Contribution of Microbial Residues Obtained from Lignin and Cellulose on Humus Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparations of A. niger Inoculum and the Fluid Medium
2.2. Experimental Design
2.3. Analysis
2.4. Statistical Analysis of the Data
3. Results
3.1. Elemental Composition of Microbial Residue
3.2. DTA of Microbial Residue
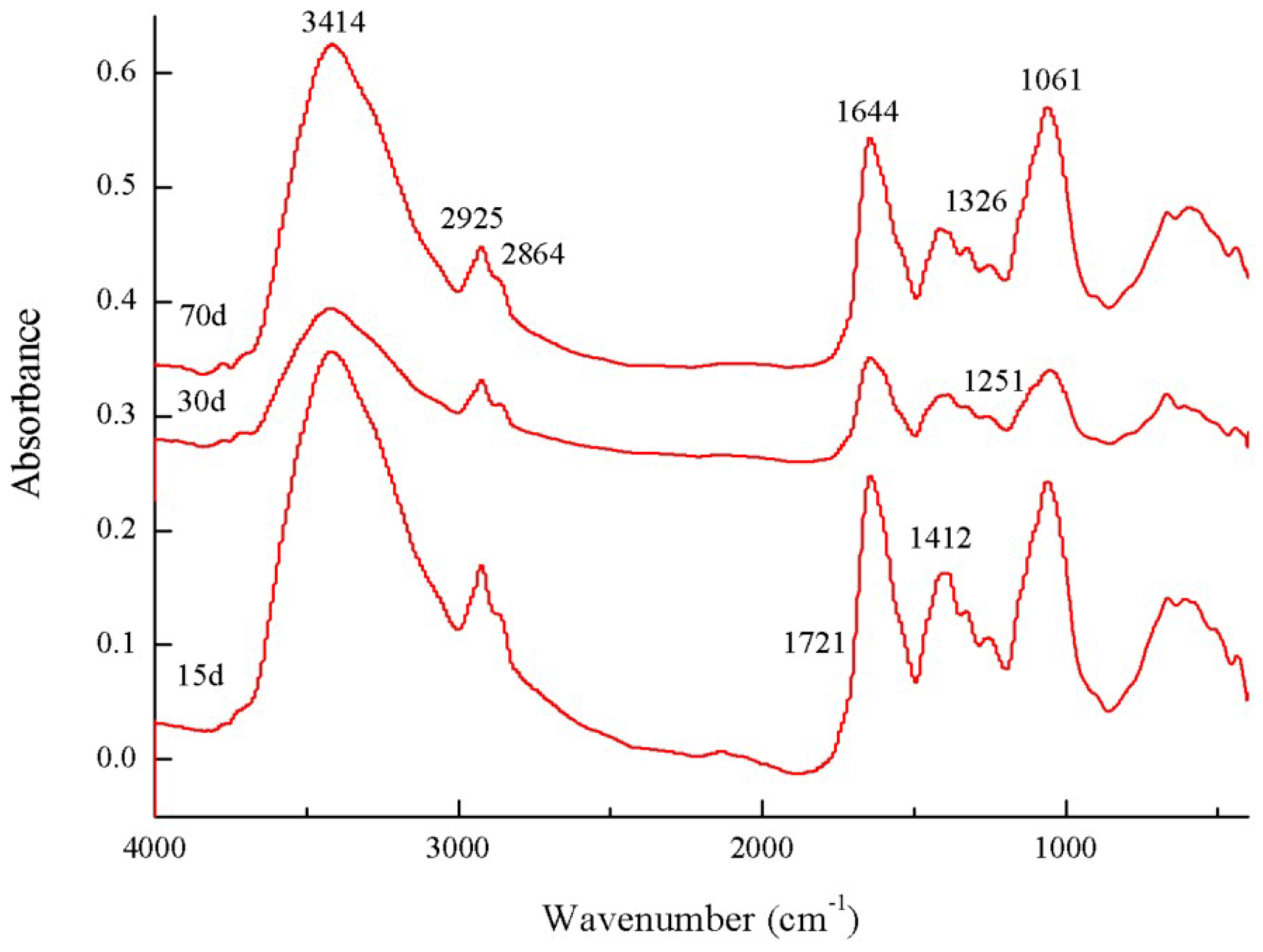
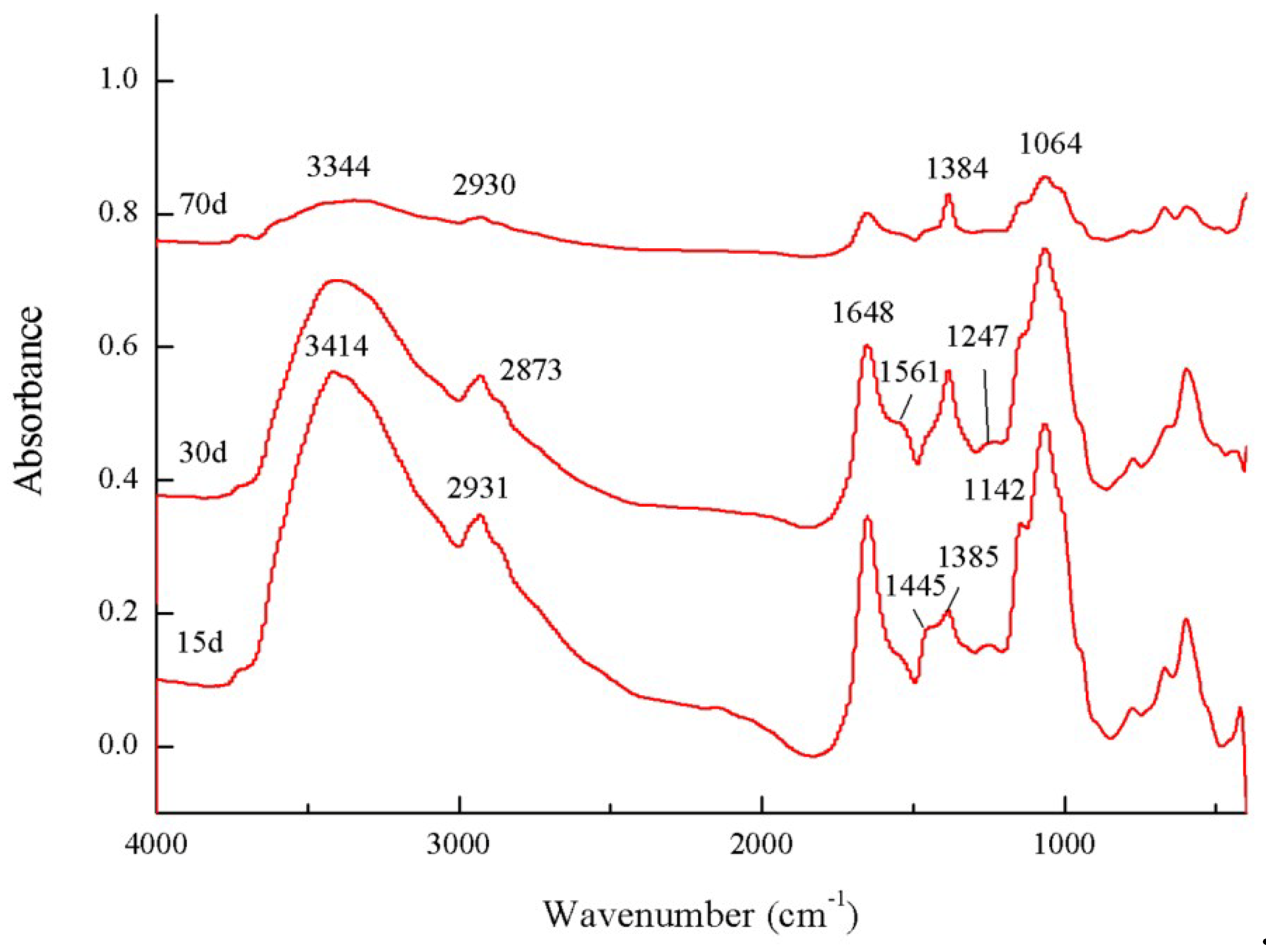
3.3. FTIR Spectra of the Microbial Residue
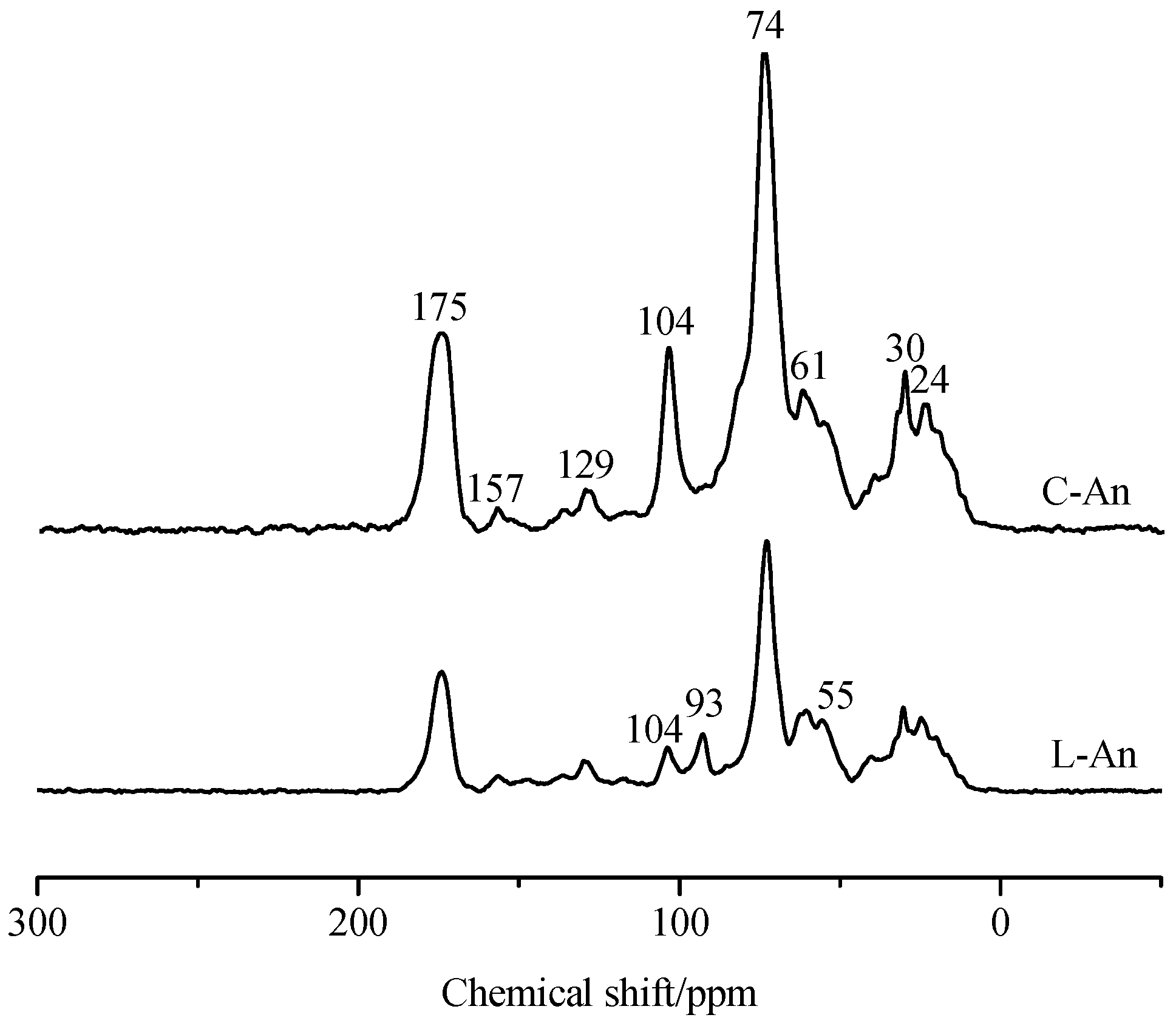
3.4. 13C CP-MAS NMR Spectra of Microbial Residue
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Weber, J.; Chen, Y.; Jamroz, E.; Miano, T. Preface: Humic substances in the environment. J. Soil Sediment 2018, 18, 2665–2667. [Google Scholar] [CrossRef]
- Semenov, V.M.; Tulina, A.S.; Semenova, N.A.; Ivannikova, L.A. Humification and nonhumification pathways of the organic matter stabilization in soil: A review. Eurasian Soil Sci. 2013, 46, 355–368. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Zhao, Y.; Wang, R.X.; Lu, Q.; Wu, J.Q.; Zhang, D.Y.; Nie, Z.F.; Wei, Z.M. Effect of the addition of exogenous precursors on humic substance formation during composting. Waste Manag. 2018, 79, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Danise, T.; Fioretto, A.; Innangi, M. Spectrophotometric methods for lignin and cellulose in forest soils as predictors for humic substances. Eur. J. Soil Sci. 2018, 69, 856–867. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Natural Fibers, Biopolymers, and Biocomposites; CRC Press, Taylor Francis Group: Boca Raton, FL, USA, 2005; p. 8. [Google Scholar]
- Datta, R.; Kelkar, A.; Baraniya, D.; Molaei, A.; Moulick, A.; Meena, R.S.; Formanek, P. Enzymatic degradation of lignin in soil: A review. Sustainability 2017, 9, 1163. [Google Scholar] [CrossRef]
- Bollók, M.; Réczey, K. Cellulase enzyme production by various fungal strains on different carbon sources. Acta Aliment Hung. 2005, 29, 154–168. [Google Scholar] [CrossRef]
- Chen, Y.N.; Chen, Y.R.; Li, Y.P.; Wu, Y.X.; Zhu, F.Z.; Zeng, G.M.; Zhang, J.C.; Li, H. Application of Fenton pretreatment on the degradation of rice straw by mixed culture of Phanerochaete chrysosporium and Aspergillus niger. Ind. Crop Prod. 2018, 112, 290–295. [Google Scholar] [CrossRef]
- Milstein, O.A.; Haars, A.; Sharma, A.; Vered, Y.; Shragina, L.; Trojanowski, J.; Flowers, H.M.; Gressel, J.; Hüttermann, A. Lignin degrading ability of selected Aspergiilus spp. Appl. Biochem. Biotech. 1984, 9, 393–394. [Google Scholar] [CrossRef]
- Barapatre, A.; Jha, H. Degradation of alkali lignin by two ascomycetes and free radical scavenging activity of the products. Biocatal. Biotransfor. 2017, 35, 269–286. [Google Scholar] [CrossRef]
- Zhao, C.; Deng, L.; Fang, H. Mixed culture of recombinant Trichoderma reesei and Aspergillus niger for cellulase production to increase the cellulose degrading capability. Biomass Bioenerg. 2018, 112, 93–98. [Google Scholar] [CrossRef]
- Hachicha, S.; Sallemi, F.; Medhioub, K.; Hachicha, R.; Ammar, E. Quality assessment of composts prepared with olive mill wastewater and agricultural wastes. Waste Manag. 2008, 28, 2593–2603. [Google Scholar] [CrossRef] [PubMed]
- Kögel-Knabner, I. The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol. Biochem. 2002, 34, 139–162. [Google Scholar] [CrossRef]
- Rigobello, E.S.; Campos, S.X.; Azevedo, E.R.D.; Dantas, A.D.B.; Vieira, E.M. Comparative characterization of humic substances extracted from freshwater and peat of different apparent molecular sizes. Rev. Ambient Água. 2017, 12, 774–785. [Google Scholar] [CrossRef][Green Version]
- Al-Faiyz, Y.S.S. CPMAS 13C NMR characterization of humic acids from composted agricultural Saudi waste. Arab. J. Chem. 2017, 10, 839–853. [Google Scholar] [CrossRef]
- Joanisse, G.D.; Bradley, R.L.; Preston, C.M. The spread of Kalmia angustifolia on black spruce forest cutovers contributes to the spatial heterogeneity of soil resources. PLoS ONE 2018, 13, e0198860. [Google Scholar] [CrossRef] [PubMed]
- Mallerman, J.; Itria, R.; Alarcón-Gutiérrez, E.; Hernández, C.; Levin, L.; Saparrat, M. Exotic litter of the invasive plant Ligustrum lucidum alters enzymatic production and lignin degradation by selected saprotrophic fungi. Can. J. For. Res. 2018, 48, 709–720. [Google Scholar] [CrossRef]
- Li, C.; Gao, S.; Zhang, J.; Zhao, L.; Wang, L. Moisture effect on soil humus characteristics in a laboratory incubation experiment. Soil Water Res. 2016, 11, 37–43. [Google Scholar] [CrossRef]
- Meehnian, H.; Jana, A.K. Cotton stalk pretreatment using Daedalea flavida, Phlebia radiata, and Flavodon flavus: Lignin degradation, cellulose recovery, and enzymatic saccharification. Appl. Biochem. Biotechnol. 2016, 181, 1465–1484. [Google Scholar] [CrossRef]
- Wei, Z.M.; Xi, B.D.; Zhao, Y.; Wang, S.P.; Liu, H.L.; Jiang, Y.H. Effect of inoculating microbes in municipal solid waste composting on characteristics of humic acid. Chemosphere 2007, 68, 368–374. [Google Scholar] [CrossRef]
- Lawoko, M.; Henriksson, G.; Gellerstedt, G. Characterisation of lignin-carbohydrate complexes (LCCs) of spruce wood (Picea abies L.) isolated with two methods. Holzforschung 2006, 30, 148–161. [Google Scholar] [CrossRef]
- Baddi, G.A.; Hafidi, M.; Gilard, V.; Revel, J.C. Characterization of humic acids produced during composting of olive mill wastes: Elemental and spectroscopic analyses. Agronomie 2003, 23, 661–666. [Google Scholar] [CrossRef]
- Prescott, C.E. Decomposition and mineralization of nutrients from litter and humus. Nutr. Acquis. Plants: Ecol. Perspect. 2005, 181, 15–41. [Google Scholar]
- Berg, B.; Soderstrom, B. Fungal biomass and nitrogen in decomposing scots pine needle litter. Soil Biol. Biochem. 1979, 11, 339–341. [Google Scholar] [CrossRef]
- Bernabé, G.A.; Kobelnik, M.; Almeida, S.; Ribeiro, C.A.; Crespi, M.S. Thermal behavior of lignin and cellulose from waste composting process. J. Therm. Anal. Calorim. 2013, 111, 589–595. [Google Scholar] [CrossRef]
- Chen, Y.N.; Huang, J.X.; Li, Y.; Zeng, G.M.; Zhang, J.C.; Huang, A.Z.; Zhang, J.; Ma, S.; Tan, X.B.; Xu, W.; et al. Study of the rice straw biodegradation in mixed culture of Trichoderma viride and A. niger by GC-MS and FTIR. Environ. Sci. Pollut. Res. 2015, 22, 9807–9815. [Google Scholar] [CrossRef] [PubMed]
- Brink, J.V.D.; Vries, R.P.D. Fungal enzyme sets for plant polysaccharide degradation. Appl. Microbiol. Biotechnol. 2011, 91, 1477–1492. [Google Scholar] [CrossRef]
- Guillen, D.; Sanchez, S.; Rodriguez-Sanoja, R. Carbohydrate binding domains: Multiplicity of biological roles. Appl. Microbiol. Biotechnol. 2011, 85, 1241–1249. [Google Scholar] [CrossRef]
- Beckham, G.T.; Matthews, J.F.; Bomble, Y.J.; Bu, L.; Adney, W.S.; Himmel, M.E.; Nimlos, M.R.; Crowley, M.F. Identification of amino acids responsible for processivity in a Family 1 carbohydrate-binding module from a fungal cellulase. J. Phys. Chem. B 2010, 114, 1447–1453. [Google Scholar] [CrossRef]
- Crawford, R.L. Lignin Biodegradation and Transformation; Wiley Interscience: New York, NY, USA, 1981; Volume 13, p. 121. [Google Scholar]
- Bi, R.; Lawoko, M.; Henriksson, G. Phoma herbarum, a soil fungus able to grow on natural lignin and synthetic lignin (DHP) as sole carbon source and cause lignin degradation. J. Ind. Microbiol. Biotechnol. 2016, 43, 1175–1182. [Google Scholar] [CrossRef]
- Leisola, M.; Brown, C.; Laurila, M.; Ulmer, D.; Fiechter, A. Polysaccharide synthesis by Phanerochaete chrysosporium during degradation of kraft lignin. Eur. J. Appl. Microbiol. Biotechnol. 1982, 15, 180–184. [Google Scholar] [CrossRef]
- Rogerson, C.T. The Filamentous Fungi. Volume II. Biosynthesis and Metabolism. Brittonia 1977, 35, 158. [Google Scholar] [CrossRef]
- Nierop, K.G.J.; Buurman, P.; Leeuw, J.W.D. Effect of vegetation on chemical composition of H horizons in incipient podzols as characterized by 13C NMR and pyrolysis-GC/MS. Geoderma 1999, 90, 111–129. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Wang, Q.; Wang, N.; Li, C.; Wang, L. First determination of Cu adsorption on soil humin. Environ. Chem. Lett. 2013, 11, 41–46. [Google Scholar] [CrossRef]
- Kanitskaya, L.V.; Medvedeva, S.A.; Babkin, V.A.; Kushnarev, D.F.; Kalabin, G.A. Destruction of aspen wood by the fungus Phanerochaete sanguinea quantitative 1H and 13C NMR spectroscopies of biologically degraded lignin. Chem. Nat. Compd. 1994, 30, 505–514. [Google Scholar] [CrossRef]
- Geng, X.; Li, K. Degradation of non-phenolic lignin by the white-rot fungus Pycnoporus cinnabarinus. Appl. Microbiol. Biotechnol. 2002, 60, 342–346. [Google Scholar] [PubMed]

| Treatments | Culture Time (days) | Element Contents | Atomic Ratios | |||||
|---|---|---|---|---|---|---|---|---|
| C (g kg−1) | H (g kg−1) | N (g kg−1) | O + S (g kg−1) | C/N | H/C | O/C | ||
| C-An | 15 | 287.4 ± 3.5d | 48.9 ± 1.0c | 36.2 ± 0.8c | 425.2 ± 5.7b | 9.3 ± 0.6b | 2.0 ± 0.4b | 1.1 ± 0.1d |
| 30 | 273.5 ± 2.8e | 46.9 ± 0.8e | 39.5 ± 0.9b | 402.6 ± 6.5c | 8.1 ± 0.8c | 2.1 ± 0.3b | 1.1 ± 0.2d | |
| 70 | 290.3 ± 4.5c | 47.9 ± 1.2d | 44.9 ± 1.1a | 383.9 ± 4.5d | 7.5 ± 0.6d | 2.0 ± 0.2b | 1.0 ± 0.1d | |
| CK-C | - | 339.5 ± 6.4b | 61.6 ± 1.8b | 4.5 ± 0.3h | 594.5 ± 6.9a | 88.1 ± 0.5b | 2.2 ± 0.3ab | 1.3 ± 0.1c |
| 15 | 144.1 ± 1.4g | 26.8 ± 0.9g | 18.1 ± 0.9f | 323.3 ± 4.5f | 9.3 ± 0.2b | 2.2 ± 0.4ab | 1.7 ± 0.1b | |
| L-An | 45 | 145.0 ± 2.6f | 27.0 ± 0.7g | 20.7 ± 1.1e | 379.2 ± 3.9e | 8.2 ± 0.4c | 2.2 ± 0.2ab | 2.0 ± 0.2a |
| 70 | 145.7 ± 1.9f | 27.8 ± 0.6f | 22.4 ± 0.7d | 389.5 ± 3.2d | 7.6 ± 0.3d | 2.3 ± 0.3a | 2.0 ± 0.1a | |
| CK-L | - | 615.0 ± 8.6a | 68.9 ± 1.7a | 7.4 ± 0.4g | 308.6 ± 4.6g | 96.7 ± 1.8a | 1.3 ± 0.1c | 0.4 ± 0.0e |
| Treatments | Culture Time (days) | Endothermic Peak | Exothermic Peak I | Exothermic Peak I | Exothermic Peak II/Exothermic Peak I | ||||
|---|---|---|---|---|---|---|---|---|---|
| Heat (kJ g−1) | Weight Loss (%) | Heat (kJ g−1) | Weight Loss (%) | Heat (kJ g−1) | Weight Loss (%) | Heat | Weight Loss | ||
| C-An | 15 | 0.99 | 7.64 | 12.17 | 44.57 | 14.54 | 25.74 | 1.20c | 0.58b |
| 30 | 0.59 | 7.94 | 11.0 | 40.56 | 17.62 | 28.35 | 1.60b | 0.70a | |
| 70 | 0.63 | 6.48 | 9.12 | 39.49 | 16.92 | 28.72 | 1.86a | 0.73a | |
| CK-C | 0 | 0.34 | 4.86 | 9.73 | 40.97 | - | - | - | - |
| L-An | 15 | 0.64 | 6.15 | 12.00 | 42.86 | 9.00 | 23.69 | 0.75d | 0.55b |
| 45 | 0.01 | 4.81 | 23.74 | 47.21 | 1.84 | 11.17 | 0.08f | 0.24d | |
| 70 | 0.95 | 6.84 | 11.60 | 37.10 | 1.99 | 16.45 | 0.17e | 0.44c | |
| CK-L | - | 0.65 | 3.42 | 11.57 | 19.80 | - | - | - | - |
| Wavenumbers (cm−1) | 3414 | 2925 | 2864 | 1721 | 1644 | 1412 | 1326 | 1251 | 1061 | (2925 + 2864)/1644 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Culture Time (days) | |||||||||||
| 15 | 65.42 | 1.63 | 0.31 | 0.03 | 13.50 | 3.35 | 0.24 | 0.41 | 15.11 | 0.144 | |
| 30 | 64.82 | 2.32 | 0.37 | 0.01 | 14.14 | 3.62 | 0.19 | 0.34 | 14.20 | 0.190 | |
| 70 | 66.09 | 1.33 | 0.13 | 0.00 | 12.91 | 2.45 | 0.24 | 0.26 | 16.60 | 0.113 | |
| Wavenumbers(cm−1) | 3414 | 2931 | 2873 | 1732 | 1648 | 1561 | 1445 | 1385 | 1247 | 1142 | 1064 | (2931 + 2873)/1648 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Culture Time (days) | |||||||||||||
| 15 | 71.19 | 1.28 | 0.21 | 0.00 | 10.23 | 0.23 | 0.78 | 0.62 | 0.30 | 1.12 | 14.03 | 0.146 | |
| 45 | 68.19 | 1.39 | 0.22 | 0.00 | 9.24 | 0.81 | 0.21 | 2.60 | 0.28 | 1.07 | 16.00 | 0.174 | |
| 70 | 59.75 | 1.39 | 0.24 | 0.00 | 10.36 | 0.30 | 0.66 | 5.51 | 0.39 | 1.25 | 20.16 | 0.157 | |
| Treatments | 0–45 ppm | 45–110 ppm | 110–140 ppm | 140–160 ppm | 160–185 ppm | 185–220 ppm | Aromaticity Index (%) | Hydrophobic C/Hydrophilic C | Alkyl-C/O-alkyl-C |
|---|---|---|---|---|---|---|---|---|---|
| Alkyl C | O-alkyl C | Aromatic C | Phenolic C | Carboxyl C | Carbonyl-C | ||||
| C-An | 19.66 | 60.84 | 4.93 | 0.99 | 13.9 | 0.67 | 5.70 | 0.40 | 0.32 |
| L-An | 21.77 | 55.17 | 8.73 | 2.77 | 14.3 | 0.04 | 9.87 | 0.55 | 0.39 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Wang, N.; Xu, J.; Zhang, X.; Dou, S. Contribution of Microbial Residues Obtained from Lignin and Cellulose on Humus Formation. Sustainability 2019, 11, 4777. https://doi.org/10.3390/su11174777
Wang S, Wang N, Xu J, Zhang X, Dou S. Contribution of Microbial Residues Obtained from Lignin and Cellulose on Humus Formation. Sustainability. 2019; 11(17):4777. https://doi.org/10.3390/su11174777
Chicago/Turabian StyleWang, Shuai, Nan Wang, Junping Xu, Xi Zhang, and Sen Dou. 2019. "Contribution of Microbial Residues Obtained from Lignin and Cellulose on Humus Formation" Sustainability 11, no. 17: 4777. https://doi.org/10.3390/su11174777
APA StyleWang, S., Wang, N., Xu, J., Zhang, X., & Dou, S. (2019). Contribution of Microbial Residues Obtained from Lignin and Cellulose on Humus Formation. Sustainability, 11(17), 4777. https://doi.org/10.3390/su11174777





