CO2 Flux Characteristics of Different Plant Communities in a Subtropical Urban Ecosystem
Abstract
:1. Introduction
2. Method
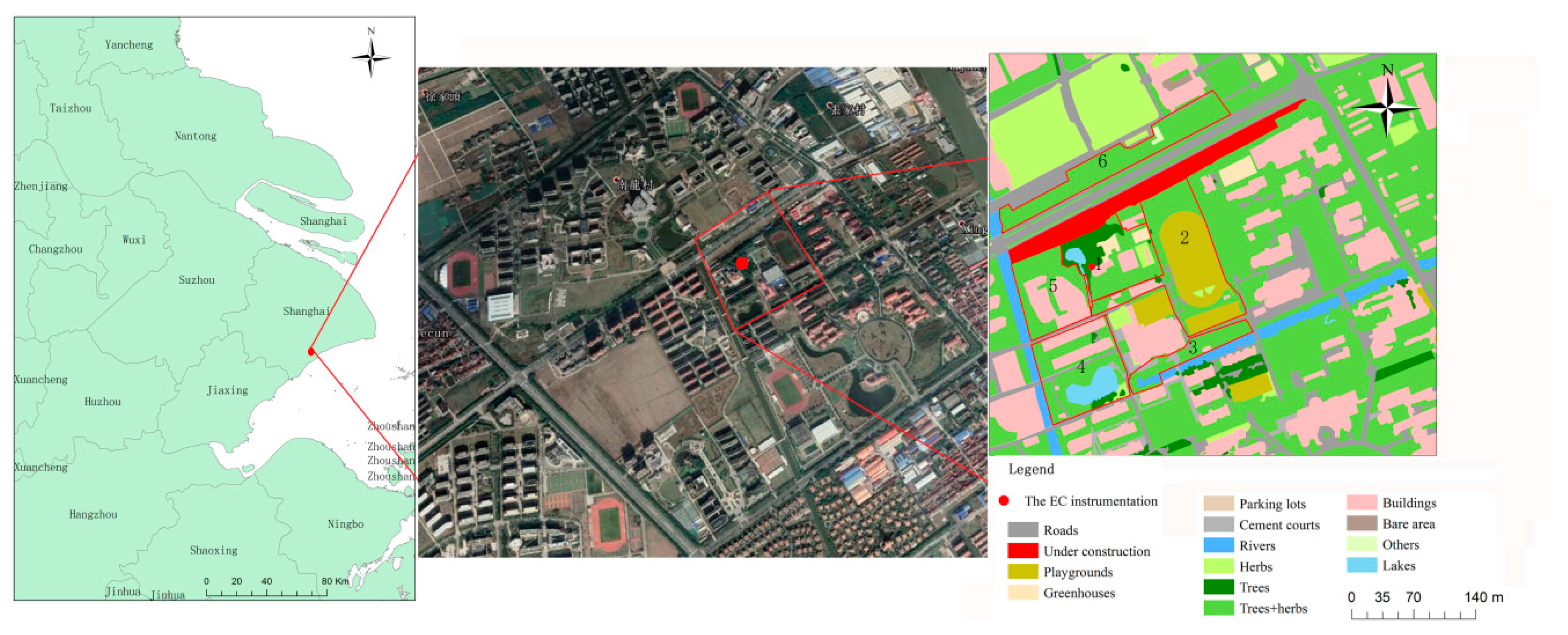
2.1. Site Description
2.2. Instrumentation and Data Quality Control
2.3. Calculation of the Footprint Function
2.4. ART-Footprint Model
3. Results
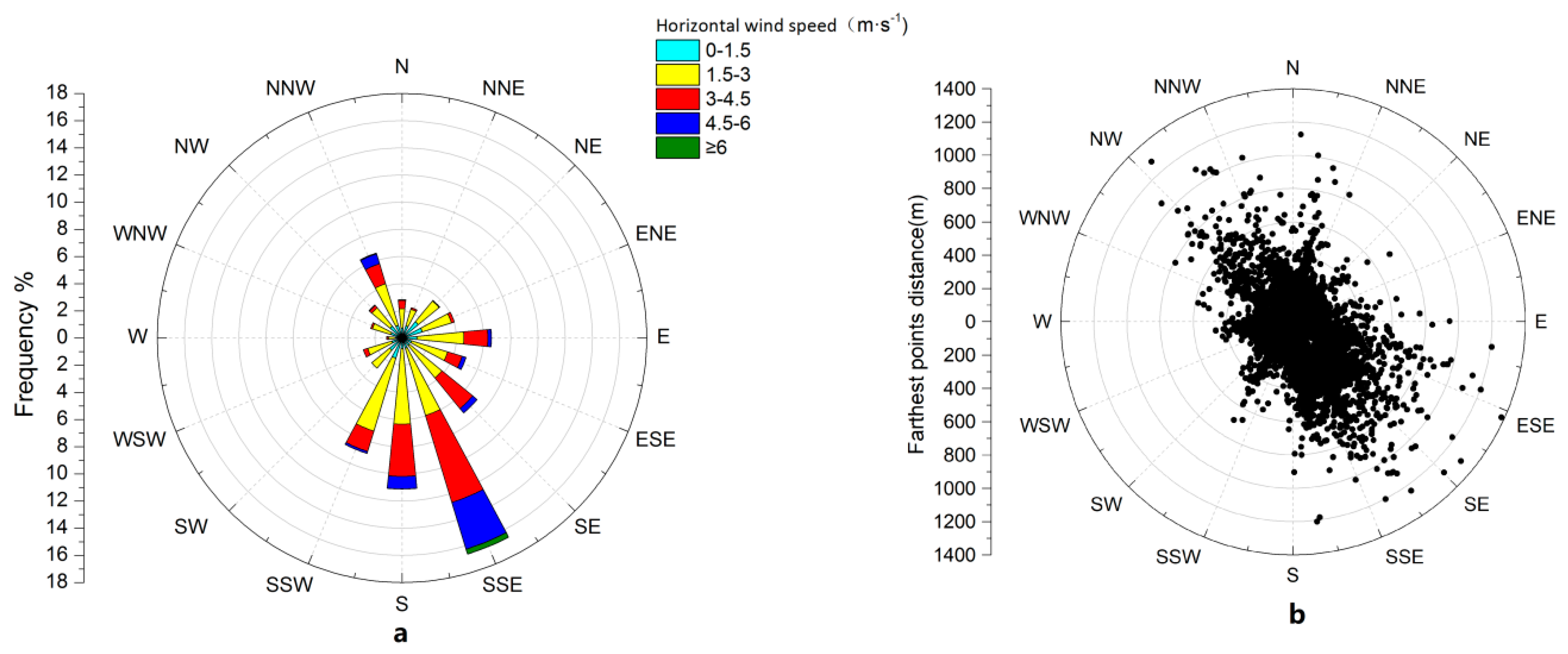
3.1. Wind Direction Characteristics and Effective Contribution Area of CO2
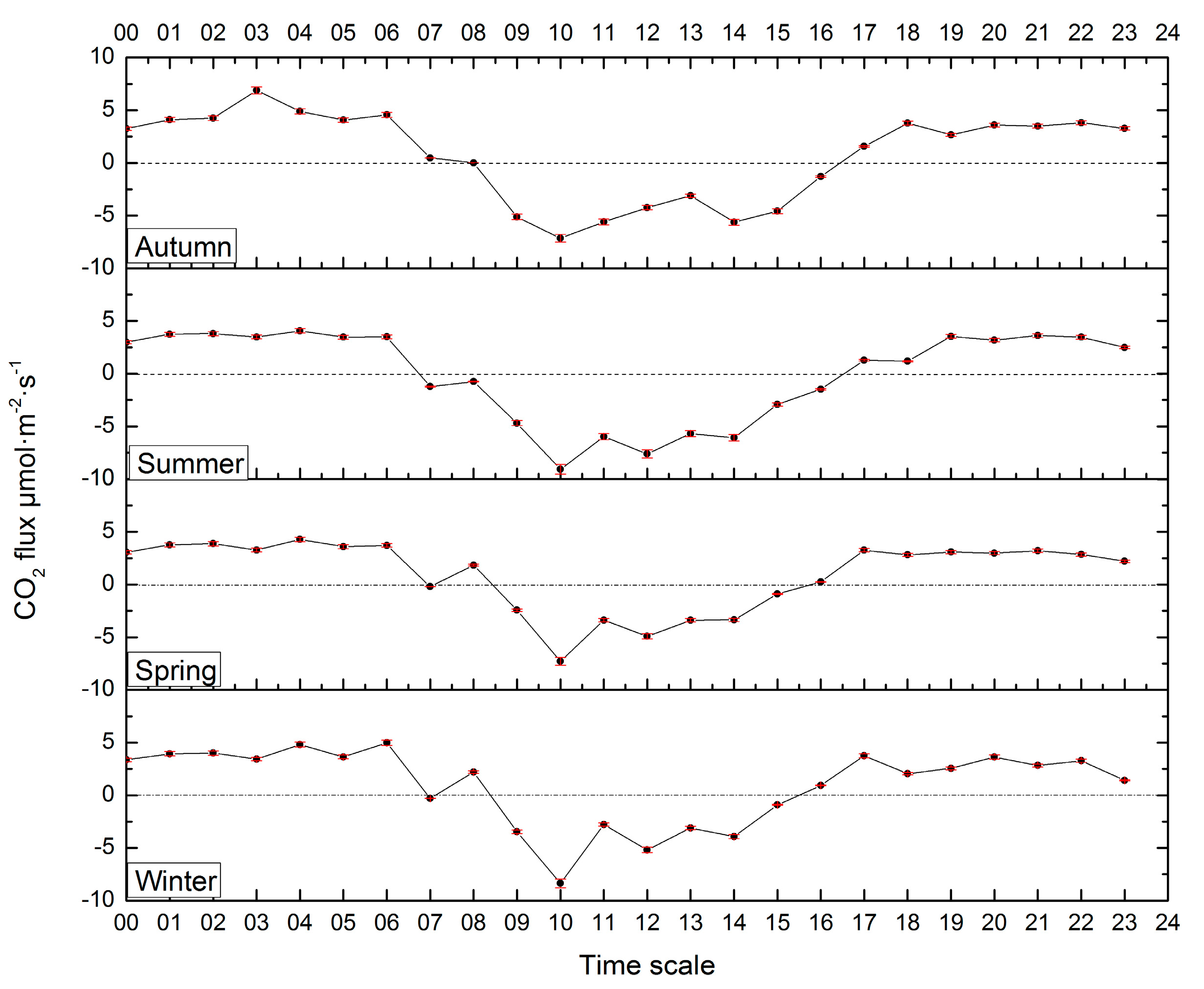
3.2. CO2 Flux Characteristics
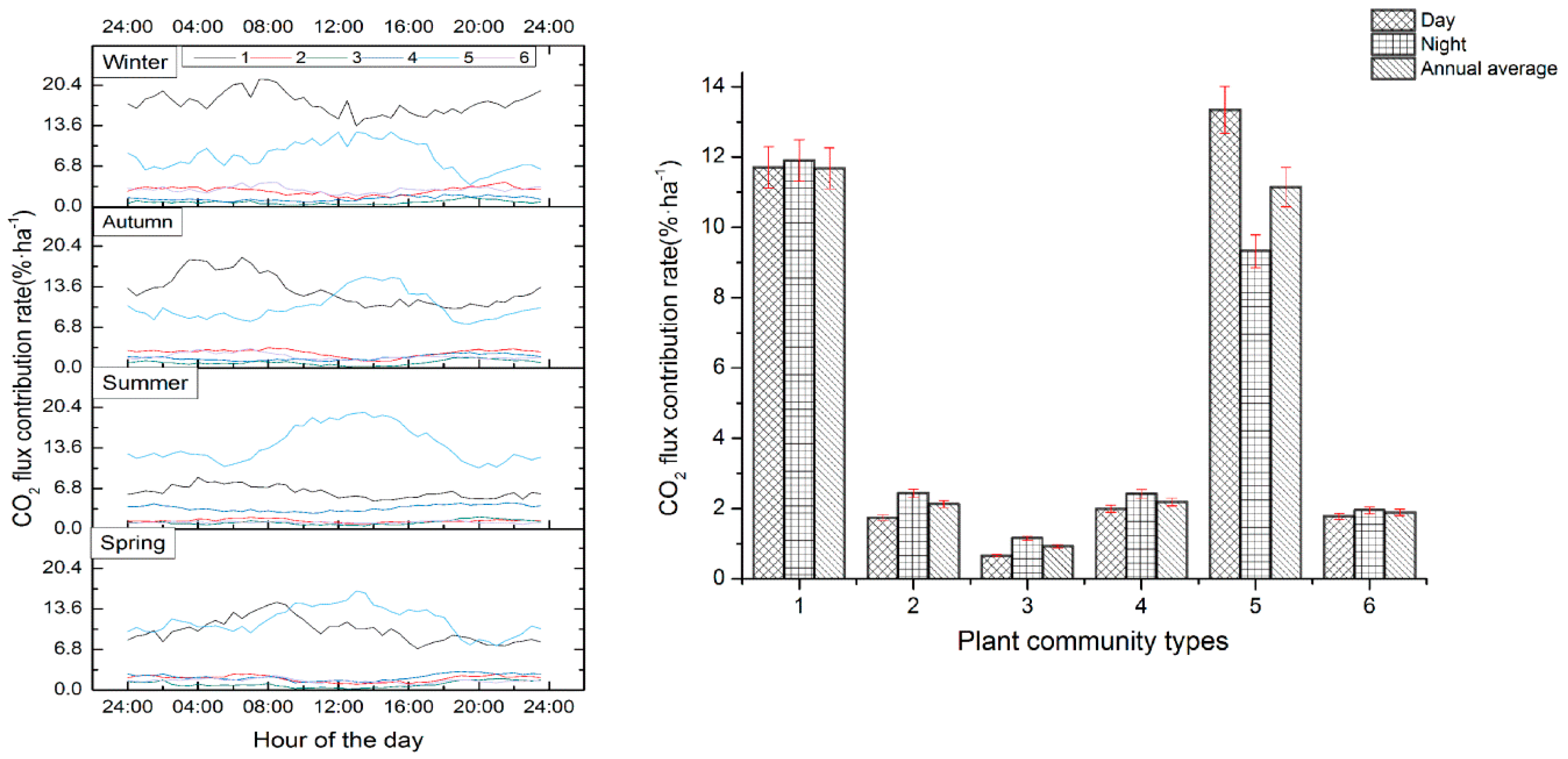
3.3. Annual Average CO2 Flux Contribution Rate of Different Vegetation Communities
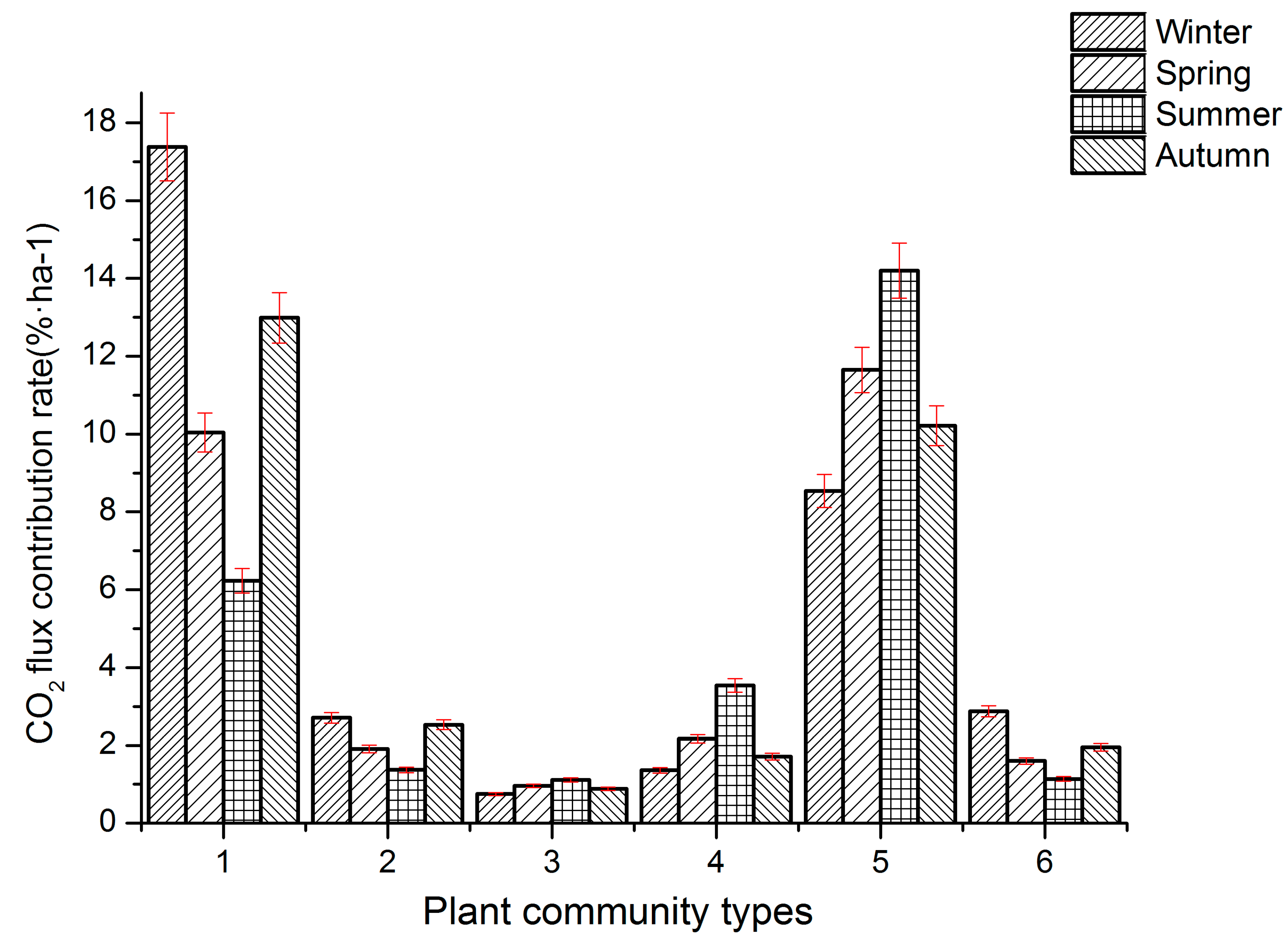
3.4. Seasonal Contribution Rates of CO2 Flux in Different Plant Communities
3.5. Variation Characteristics of CO2 Flux in Different Plant Communities
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Velasco, E.; Perrusquia, R.; Jiménez, E.; Hernández, F.; Camacho, P.; Rodríguez, S.; Retama, A.; Molina, L.T. Sources and sinks of carbon dioxide in a neighborhood of Mexico City. Atmos. Environ. 2014, 97, 226–238. [Google Scholar] [CrossRef]
- Lal, R. Urban Ecosystems and Climate Change; Springer: Dordrecht, The Netherlands, 2012; pp. 3–19. [Google Scholar]
- Wang, Z.; Wang, Y. Carbon flux dynamics and its environmental controlling factors in a desert steppe. Acta Ecol. Sin. 2011, 31, 49–54. [Google Scholar] [CrossRef]
- Baldocchi, D. ‘Breathing’ of the terrestrial biosphere: Lessons learned from a global network of carbon dioxide flux measurement systems. Aust. J. Bot. 2008, 56, 1–26. [Google Scholar] [CrossRef]
- Rodda, S.; Thumaty, K.; Jha, C.; Dadhwal, V. Seasonal Variations of Carbon Dioxide, Water Vapor and Energy Fluxes in Tropical Indian Mangroves. Forests 2016, 7, 35. [Google Scholar] [CrossRef]
- Neftel, A.; Spirig, C.; Ammann, C. Application and test of a simple tool for operational footprint evaluations. Environ. Pollut. 2008, 152, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Liu, H.; Xu, L. Evaluating of simulated carbon flux phenology over a cropland ecosystem in a semiarid area of China with SiBcrop. Int. J. Biometeorol. 2017, 61, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Gahagan, A.; Giardina, C.P.; King, J.S.; Binkley, D.; Pregitzer, K.S.; Burton, A.J. Carbon fluxes, storage and harvest removals through 60 years of stand development in red pine plantations and mixed hardwood stands in Northern Michigan, USA. For. Ecol. Manag. 2015, 337, 88–97. [Google Scholar] [CrossRef]
- Hammerle, A.; Haslwanter, A.; Schmitt, M.; Bahn, M.; Tappeiner, U.; Cernusca, A.; Wohlfahrt, G. Eddy covariance measurements of carbon dioxide, latent and sensible energy fluxes above a meadow on a mountain slope. Bound. Layer Meteorol. 2007, 122, 397–416. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Yang, Y. Modeling and Predicting Carbon and Water Fluxes Using Data-Driven Techniques in a Forest Ecosystem. Forests 2017, 8, 498. [Google Scholar] [CrossRef]
- Bergeron, O.; Strachan, I.B. CO2 sources and sinks in urban and suburban areas of a northern mid-latitude city. Atmos. Environ. 2011, 45, 1564–1573. [Google Scholar] [CrossRef]
- Ramamurthy, P.; Pardyjak, E.R. Toward understanding the behavior of carbon dioxide and surface energy fluxes in the urbanized semi-arid Salt Lake Valley, Utah, USA. Atmos. Environ. 2011, 45, 73–84. [Google Scholar] [CrossRef]
- Khatib, H. IEA World Energy Outlook 2011—A comment. Energy Policy 2012, 48, 737–743. [Google Scholar] [CrossRef]
- Bellucco, V.; Marras, S.; Grimmond, C.S.B.; Järvi, L.; Sirca, C.; Spano, D. Modelling the biogenic CO2 exchange in urban and non-urban ecosystems through the assessment of light-response curve parameters. Agric. For. Meteorol. 2017, 236, 113–122. [Google Scholar] [CrossRef]
- Ward, H.C.; Evans, J.G.; Grimmond, C.S.B. Multi-season eddy covariance observations of energy, water and carbon fluxes over a suburban area in Swindon, UK. Atmos. Chem. Phys. 2013, 13, 4645–4666. [Google Scholar] [CrossRef] [Green Version]
- Christen, A.; Coops, N.C.; Crawford, B.R.; Kellett, R.; Liss, K.N.; Olchovski, I.; Tooke, T.R.; van der Laan, M.; Voogt, J.A. Validation of modeled carbon-dioxide emissions from an urban neighborhood with direct eddy-covariance measurements. Atmos. Environ. 2011, 45, 6057–6069. [Google Scholar] [CrossRef]
- Crawford, B.; Grimmond, C.S.B.; Christen, A. Five years of carbon dioxide fluxes measurements in a highly vegetated suburban area. Atmos. Environ. 2011, 45, 896–905. [Google Scholar] [CrossRef]
- Grimmond, C.S.B.; King, T.S.; Cropley, F.D.; Nowak, D.J.; Souch, C. Local-scale fluxes of carbon dioxide in urban environments: Methodological challenges and results from Chicago. Environ. Pollut. 2002, 116, S243–S254. [Google Scholar] [CrossRef]
- Velasco, E.; Roth, M. Cities as Net Sources of CO2: Review of Atmospheric CO2 Exchange in Urban Environments Measured by Eddy Covariance Technique. Geogr. Compass 2010, 4, 1238–1259. [Google Scholar] [CrossRef]
- Gu, L.; Zhou, Y.; Mei, T.; Zhou, G.; Xu, L. Carbon Footprint Analysis of Bamboo Scrimber Flooring—Implications for Carbon Sequestration of Bamboo Forests and Its Products. Forests 2019, 10, 51. [Google Scholar] [CrossRef]
- Christen, A. Atmospheric measurement techniques to quantify greenhouse gas emissions from cities. Urban Clim. 2014, 10, 241–260. [Google Scholar] [CrossRef]
- Weissert, L.F.; Salmond, J.A.; Schwendenmann, L. A review of the current progress in quantifying the potential of urban forests to mitigate urban CO2 emissions. Urban Clim. 2014, 8, 100–125. [Google Scholar] [CrossRef]
- Grimmond, C.S.B.; Christen, A. Flux measurements in urban ecosystems. FluxLetter 2012, 5, 1–8. [Google Scholar]
- Velasco, E.; Roth, M.; Norford, L.; Molina, L.T. Does urban vegetation enhance carbon sequestration? Landsc. Urban Plan. 2016, 148, 99–107. [Google Scholar] [CrossRef]
- Velasco, E.; Roth, M.; Tan, S.H.; Quak, M.; Nabarro, S.D.A.; Norford, L. The role of vegetation in the CO2 flux from a tropical urban neighbourhood. Atmos. Chem. Phys. 2013, 13, 10185–10202. [Google Scholar] [CrossRef]
- Coutts, A.M.; Beringer, J.; Tapper, N.J. Characteristics influencing the variability of urban CO2 fluxes in Melbourne, Australia. Atmos. Environ. 2007, 41, 51–62. [Google Scholar] [CrossRef]
- Vesala, T.; Järvi, L.; Launiainen, S.; Sogachev, A.; Rannik, U.; Mammarella, I.; Siivola, E.; Keronen, P.; Rinne, J.; Riikonen, A.; et al. Surface—Atmosphere interactions over complex urban terrain in Helsinki, Finland. Tellus Ser. B-Chem. Phys. Meteorol. 2008, 60B, 188–199. [Google Scholar] [CrossRef]
- Song, T.; Wang, Y. Carbon dioxide fluxes from an urban area in Beijing. Atmos. Res. 2012, 106, 139–149. [Google Scholar] [CrossRef]
- Guo, Z.J.; Gong, Y.; Zhang, K.D.; Zhang, L.P.; He, Y.; Xu, L.; Zhao, M. CO2 flux footprints of impervious layer on complex land surface: A case study at the Fengxian College Park, Shanghai. Acta Sci. Circumstantiae 2018, 38, 772–779. [Google Scholar]
- Gong, Y.; Zhao, M.; Yao, X.; Guo, Z.J.; He, Y.; Zhang, L.P. Analysis and comparison of carbon flux contribution zones in urban ecological system based on Hsieh and Kljun models. J. Environ. Eng. Technol. 2017, 7, 225–231. [Google Scholar]
- Zhang, K.D.; Gong, Y.; Guo, Z.J.; Wei, Y.Y.; Yao, G.F.; Xu, L.; Zhao, M. Flux source area and CO2 flux characteristics of subtropical urban ecosystem in 2011 and 2016: A case study of Shanghai Fengxian University City. J. Shanghai Norm. Univ. (Nat. Sci.) 2018, 47, 458–465. [Google Scholar]
- Wu, Z.Y. China Vegetation; Science Press: Beijing, China, 1980; ISBN 9787030024220. [Google Scholar]
- Liu, Y.F.; Song, X.; Yu, G.R.; Sun, X.M.; Wen, X.F.; Chen, Y.R. Seasonal variation of CO2 flux and its environmental factors in evergreen coniferous plantation. Sci. China (Ser. D Earth Sci.) 2005, 48, 123–132. [Google Scholar]
- Kljun, N.; Calanca, P.; Rotach, M.W.; Schmid, H.P. A simple parameterisationfor flux footprint predictions. Bound. Layer Meteorol. 2004, 112, 503–523. [Google Scholar] [CrossRef]
- Gu, L.; Post, W.M.; Baldocchi, D.D.; Black, T.A.; Suyker, A.E.; Verma, S.B.; Vesala, T.; Wofsy, S.C. Characterizing the Seasonal Dynamics of Plant Community Photosynthesis Across a Range of Vegetation Types; Springer: New York, NY, USA, 2009; pp. 35–58. [Google Scholar]
- Gong, Y. A Study on Spatial Differentiation of Carbon Flux Based on Footprint Model: A Case Study of Fengxian University City in Shanghai. Master’s Thesis, Shanghai Normal University, Shanghai, China, 2017. [Google Scholar]
- Pan, R.Z. Plant Physiology, 6th ed.; Higher Education Press: Beijing, China, 2008; ISBN 7040239744. [Google Scholar]
- Zhang, C.H. Ecological habits and cultivation techniques of camphor. Mod. Agric. Technol. 2012, 195–222. [Google Scholar] [CrossRef]
- Fu, R.S.; Huang, X.L.; Luo, W.X.; Chen, S.L.; Huang, Q.P. Ecological habits and reproduction of foliage plants in palm family. Fujian J. Agric. Sci. 1999, 14, 80–84. [Google Scholar]
- Chai, X.; Li, Y.N.; Duan, C.; Zhang, T.; Zong, N.; Shi, P.L.; He, Y.T.; Zhang, X.Z. CO2 flux dynamics and its limiting factors in the alpine shrub-meadow and steppe-meadow on the Qinghai-Xizang Plateau. Chin. J. Plant Ecol. 2018, 42, 6–19. [Google Scholar]
- Gong, Y.; Guo, Z.J.; Zhang, K.D.; Xu, L.; Wei, Y.Y.; Zhao, M. Impact of vegetation on CO2 flux of a subtropical urban ecosystem. Acta Ecol. Sin. 2019, 39, 530–541. [Google Scholar]
- Gong, Y.; Zhao, M.; Yao, X.; Guo, Z.J.; He, Y.; Zhang, L.P.; Lin, W.P. Study on carbon flux characteristics of the underlying surface of urban ecosystem-a case study of Shanghai Fengxian University City. Resour. Environ. Yangtze Basin 2017, 26, 91–99. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Gong, Y.; Fa, H.; Zhao, M. CO2 Flux Characteristics of Different Plant Communities in a Subtropical Urban Ecosystem. Sustainability 2019, 11, 4879. https://doi.org/10.3390/su11184879
Zhang K, Gong Y, Fa H, Zhao M. CO2 Flux Characteristics of Different Plant Communities in a Subtropical Urban Ecosystem. Sustainability. 2019; 11(18):4879. https://doi.org/10.3390/su11184879
Chicago/Turabian StyleZhang, Kaidi, Yuan Gong, Hao Fa, and Min Zhao. 2019. "CO2 Flux Characteristics of Different Plant Communities in a Subtropical Urban Ecosystem" Sustainability 11, no. 18: 4879. https://doi.org/10.3390/su11184879
APA StyleZhang, K., Gong, Y., Fa, H., & Zhao, M. (2019). CO2 Flux Characteristics of Different Plant Communities in a Subtropical Urban Ecosystem. Sustainability, 11(18), 4879. https://doi.org/10.3390/su11184879




