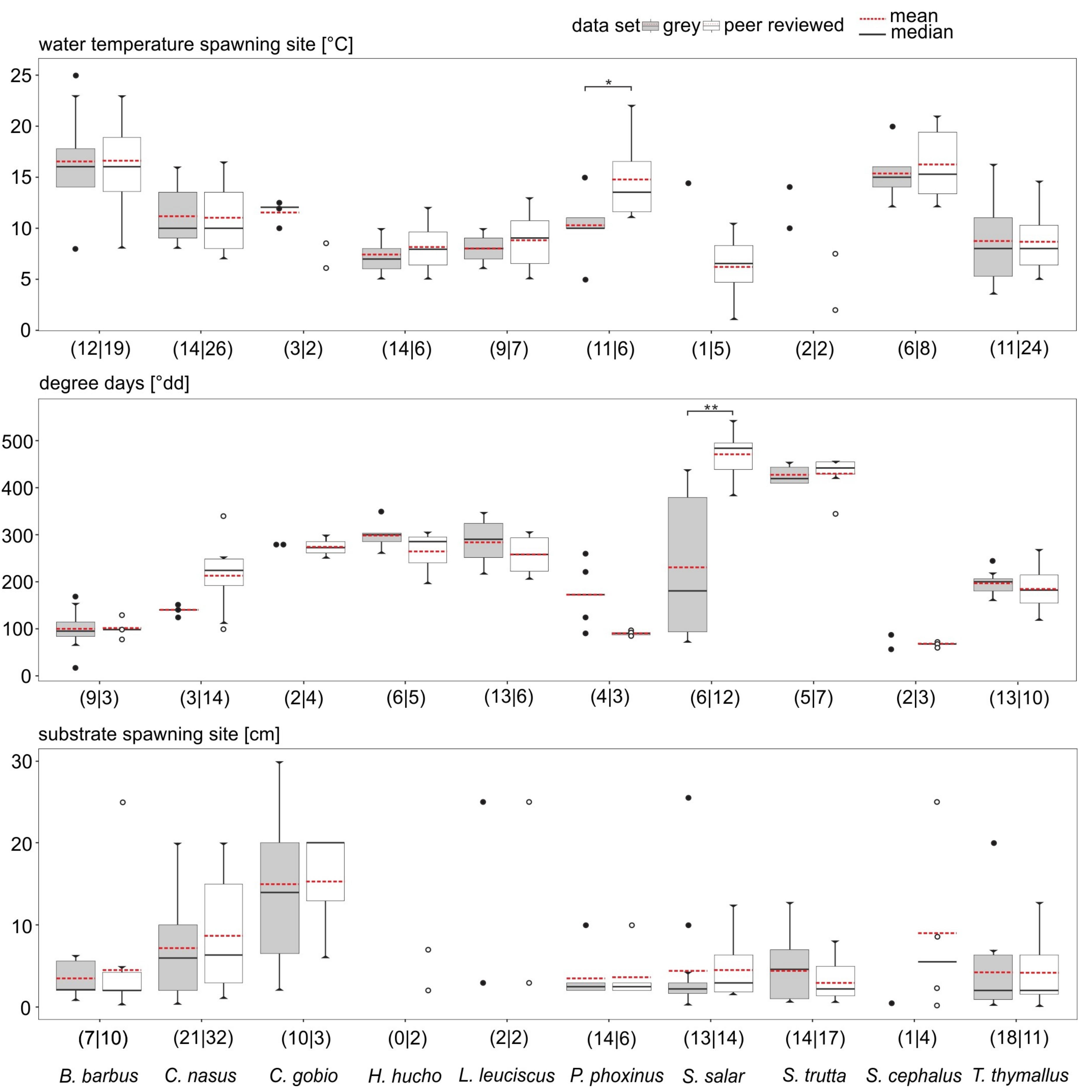
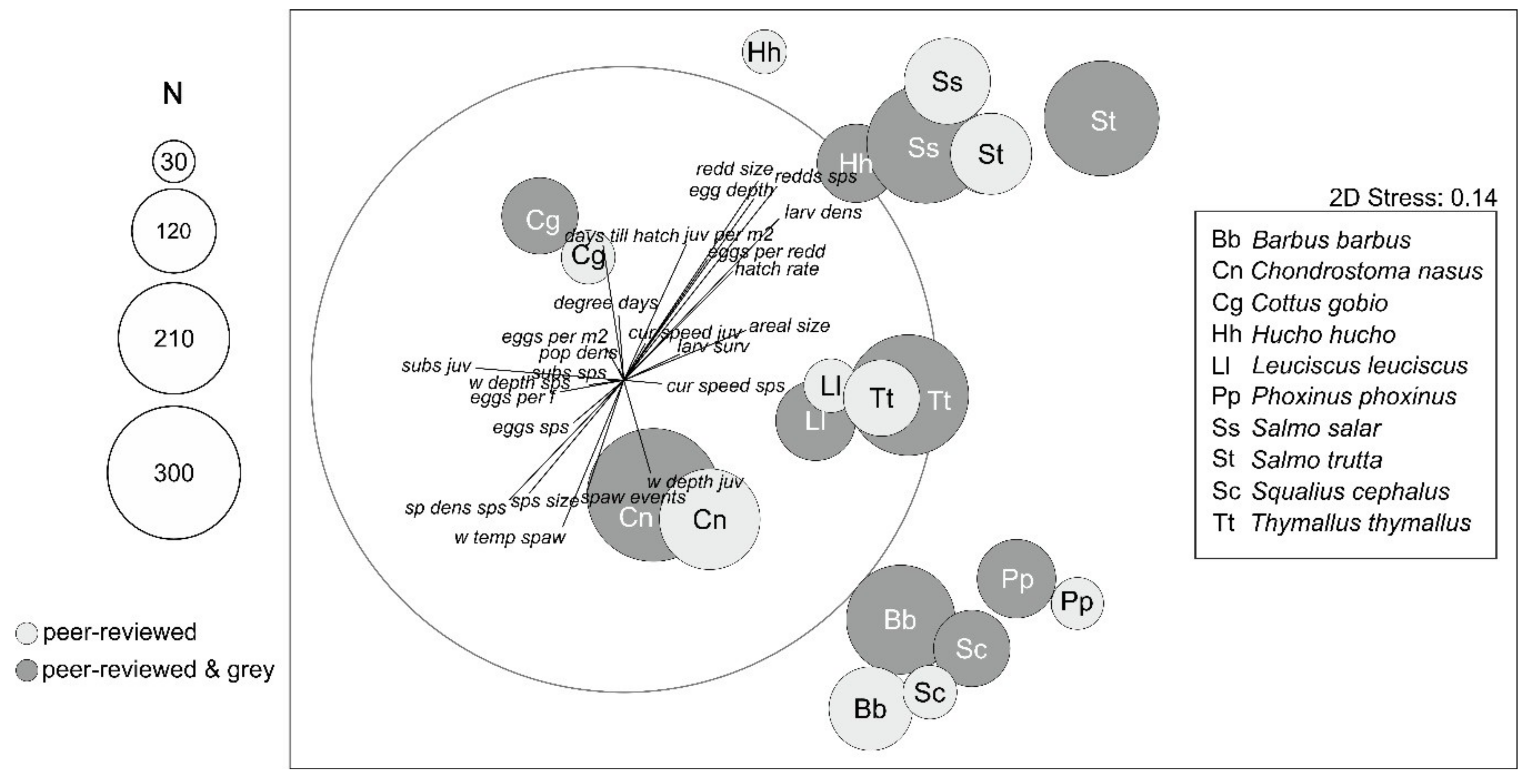
3.2. Data Comparability and Variability from Different Sources
3.3. Data Quality Differences Among Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Species Name | Environmental Parameter | ||||
|---|---|---|---|---|---|
| German | English | Latin | |||
| Barbe | or | common barbel | or | Barbus barbus | + areal size |
| Nase | or | common nase | or | Chondrostoma nasus | + current speed juvenile |
| Mühlkoppe or Groppe * | or | European bullhead | or | Cottus gobio | + current speed spawning site |
| Huchen | or | Danube salmon | or | Hucho hucho | + days till hatch |
| Hasel | or | European dace | or | Leuciscus leuciscus | + degree days |
| Elritze | or | European minnow | or | Phoxinus phoxinus | + egg depth |
| Atlantischer Lachs | or | Atlantic salmon | or | Salmo salar | + eggs per female |
| Bachforelle | or | brown trout | or | Salmo trutta | + eggs per m2 |
| Döbel or Aitel * | or | European chub | or | Squalius cephalus | + eggs per redd |
| Äsche | or | European grayling | or | Thymallus thymallus | + eggs spawning site |
| + hatch rate | |||||
| + juvenile per m2 | |||||
| + larval density | |||||
| + larval survival | |||||
| + population density | |||||
| + nest size | |||||
| + redd size | |||||
| + redds spawning site | |||||
| + spawner density spawning site | |||||
| + spawning events | |||||
| + spawning site size | |||||
| + substrate juvenile | |||||
| + substrate spawning site | |||||
| + water depth juvenile | |||||
| + water depth spawning site | |||||
| + water temperature spawning | |||||
| + habitat | |||||
| + spawning | |||||
| + eggs | |||||
| + individual density | |||||
| + hatch success | |||||
| + substrate +substrate quality | |||||
| + juvenile | |||||
| + fecundity | |||||
| + autecology | |||||
References
- Aarts, B.G.; Van Den Brink, F.W.; Nienhuis, P.H. Habitat loss as the main cause of the slow recovery of fish faunas of regulated large rivers in Europe: The transversal floodplain gradient. River Res. Appl. 2004, 20, 3–23. [Google Scholar] [CrossRef]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Ormerod, S.J.; Dobson, M.; Hildrew, A.G.; Townsend, C. Multiple stressors in freshwater ecosystems. Freshw. Biol. 2010, 55, 1–4. [Google Scholar] [CrossRef]
- Arthington, A.H.; Dulvy, N.K.; Gladstone, W.; Winfield, I.J. Fish conservation in freshwater and marine realms: Status, threats and management. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 838–857. [Google Scholar] [CrossRef]
- Bierschenk, B.M.; Pander, J.; Mueller, M.; Geist, J. Fish injury and mortality at pumping stations: A comparison of conventional and fish-friendly pumps. Mar. Freshw. Res. 2018, 70, 449–458. [Google Scholar] [CrossRef]
- Auerswald, K.; Moyle, P.; Seibert, S.P.; Geist, J. HESS Opinions: Socio-economic and ecological trade-offs of flood management - benefits of a transdisciplinary approach. Hydrol. Earth Syst. Sci. 2019, 23, 1035–1044. [Google Scholar] [CrossRef]
- Grill, G.; Lehner, B.; Thieme, M.; Geenen, B.; Tickner, D.; Antonelli, F.; Babu, S.; Borrelli, L.; Crochetiere, C.H.; Macedo, H.E.; et al. Mapping the world’s free-flowing rivers. Nature 2019, 569, 215. [Google Scholar] [CrossRef]
- Lucas, M.C.; Frear, P.A. Effects of a flow-gauging weir on the migratory behaviour of adult barbel, a riverine cyprinid. J. Fish Biol. 1997, 50, 382–396. [Google Scholar] [CrossRef]
- Caudill, C.C.; Daigle, W.R.; Keefer, M.L.; Boggs, C.T.; Jepson, M.A.; Burke, B.J.; Zabel, R.W.; Bjornn, T.C.; Peery, C.A. Slow dam passage in adult Columbia River salmonids associated with unsuccessful migration: Delayed negative effects of passage obstacles or condition-dependent mortality? Can. J. Fish. Aquat. Sci. 2007, 64, 979–995. [Google Scholar] [CrossRef]
- Wolter, C.; Buijse, A.D.; Parasiewicz, P. Temporal and spatial patterns of fish response to hydromorphological processes. River Res. Appl. 2016, 32, 190–201. [Google Scholar] [CrossRef]
- Mueller, M.; Pander, J.; Geist, J. The effects of weirs on structural stream habitat and biological communities. J. Appl. Ecol. 2011, 48, 1450–1461. [Google Scholar] [CrossRef]
- Santucci, V.J., Jr.; Gephard, S.R.; Pescitelli, S.M. Effects of multiple low-head dams on fish, macroinvertebrates, habitat, and water quality in the Fox River, Illinois. N. Am. J. Fish. Manag. 2005, 25, 975–992. [Google Scholar] [CrossRef]
- Catalano, M.J.; Bozek, M.A.; Pellett, T.D. Effects of dam removal on fish assemblage structure and spatial distributions in the Baraboo River, Wisconsin. N. Am. J. Fish. Manag. 2007, 27, 519–530. [Google Scholar] [CrossRef]
- Spens, J.; Englund, G.; Lundqvist, H. Network connectivity and dispersal barriers: Using geographical information system (GIS) tools to predict landscape scale distribution of a key predator (Esox lucius) among lakes. J. Appl. Ecol. 2007, 44, 1127–1137. [Google Scholar] [CrossRef]
- Slawski, T.M.; Veraldi, F.M.; Pescitelli, S.M.; Pauers, M.J. Effects of tributary spatial position, urbanization, and multiple low-head dams on warmwater fish community structure in a Midwestern stream. N. Am. J. Fish. Manag. 2008, 28, 1020–1035. [Google Scholar] [CrossRef]
- Nislow, K.H.; Hudy, M.; Letcher, B.H.; Smith, E.P. Variation in local abundance and species richness of stream fishes in relation to dispersal barriers: Implications for management and conservation. Freshw. Biol. 2011, 56, 2135–2144. [Google Scholar] [CrossRef]
- Langhans, S.; Gessner, J.; Hermoso, V.; Wolter, C. Coupling systematic planning and expert judgement enhances the efficiency of river restoration. Sci. Total Environ. 2016, 560, 266–273. [Google Scholar] [CrossRef]
- Geist, J. Seven steps towards improving freshwater conservation. Aquat. Conserv. Mar. Freshw. Ecosyst. 2015, 25, 447–453. [Google Scholar] [CrossRef]
- Pander, J.; Geist, J. Ecological indicators for stream restoration success. Ecol. Indic. 2013, 30, 106–118. [Google Scholar] [CrossRef]
- Geist, J.; Hawkins, S.J. Habitat recovery and restoration in aquatic ecosystems: Current progress and future challenges. Aquat. Conserv. 2016, 26, 942–962. [Google Scholar] [CrossRef]
- Van Looy, K.; Tonkin, J.D.; Floury, M.; Leigh, C.; Soininen, J.; Larsen, S.; Heino, J.; Poff, N.L.; Delong, M.; Jähnig, S.C.; et al. The three Rs of river ecosystem resilience: Resources, Recruitment and Refugia. River Res. Appl. 2019, 35, 107–120. [Google Scholar] [CrossRef]
- Casas-Mulet, R.; Saltveit, S.J.; Alfredsen, K. The survival of Atlantic salmon (Salmo salar) eggs during dewatering in a river subjected to hydropeaking. River Res. Appl. 2015, 31, 433–446. [Google Scholar] [CrossRef]
- Radinger, J.; Kail, J.; Wolter, C. Differences among expert judgments of fish habitat suitability and implications for river management. River Res. Appl. 2017, 33, 538–547. [Google Scholar] [CrossRef]
- Duerregger, A.; Pander, J.; Palt, M.; Mueller, M.; Nagel, C.; Geist, J. The importance of stream interstitial conditions for the early-life-stage development of the European nase (Chondrostoma nasus L.). Ecol. Freshw. Fish 2018, 27, 920–932. [Google Scholar] [CrossRef]
- Morandi, B.; Kail, J.; Toedter, A.; Wolter, C.; Piégay, H. Diverse approaches to implement and monitor river restoration: A comparative perspective in France and Germany. Environ. Manag. 2017, 60, 931–946. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.T.; Lucas, M.C.; Castro-Santos, T.; Katopodis, C.; Baumgartner, L.J.; Thiem, J.D.; Aarestrup, K.; Pompeu, P.S.; O’Brien, G.C.; Braun, D.C.; et al. The future of fish passage science, engineering, and practice. Fish Fish. 2018, 19, 340–362. [Google Scholar] [CrossRef]
- Geist, J. Integrative freshwater ecology and biodiversity conservation. Ecol. Indic. 2011, 11, 1507–1516. [Google Scholar] [CrossRef]
- Mueller, M.; Pander, J.; Geist, J. Comprehensive analysis of >30 years of data on stream fish population trends and conservation status in Bavaria, Germany. Biol. Conserv. 2018, 226, 311–320. [Google Scholar] [CrossRef]
- McDonald, J.H. Handbook of Biological Statistics, 3rd ed.; Sparky House Publishing: Baltimore, MD, USA, 2014; pp. 157–164. [Google Scholar]
- RC Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: http://www.R-project.org (accessed on 6 July 2018).
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 2nd ed.; Sage: Thousand Oaks, CA, USA, 2011; Available online: http://socserv.socsci.mcmaster.ca/jfox/Books/Companion (accessed on 20 February 2018).
- Wickham, H.; François, R.; Henry, L.; Müller, K. dplyr: A Grammar of Data Manipulation. R package version 0.7.5. 2018. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 6 July 2018).
- Chang, W. Extrafont: Tools for Using Fonts. R package version 0.17. 2014. Available online: https://CRAN.R-project.org/package=extrafont (accessed on 20 February 2018).
- Rudis, B.; Bolker, B.; Schulz, J.; Kothari, A.; Sidi, J. ggalt: Extra Coordinate Systems, ‘Geoms’, Statistical Transformations, Scales and Fonts for ‘ggplot2’. R package version 0.6.0. 2017. Available online: https://github.com/hrbrmstr/ggalt (accessed on 6 July 2018).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer-Verlag: New York, NY, USA, 2009; p. 213. [Google Scholar] [CrossRef]
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Liaw, W.H.A.; Lumley, T.; Maechler, M.; Magnusson, A.; Moeller, S.; Schartz, M.; et al. gplots: Various R Programming Tools for Plotting Data. R package version 3.0.1. 2016. Available online: https://CRAN.R-project.org/package=gplots (accessed on 6 July 2018).
- RC Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.R-project.org/ (accessed on 20 February 2018).
- Wickham, H. The Split-Apply-Combine Strategy for Data Analysis. J. Stat. Softw. 2011, 40, 1–29. Available online: http://www.jstatsoft.org/v40/i01/ (accessed on 20 February 2018). [CrossRef]
- Neuwirth, E. RColorBrewer: ColorBrewer Palettes. R package version 1.1-2. 2014. Available online: https://CRAN.R-project.org/package=RColorBrewer (accessed on 20 February 2018).
- Wickham, H.; Henry, L. Tidyr: Easily Tidy Data with ‘spread()’ and ‘gather()’ Functions. R package version 0.8.1. 2018. Available online: https://CRAN.R-project.org/package=tidyr (accessed on 6 July 2018).
- McCune, B.; Grace, J.B. Analysis of Ecological Communities, 1st ed.; MjM Software Design: Gleneden Beach, OR, USA, 2002; p. 300. [Google Scholar]
- Anders, P.J. Conservation aquaculture and endangered species. Fisheries 1998, 23, 28–31. [Google Scholar]
- Schiemer, F.; Keckeis, H.; Kamler, E. The early life history stages of riverine fish: Ecophysiological and environmental bottlenecks. Comp. Biochem. Physiol. 2002, 133, 439–449. [Google Scholar] [CrossRef]
- Rhodes, J.R.; Ng, C.F.; de Villiers, D.L.; Preece, H.J.; McAlpine, C.A.; Possingham, H.P. Using integrated population modelling to quantify the implications of multiple threatening processes for a rapidly declining population. Biol. Conserv. 2011, 144, 1081–1088. [Google Scholar] [CrossRef]
- Denic, M.; Geist, J. Habitat suitability analysis for lacustrine brown trout (Salmo trutta) in Lake Walchensee, Germany: Implications for the conservation of an endangered flagship species. Aquat. Conserv. 2010, 20, 9–17. [Google Scholar] [CrossRef]
- Mertens, M.; Bösiger, R.; Imhof, P.; Knutti, A.; Küry, D.; Staub, E. Der Lachs—Ein Fisch kehrt zurück, 1st ed.; Haupt Verlag: Bern, Switzerland, 2011; p. 264. [Google Scholar]
- Butler, J.R.; Radford, A.; Riddington, G.; Laughton, R. Evaluating an ecosystem service provided by Atlantic salmon, sea trout and other fish species in the River Spey, Scotland: The economic impact of recreational rod fisheries. Fish. Res. 2009, 96, 259–266. [Google Scholar] [CrossRef]
- ICES. Baltic Sea Ecoregion—Fisheries overview. Report of the ICES Advisory Committee, Section 4.2. 2018; 23. [Google Scholar] [CrossRef]
- Huet, M. Profiles and biology of western European streams as related to fish management. Trans. Am. Fish. Soc. 1959, 88, 155–163. [Google Scholar] [CrossRef]
- Lasne, E.; Bergerot, B.; Lek, S.; Laffaille, P. Fish zonation and indicator species for the evaluation of the ecological status of rivers: Example of the Loire basin (France). River Res. Appl. 2007, 23, 877–890. [Google Scholar] [CrossRef]
- Sternecker, K.; Wild, R.; Geist, J. Effects of substratum restoration on salmonid habitat quality in a subalpine stream. Environ. Biol. Fish. 2013, 96, 1341–1351. [Google Scholar] [CrossRef]
- Brandner, J.; Auerswald, K.; Cerwenka, A.F.; Schliewen, U.K.; Geist, J. Comparative feeding ecology of invasive Ponto-Caspian gobies. Hydrobiologia 2013, 703, 113–131. [Google Scholar] [CrossRef]
- Brandner, J.; Cerwenka, A.F.; Schliewen, U.K.; Geist, J. Bigger is better: Characteristics of round gobies forming an invasion front in the Danube River. PLoS ONE 2013, 8, e73036. [Google Scholar] [CrossRef] [PubMed]
- Cerwenka, A.; Brandner, J.; Schliewen, U.; Geist, J. Population trends of invasive alien gobies in the upper Danube River: 10 years after first detection of the globally invasive round goby (Neogobius melanostomus). Aquat. Invasions 2018, 13, 525–535. [Google Scholar] [CrossRef]
- Decker, E.; Linke, S.; Hermoso, V.; Geist, J. Incorporating ecological functions in conservation decision making. Ecol. Evol. 2017, 7, 8273–8281. [Google Scholar] [CrossRef]
- Schmutz, S.; Zitek, A.; Zobl, S.; Jungwirth, M.; Knopf, N.; Kraus, E.; Bauer, T.; Kaufmann, T. Integrated approach to the conservation and restoration of Danube salmon, Hucho hucho, populations in Austria. In Conservation of Freshwater Fishes: Options for the Future, 1st ed.; Cowx, I.G., Collares-Pereira, M.J., Coelho, M.M., Eds.; Fishing News Books: Oxford, UK, 2002; pp. 157–173. [Google Scholar]
- Lewis, C.A.; Lester, N.P.; Bradshaw, A.D.; Fitzgibbon, J.E.; Fuller, K.; Hakanson, L.; Richards, C. Considerations of scale in habitat conservation and restoration. Can. J. Fish. Aquat. Sci. 1996, 53, 440–445. [Google Scholar] [CrossRef]
- Fletcher, W.J.; Shaw, J.; Metcalf, S.J.; Gaughan, D.J. An ecosystem based fisheries management framework: The efficient, regional-level planning tool for management agencies. Mar. Pol. 2010, 34, 1226–1238. [Google Scholar] [CrossRef]
- Burgman, M.A.; Breininger, D.R.; Duncan, B.W.; Ferson, S. Setting reliability bounds on habitat suitability indices. Ecol. Appl. 2001, 11, 70–78. [Google Scholar] [CrossRef]
- Bonar, S.A.; Hubert, W.A. Standard sampling of inland fish: Benefits, challenges, and a call for action. Fisheries 2002, 27, 10–16. [Google Scholar] [CrossRef]
- Trudgill, D.L.; Honek, A.; Li, D.; Van Straalen, N.M. Thermal time–concepts and utility. Ann. Appl. Biol. 2005, 146, 1–14. [Google Scholar] [CrossRef]
- Blott, S.J.; Pye, K. Particle size scales and classification of sediment types based on particle size distributions: Review and recommended procedures. Sedimentology 2012, 59, 2071–2096. [Google Scholar] [CrossRef]
- EN933-1. European Standard. Tests for Geometrical Properties of Aggregates—Part 1: Determination of Particle Size Distribution—Sieving Methods, 1st ed.; The Office for Standards, Metrology and testing (ÚNMZ): Prague, Czech Republic, 2012; p. 10. [Google Scholar]
- Udden, J.A. Mechanical composition of clastic sediments. Bull. Geol. Soc. Am. 1914, 25, 655–744. [Google Scholar] [CrossRef]
- Wentworth, C.K. A scale of grade and class terms for clastic sediments. J. Geol. 1922, 30, 377–392. [Google Scholar] [CrossRef]
- Udden, J.A. The Mechanical Composition of Wind Deposits; Augustana Library Publications No 1: Rock Island, IL, USA, 1898; p. 69. [Google Scholar]
- Frissell, C.A.; Liss, W.J.; Warren, C.E.; Hurley, M.D. A hierarchical framework for stream habitat classification: Viewing streams in a watershed context. Environ. Manag. 1986, 10, 199–214. [Google Scholar] [CrossRef]
- Flather, C.H.; Hayward, G.D.; Beissinger, S.R.; Stephens, P.A. Minimum viable populations: Is there a ‘magic number’for conservation practitioners? Trends Ecol. Evol. 2011, 26, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Brook, B.W.; Bradshaw, C.J.; Traill, L.W.; Frankham, R. Minimum viable population size: Not magic, but necessary. Trends Ecol. Evol. 2011, 26, 619–620. [Google Scholar] [CrossRef]
- Jamieson, I.G.; Allendorf, F.W. How does the 50/500 rule apply to MVPs? Trends Ecol. Evol. 2012, 27, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Frankham, R.; Brook, B.W.; Bradshaw, C.J.; Traill, L.W.; Spielman, D. 50/500 rule and minimum viable populations: Response to Jamieson and Allendorf. Trends Ecol. Evol. 2013, 28, 187–188. [Google Scholar] [CrossRef]
- Conn, V.S.; Valentine, J.C.; Cooper, H.M.; Rantz, M.J. Grey literature in meta-analyses. Nurs. Res. 2003, 52, 256–261. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Bayliss, H.R. Shades of grey: Two forms of grey literature important for reviews in conservation. Biol. Conserv. 2015, 191, 827–829. [Google Scholar] [CrossRef]
- Paez, A. Gray literature: An important resource in systematic reviews. J. Evid. Based Med. 2017, 10, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Gerstmeier, R.; Romig, T. Die Süßwasserfische Europas, 1st ed.; Franckh-Kosmos Verlags-GmbH und Co.: Stuttgart, Germany, 1998; p. 367. [Google Scholar]
- Kottelat, M.; Freyhof, J. Handbook of European Freshwater Fishes, 1st ed.; Publications Kottelat: Cornol, Switzerland, 2007; p. 646. [Google Scholar]
| Selected Ecological Parameter | Acronym | Description |
|---|---|---|
| Population | ||
| population density (individuals m2, individuals m−1, kg ha−1, individuals per fish school) | pop dens | reported density of individuals in a population |
| areal size (m2, km) | areal size | reported areal size used by a population |
| Spawner | ||
| spawner density (individuals m−2) | sp dens sps | spawner density on spawning ground during spawning |
| spawning events per female (events female−1) | spaw events | spawning events per female per year |
| Spawning site | ||
| spawning site size (m2) | sps size | size of spawning site used by fish population |
| current speed spawning site (m s−1) | cur speed sps | measured current speed at spawning ground during spawning |
| substrate spawning site (cm, %, term) | subs sps | substrate at spawning site |
| water depth spawning site (m) | w depth sps | water depth at spawning ground during spawning |
| water temperature spawning (°C) | w temp spaw | water temperature measured during spawning |
| Redd/Nest | ||
| redds at spawning site (redds m−1, redds m−2, redds spawning site−1) | redds sps | total number of redds/nests per spawning site |
| redd size (m2) | redd size | size of a redd/nest |
| Egg and Larvae | ||
| egg depth (cm) | egg depth | depth of eggs in interstitial zone |
| eggs (eggs m−2) | eggs per m2 | eggs density found at spawning ground |
| eggs per female (eggs female−1) | eggs per f | number of eggs per female |
| eggs per redd (eggs redd−1) | eggs per redd | number of eggs in a redd/nest |
| eggs at spawning site (eggs sps−1) | eggs sps | total number of eggs counted for a spawning site |
| days till hatch (days) | days till hatch | days till hatch of fish larvae |
| degree days (days) | degree days | degree days till hatch of fish larvae |
| hatch rate/success (%) | hatch rate | hatching rates of eggs |
| larval survival (%) | larv surv | percentage of larvae surviving till yolk sack depletion |
| larvae density (individuals m−2) | larv dens | larvae density in larval habitats |
| Juvenile | ||
| * juvenile survival (%) | juv surv | percentage of juvenile surviving first year post hatching |
| juveniles per m2 (juveniles m−2) | juv per m2 | juvenile density in juvenile habitat |
| current speed juvenile (m s−1) | cur speed juv | measured current speed in juvenile habitat |
| substrate juvenile (cm, term) | subs juv | substrate in juvenile habitat |
| water depth juvenile (m) | w depth juv | water depth in juvenile habitat |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smialek, N.; Pander, J.; Mueller, M.; van Treeck, R.; Wolter, C.; Geist, J. Do We Know Enough to Save European Riverine Fish?—A Systematic Review on Autecological Requirements During Critical Life Stages of 10 Rheophilic Species at Risk. Sustainability 2019, 11, 5011. https://doi.org/10.3390/su11185011
Smialek N, Pander J, Mueller M, van Treeck R, Wolter C, Geist J. Do We Know Enough to Save European Riverine Fish?—A Systematic Review on Autecological Requirements During Critical Life Stages of 10 Rheophilic Species at Risk. Sustainability. 2019; 11(18):5011. https://doi.org/10.3390/su11185011
Chicago/Turabian StyleSmialek, Nicole, Joachim Pander, Melanie Mueller, Ruben van Treeck, Christian Wolter, and Juergen Geist. 2019. "Do We Know Enough to Save European Riverine Fish?—A Systematic Review on Autecological Requirements During Critical Life Stages of 10 Rheophilic Species at Risk" Sustainability 11, no. 18: 5011. https://doi.org/10.3390/su11185011
APA StyleSmialek, N., Pander, J., Mueller, M., van Treeck, R., Wolter, C., & Geist, J. (2019). Do We Know Enough to Save European Riverine Fish?—A Systematic Review on Autecological Requirements During Critical Life Stages of 10 Rheophilic Species at Risk. Sustainability, 11(18), 5011. https://doi.org/10.3390/su11185011





