Plant Biodiversity Knowledge Varies by Gender in Sustainable Amazonian Agricultural Systems Called Chacras
Abstract
:1. Introduction
2. Materials and Methods
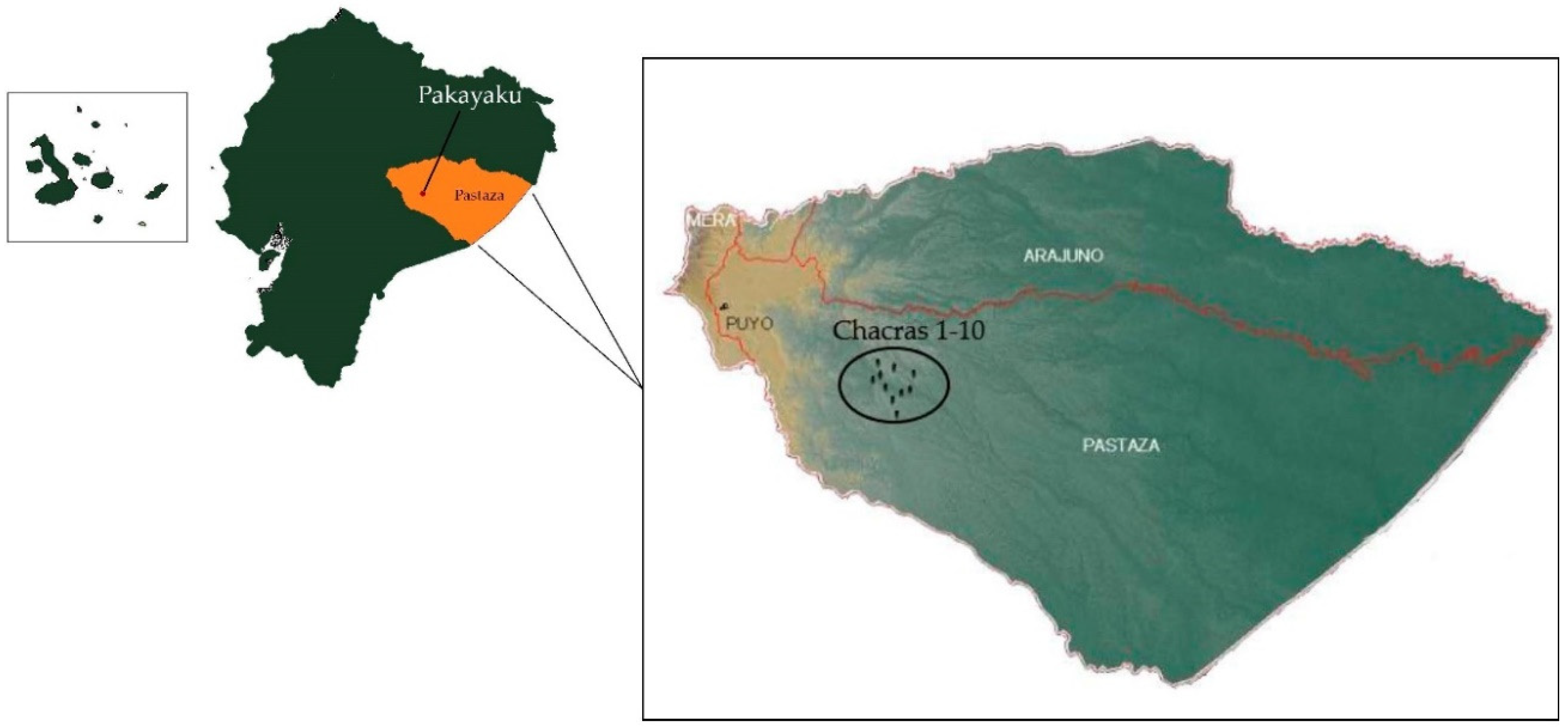
2.1. Study Area and Permissions
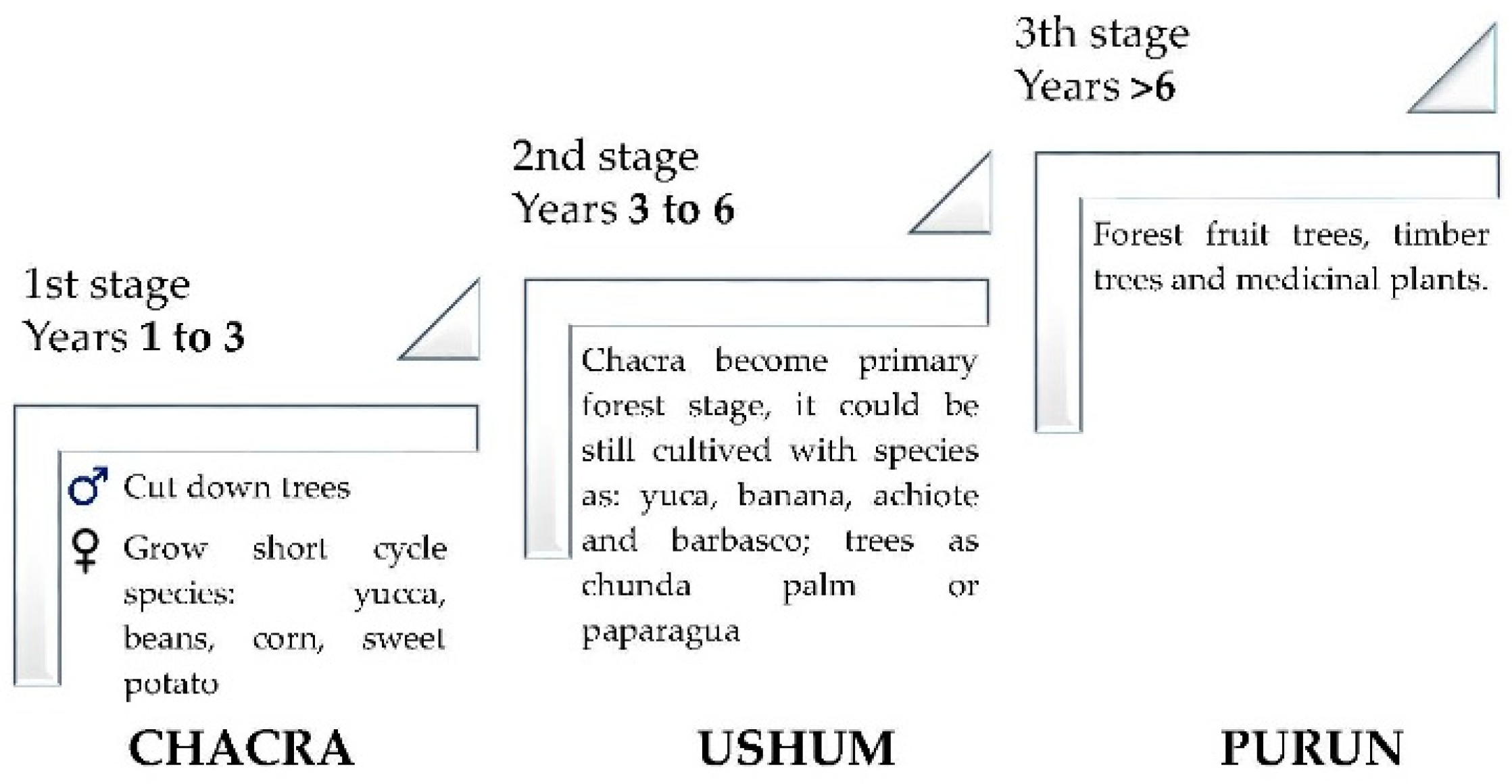
2.2. Plant Material
2.3. Workshop Design
Selection of the People and Participation
2.4. Data Digitalization
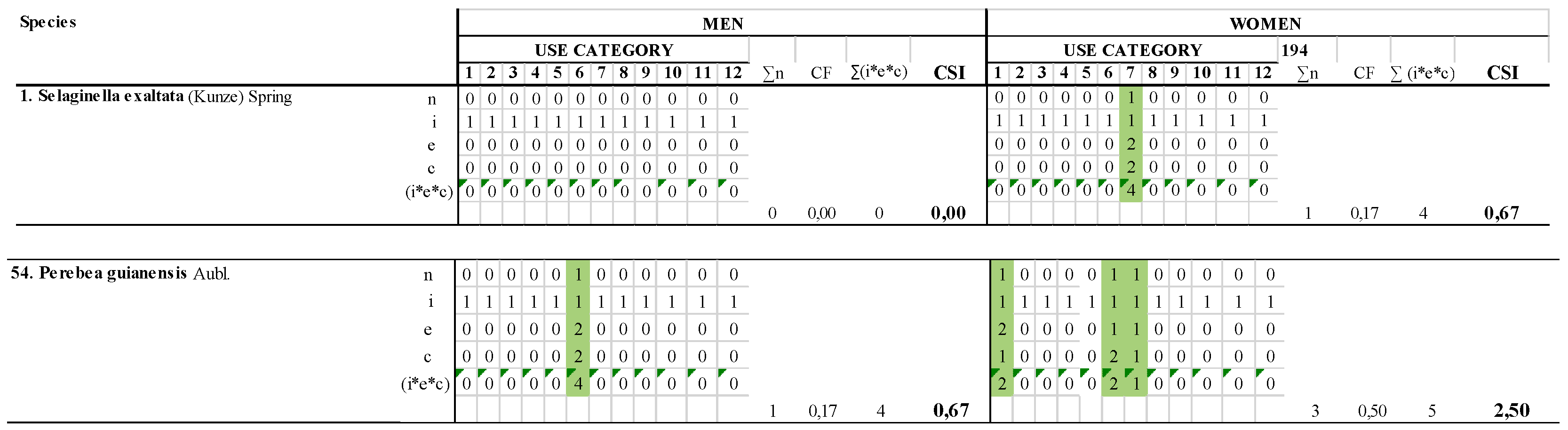
- Row i (management): Significance of the plant from an agronomical point of view. For wild plants, value, = 1. For plants referenced as cultivated in the Encyclopedia of Useful Plants of Ecuador [29] value, = 2.
- Row e (preference): Proportion of the workshop participants who selected that use category as “the most preferred”. It is expressed as per participant, not in percentage. Maximum value 2 was given to the preferred category. Non-preferred categories value = 1. Non-used categories = 0.
- Row c (frequency): Proportion of the workshop who selected that use category as “the most frequently used in the community”. It is expressed as per participant, not in percentage. Maximum value 2 was given to the selected category. Non-selected categories value = 1. Non-used categories = 0.
- Row (i*c*e): Value obtained for a category of use of the species in the workshop. Maximum value (2 × 2 × 2) = 8. Minimum value (1 × 1 × 1) = 1 Non-used categories = 0.
- Column ∑n: Minimum value 0. maximum value = 12. H = the highest value obtained in the compared workshops by one species.
- Column ∑(i*e*c): Global value of all the uses of the species. The maximum value that one species can obtain is ((2 × 2 × 2) + [11 × (2 × 1 × 1)) = 30.
2.5. Quantitative Analysis of Data: Cultural Significance Indexes (CSI) Calculations and Statistics
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Herren, H.R. The Sustainable Development Goals: Challenge or opportunity. In Farming, Food and Nature; Taylor and Francis: London, UK, 2018; pp. 171–173. [Google Scholar]
- Peña-Venegas, P.; Mazorra Valderrama, A.; Acosta Muñoz, L.E.; Pérez Rúa, M.N. Seguridad Alimentaria en Comunidades Indígenas del Amazonas: Ayer y Hoy; Instituto Sinchi: Bogotá, Colombia, 2009. [Google Scholar]
- Bioversity International. Sustainable Agriculture for Food and Nutrition Security; Bioversity International: Rome, Italy, 2011; ISBN 978-92-9043-898-4. [Google Scholar]
- Ortiz, R.; Nowak, A.; Lavado, A.; Parker, L. Food Security in the Amazon—A Report for the Amazonia Security Agenda Project; Global Canopy Foundation: Oxford, UK, 2013. [Google Scholar]
- Tapia-Armijos, M.F.; Homeier, J.; Espinosa, C.I.; Leuschner, C.; De La Cruz, M. Deforestation and forest fragmentation in south Ecuador since the 1970s—Losing a hotspot of biodiversity. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- FAO-CEPAL. Seguridad Alimentraria, Nutrición y Erradicación del Hambre, Celac 2025: Elementos Para el Debate y la Cooperación Regionales; United Nations: New York, NY, USA, 2016. [Google Scholar]
- Calero, C.J. Seguridad Alimentaria en Ecuador Desde un Enfoque de Acceso a Alimentos. Master’s Thesis, FLACSO Ecuador, Quito, Ecuador, 2011. [Google Scholar]
- Ortiz, T.P. El laberinto de la autonomía indígena en el Ecuador. Las circunscripciones territoriales indígenas en la Amazonía Central, 2010–2012. Lat. Am. Caribb. Ethn. Stud. 2015, 10, 60–86. [Google Scholar]
- COP-6-CDB UNEP/CBD/COP/6. Decisions. Available online: https://www.cbd.int/kb/record/meetingDocument/2303?RecordType=meetingDocument&Event=COP-06 (accessed on 24 June 2019).
- Ulloa, A. The Ecological Native: Indigenous Peoples’ Movements and Eco-Governmentality in Colombia; Routledge: Abingdon, UK, 2013; ISBN 9780203958674. [Google Scholar]
- Indigenous-Women-of-America. Memoria: IV Encuentro Continental de las Mujeres Indígenas de las Américas: Lima, 4–7 abril del 2004: Un Solo Continente, un Solo Espíritu; Chirapaq, Centro de Culturas Indígenas de Perú: Lima, Perú, 2004; ISBN 9873-676-11-2. [Google Scholar]
- Vera, R.R.; Cota-Sánchez, J.H.; Grijalva Olmedo, J.E.; Coq-Huelva, D.; Higuchi, A.; Alfalla-Luque, R.; Burgos-Morán, R.; Arias-Gutiérrez, R.; Panduro, A.; Mass, W.; et al. La chagra: Patrimonio colectivo de las comunidades indígenas amazónicas. Sustainability 2017, 9, 21–37. [Google Scholar] [CrossRef]
- Garí, J. Biodiversity and Indigenous Agroecology in Amazonia: The Indigenous Peoples of Pastaza. Ecol. Res. 2001, 5, 21–37. [Google Scholar]
- Santi, E. Por la Tierra, Por la Vida, Despertemos. Confrontación Entre visiones Sobre el Territorio; PPGCSPA: Sao Louis, Brazil, 2016. [Google Scholar]
- Panduro, A.; Mass, W.; Reig, M.C.; Pinedo, J.M. Chacras Amazónicas; Programa de Cooperación Hispano Peruano, Agencia Española de cooperación Internacional para el Desarrollo y Universidad de Córdoba: Iquitos, Perú, 2010; ISBN 9786124565045. [Google Scholar]
- Vera, R.R.; Cota-Sánchez, J.H.; Grijalva Olmedo, J.E. Biodiversity, dynamics, and impact of chakras on the Ecuadorian Amazon. J. Plant. Ecol. 2017, 12, 34–44. [Google Scholar] [CrossRef]
- Robert, A.V. Are women reservoirs of traditional plant knowledge? Gender, ethnobotany and globalization in northeast Brazil. Singap. J. Trop. Geogr. 2007, 28, 7–20. [Google Scholar]
- Pfeiffer, J.; Butz, R. Assessing cultural and ecological variation in ethnobiological research: The importance of gender. J. Ethnobiol. 2005, 25, 240–279. [Google Scholar] [CrossRef]
- Müller, J.G.; Boubacar, R.; Guimbo, I.D. The “How” and “Why” of Including Gender and Age in Ethnobotanical Research and Community-Based Resource Management. Ambio 2014, 28, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Howard, P.; Cuijpers, W. Gender and the Management and Conservation of Plant Biodiversity. In Encyclopaedia of Life Support Systems (EOLSS); Doelle, H.W., DaSilva, E., Eds.; EOLSS: Oxford, UK, 2002. [Google Scholar]
- Luce, D. Gender and Global Biodiversity From ’Women and Plants’ to International Law. Ph.D. Thesis, University of Ottawa, Ottawa, Canada, 2010. [Google Scholar]
- Zent, E.L. Women and Plants: Gender Relations in Biodiversity Management and Conservation. J. Ethnobiol. 2008, 25, 151–154. [Google Scholar] [CrossRef]
- Arias Gutiérrez, R.I.; González Sousa, R.; Herrera Sorzano, A.; Alemán Pérez, R.D. Diagnóstico integral de comunidades Kichwa amazónicas ecuatorianas para la elaboración de la estrategia de desarrollo sostenible. II. Indicadores socio-económicos. Centro Agrícola 2015, 42, 73–79. [Google Scholar]
- Santi Gualinga, F. Diagnóstico de la Realidad Socio-Económica de las Comunidades Kichwa de la Cuenca del Bobonaza con enfoque de Género. Bachelor’s Thesis, Universidad de Cuenca, Cuenca, Ecuador, 2015. [Google Scholar]
- GADR-Sarayaku. PODT Sarayaku. Plan. de Desarrollo y Ordenamiento Territorial de la Parroquia de Sarayakyu; GAD Pastaza: Puyo, Ecuador, 2009. [Google Scholar]
- Luzuriaga-Quichimbo, C.X. Estudio Etnobotánico en Comunidades Kichwas Amazónicas de Pastaza. Ph.D. Thesis, Universidad de Extremadura, Ecuador, Badajoz, Spain, 2017. [Google Scholar]
- Chávez, G.; Lara, R.; Moreno, M.-A. El Pueblo del Cenit.Identidad y Construcción Étnica: Informe antropológico —Jurídico Sobre los Impactos Sociales y Culturales de la Presencia de la Compañía CGC en Sarayaku; FLACSO Sede Académica de Ecuador: Quito, Ecuador, 2005. [Google Scholar]
- United-Nations Convention on Biological Diversity. Available online: https://www.cbd.int/convention/ (accessed on 24 June 2019).
- De la Torre, L.; Navarrete, H.; Muriel, P.; Marcia, M.; Balslev, H. Enciclopedia De Plantas Utiles Del Ecuador; Herbario QCA & Herbario AAU: Quito, Ecuador, 2008; Volume 1, ISBN 978-9978-77-135-8. [Google Scholar]
- Hoffman, B.; Gallaher, T. Importance indices in ethnobotany. Ethnobot. Res. Appl. 2007, 5, 201–218. [Google Scholar] [CrossRef]
- Da Silva, V.A.; Andrade, L.D.H.C.; de Albuquerque, U.P. Revising the Cultural Significance Index: The Case of the Fulni-ô in Northeastern Brazil. Field Methods 2006. [Google Scholar] [CrossRef]
- Paymal, N.; Sosa, C. Mundos Amazónicos: Pueblos y Culturas de la Amazonia Ecuatoriana; Fundación Sinchi Sach: Quito, Ecuador, 1993; ISBN 9978823654. [Google Scholar]
- Whitten, N.W. Arte, Cultura y Poder de los Canelos Quichua de la Amazonia Ecuatoriana; Departamento de Etnografía del Banco Central de Ecuador: Quito, Ecuador, 1987. [Google Scholar]
- Cerón, C.; Reyes, C.I.; Jiménez, E.D.; Simba, D.J. Plantas útiles de los Kichwa, Centro-Norte de la Amazonía Ecuatoriana. Cinchonia 2012, 12, 1–202. [Google Scholar]
- Arias-Gutierrez, R.I.; Herrera Sorzano, A.; Gonzalez Sousa, R. Amazonian indigenous settlement and local development in Pastaza, Ecuador. Noved. Población 2016, 16, 544. [Google Scholar]
- Pérez Quintana, M.; Arias Gutiérrez, R.; Sablón Cossio, N. Amazonia, Healthy Food and Rural Communities, Pastaza-Ecuador. 2017. Available online: https://www.researchgate.net/publication/312555679_Amazonia_healthy_food_and_rural_communities_Pastaza-Ecuador (accessed on 24 June 2019).
- Torres-Avilez, W.; Medeiros, P.M.D.; Albuquerque, U.P. Effect of Gender on the Knowledge of Medicinal Plants: Systematic Review and Meta-Analysis. Evidence Based Complement. Altern. Med. 2016, 2016, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
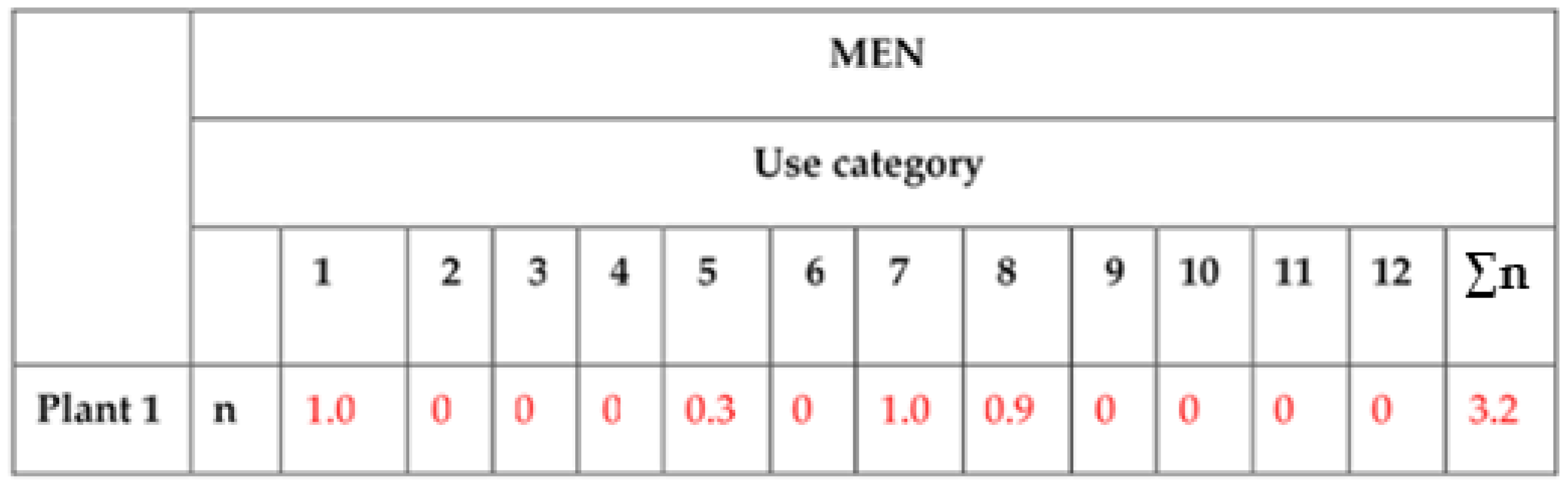
| Reference Number: Plant 1 (See List in Materials and Methods) | |||
|---|---|---|---|
| Nº People that Consider it Useful Plant: 10 Sex: Men | |||
| Category | Nº Persons Who Cite it in: | Select the Most Frequent Use (Only 1) & Sign as +F | Select the Preferred Use (Only 1) & Sign it as +P |
| 1 Food for human | 10 | +F | |
| 2 Food for animal | |||
| 3 Utensils and tools | |||
| 4 Handcrafts | |||
| 5 Construction | 3 | ||
| 6 Cultural uses | |||
| 7 Human medicine | 10 | +P | |
| 8 Veterinary uses | 9 | ||
| 9 Poisonous plants | |||
| 10 Ornamental plants | |||
| 11 Environmental uses | |||
| 12 Plants for fuel | |||
| Men | Women | |||
|---|---|---|---|---|
| ∑n | CSIm | ∑n | CSIw | |
| 1. Selaginella exaltata (Kunze) Spring | 0 | 0.00 | 1 | 0.67 |
| 2. Danaea ulei Christ | 1 | 0.67 | 0 | 0.00 |
| 3. Siparuna sp. | 2 | 1.33 | 3 | 3.00 |
| 4. Annona muricata L. | 1 | 1.33 | 2 | 3.33 |
| 5. Guatteria multinervis Wall. | 3 | 3.00 | 3 | 3.00 |
| 6. Compsoneura sprucei (A. DC.) Warb. | 1 | 2.00 | 1 | 0.67 |
| 7. Colocasia sculenta (L.) Schott | 3 | 6.00 | 3 | 6.00 |
| 8. Homalomena crinipes Engl. | 2 | 1.67 | 3 | 3.00 |
| 9. Homalomena picturata (Linden & André) Regel | 2 | 1.67 | 3 | 3.00 |
| 10. Philodendron schmidtiae Croat & Cerón | 1 | 0.67 | 2 | 1.67 |
| 11. Xanthosoma saggitifolium (L.) Schott | 2 | 1.67 | 2 | 1.67 |
| 12. Dioscorea trifida L.f. | 2 | 3.33 | 2 | 3.33 |
| 13. Carludovica palmata Ruiz & Pav. | 2 | 3.33 | 6 | 18.00 |
| 14. Cyclanthus bipartitus Poit. ex A. Rich. | 2 | 1.33 | 4 | 4.00 |
| 15. Aphandra natalia (Balslev & A.J. Hend.) Barfod | 2 | 2.67 | 4 | 8.00 |
| 16. Bactris gasipaes Kunth | 4 | 8.00 | 3 | 6.00 |
| 17. Geonoma macrostachys Mart. | 1 | 1.33 | 3 | 6.00 |
| 18. Iriartea deltoidea Ruiz & Pav. | 2 | 3.33 | 6 | 18.00 |
| 19. Mauritia flexuosa L. f. | 2 | 3.33 | 3 | 6.00 |
| 20. Oenocarpus batatua Mart. | 4 | 9.33 | 6 | 18.00 |
| 21. Ananas comosus (L.) Merr. | 0 | 0.00 | 2 | 3.33 |
| 22. Ananas lucidus Mill. | 3 | 6.00 | 2 | 1.33 |
| 23. Paspalum pilosum Lam. | 0 | 0.00 | 0 | 0.00 |
| 24. Saccharum officinarum L. | 2 | 3.33 | 2 | 3.33 |
| 25. Rhynchospora radicans (Schltdl. & Cham.) H. Pfeiff. | 0 | 0.00 | 0 | 0.00 |
| 26. Scleria melaleuca Rchb. ex Schltdl. & Cham. | 0 | 0.00 | 1 | 0.67 |
| 27. Tripogandra serrulata (Vahl) Handlos | 1 | 0.67 | 1 | 0.67 |
| 28. Costus scaber Ruiz & Pav. | 2 | 1.67 | 3 | 2.50 |
| 29. Heliconia chartacea Lane ex Barreiros | 1 | 0.67 | 1 | 0.67 |
| 30. Heliconia episcopalis Vell. | 2 | 1.67 | 2 | 1.33 |
| 31. Heliconia hirsuta L. f. | 0 | 0.00 | 1 | 0.67 |
| 32. Heliconia rostrata Ruiz & Pav. | 2 | 1.67 | 2 | 1.33 |
| 33. Heliconia shumanniana Loes. | 0 | 0.00 | 2 | 1.67 |
| 34. Heliconia velutina L. Andersson | 1 | 0.67 | 1 | 0.67 |
| 35. Calathea lutea (Aubl.) Schult. | 1 | 0.67 | 1 | 0.67 |
| 36. Musa acuminata Colla | 1 | 1.33 | 3 | 6.00 |
| 37. Zingiber officinale Roscoe | 1 | 1.33 | 2 | 3.33 |
| 38. Tetracera volubilis L. | 0 | 0.00 | 1 | 0.67 |
| 39. Garcinia macrophylla Mart. | 2 | 1.67 | 3 | 3.00 |
| 40. Alchornea triplinervia (Spreng.) Müll. Arg. | 1 | 0.67 | 1 | 0.67 |
| 41. Croton lecheri Müll. Arg. | 2 | 1.67 | 2 | 1.67 |
| 42. Manihot esculenta Crantz | 2 | 3.33 | 2 | 3.33 |
| 43. Vismia baccifera (L.) Triana & Planch. | 0 | 2.00 | 2 | 1.67 |
| 44. Lunania parviflora Spruce ex Benth. | 1 | 0.67 | 4 | 4.00 |
| 45. Bauhinia tarapotensis Benth. | 4 | 4.67 | 2 | 1.67 |
| 46. Erythrina poeppigiana (Walp.) O.F. Cook | 1 | 0.67 | 3 | 3.00 |
| 47. Inga alba (Sw.) Willd. | 3 | 2.50 | 2 | 1.33 |
| 48. Inga auristellae Harms | 3 | 3.00 | 2 | 1.33 |
| 49. Inga edulis Mart. | 2 | 3.33 | 3 | 6.00 |
| 50. Inga sapindoides Willd. | 3 | 2.50 | 2 | 1.67 |
| 51. Lonchocarpus utilis A.C. Sm. | 1 | 1.33 | 0 | 0.00 |
| 52. Piptadenia sp. | 1 | 0.67 | 2 | 1.67 |
| 53. Clarisia racemosa Ruiz & Pav. | 2 | 1.67 | 2 | 1.67 |
| 54. Perebea guianensis Aubl. | 1 | 0.67 | 3 | 2.50 |
| 55. Perebea xanthochyma H. Karst. | 2 | 1.67 | 2 | 1.67 |
| 56. Cecropia engleriana Snethl. | 0 | 0.00 | 1 | 0.67 |
| 57. Cecropia ficifolia Warb. ex Snethl. | 1 | 0.67 | 2 | 2.00 |
| 58. Aciotis purpurascens (Aubl.) Triana | 0 | 0.00 | 1 | 0.67 |
| 59. Bellucia pentamera Naudin | 1 | 0.67 | 1 | 0.67 |
| 60. Clidemia dentata Pav. ex D. Don | 1 | 0.67 | 1 | 0.67 |
| 61. Clidemia octona (Bonpl.) L.O. Williams | 1 | 0.00 | 1 | 0.67 |
| 62. Graffenrieda gracilis (Triana) L.O. Williams | 1 | 0.00 | 1 | 0.67 |
| 63. Leandra catequensis Gleason | 0 | 0.00 | 1 | 0.67 |
| 64. Miconia aureoides Cogn. | 1 | 0.67 | 2 | 1.33 |
| 65. Miconia paleacea Cogn. | 1 | 0.67 | 1 | 0.67 |
| 66. Miconia punctata (Desr.) D. Don ex DC. | 1 | 0.67 | 3 | 2.50 |
| 67. Bixa orellana L. | 4 | 9.33 | 4 | 9.33 |
| 68. Apeiba aspera Aubl. | 1 | 0.67 | 1 | 0.67 |
| 69. Ochroma pyramidale (Cav. ex Lam.) Urb. | 2 | 1.67 | 5 | 7.50 |
| 70. Theobroma cacao L. | 2 | 2.67 | 2 | 3.33 |
| 71. Theobroma subincanum Mart. | 3 | 6.00 | 3 | 6.00 |
| 72. Carica papaya L. | 2 | 3.33 | 3 | 5.00 |
| 73. Minquartia guianensis Aubl. | 1 | 0.67 | 2 | 1.33 |
| 74. Agonandra sp. | 1 | 0.67 | 3 | 3.00 |
| 75. Cyathula prostrata (L.) Blume | 1 | 0.67 | 0 | 0.00 |
| 76. Phytolacca sp. | 0 | 0.00 | 2 | 1.33 |
| 77. Gustavia longifolia Poepp. ex O. Berg | 1 | 1.33 | 1 | 1.33 |
| 78. Pouteria caimito (Ruiz & Pav.) Radlk. | 3 | 6.00 | 2 | 3.33 |
| 79. Capsicum sp. | 3 | 6.00 | 4 | 9.33 |
| 80. Nicotiana tabacum L. | 1 | 1.33 | 1 | 1.33 |
| 81. Solanum quitoense Lam. | 2 | 3.33 | 3 | 6.00 |
| 82. Witheringa solanacea L’Hér. | 0 | 0.00 | 1 | 0.67 |
| 83. Aspidosperma excelsum Benth. | 1 | 0.67 | 1 | 0.67 |
| 84. Chelonanthus alatus (Aubl.) Pulle | 0 | 0.00 | 1 | 0.67 |
| 85. Spermacoce exilis (L.O. Williams) C.D. Adams | 0 | 0.00 | 1 | 0.67 |
| 86. Spermacoce remota Lam. | 1 | 0.67 | 1 | 0.67 |
| 87. Uncaria guianensis (Aubl.) J.F. Gmel. | 1 | 0.67 | 3 | 4.00 |
| 88. Warszewiczia coccinea (Vahl) Klotzsch | 1 | 1.33 | 2 | 2.67 |
| 89. Justicia comata (L.) Lam. | 0 | 0.00 | 1 | 0.67 |
| 90. Jacaranda copaia (Aubl.) D. Don | 2 | 1.33 | 2 | 1.33 |
| 91. Besleria sp. | 0 | 0.00 | 1 | 0.67 |
| 92. Hyptis obtusiflora C. Presl ex Benth. | 0 | 0.00 | 1 | 0.67 |
| 93. Cordia alliodora (Ruiz & Pav.) Oken | 1 | 0.67 | 1 | 0.67 |
| 94. Adenostemma fosbergii R.M. King & H. Rob. | 1 | 0.67 | 1 | 0.67 |
| 95. Conyza sumatrensis (Retz.) E. Walker | 0 | 0.00 | 1 | 0.67 |
| 96. Erechtites hieraciifolius (L.) Raf. ex DC. | 1 | 0.67 | 1 | 0.67 |
| 97. Piptocoma discolor (Kunth) Pruski | 4 | 4.00 | 3 | 2.50 |
| TOTAL | 135 | 164.67 | 294 | 267.50 |
| CSIm | CSIw | CSIm + CSIw | |
|---|---|---|---|
| +20. Oenocarpus batatua Mart. | 9.33 | 18.00 | 27.33 |
| +13. Carludovica palmata Ruiz & Pav. | 3.33 | 18.00 | 21.33 |
| +18. Iriartea deltoidea Ruiz & Pav. | 3.33 | 18.00 | 21.33 |
| +67. Bixa orellana L. | 9.33 | 9.33 | 18.67 |
| +79. Capsicum sp. | 6.00 | 9.33 | 15.33 |
| +16. Bactris gasipaes Kunth | 8.00 | 6.00 | 14.00 |
| +7. Colocasia sculenta (L.) Schott | 6.00 | 6.00 | 12.00 |
| +71. Theobroma subincanum Mart. | 6.00 | 6.00 | 12.00 |
| +15. Aphandra natalia (Balslev & A.J. Hend.) Barfod | 2.67 | 8.00 | 10.67 |
| +78. Pouteria caimito (Ruiz & Pav.) Radlk. | 6.00 | 3.33 | 9.33 |
| +19. Mauritia flexuosa L. f. | 3.33 | 6.00 | 9.33 |
| +49. Inga edulis Mart. | 3.33 | 6.00 | 9.33 |
| +81. Solanum quitoense Lam. | 3.33 | 6.00 | 9.33 |
| +69. Ochroma pyramidale (Cav. ex Lam.) Urb. | 1.67 | 7.50 | 9.17 |
| +72. Carica papaya L. | 3.33 | 5.00 | 8.33 |
| 22. Ananas lucidus Mill. | 6.00 | 1.33 | 7.33 |
| +17. Geonoma macrostachys Mart. | 1.33 | 6.00 | 7.33 |
| +36. Musa acuminata Colla | 1.33 | 6.00 | 7.33 |
| 12. Dioscorea trifida L.f. | 3.33 | 3.33 | 6.67 |
| +24. Saccharum officinarum L. | 3.33 | 3.33 | 6.67 |
| +42. Manihot esculenta Crantz | 3.33 | 3.33 | 6.67 |
| 97. Piptocoma discolor (Kunth) Pruski | 4.00 | 2.50 | 6.50 |
| 45. Bauhinia tarapotensis Benth. | 4.67 | 1.67 | 6.33 |
| 5. Guatteria multinervis Wall | 3.00 | 3.00 | 6.00 |
| +70. Theobroma cacao L. | 2.67 | 3.33 | 6.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luzuriaga-Quichimbo, C.X.; Hernández del Barco, M.; Blanco-Salas, J.; Cerón-Martínez, C.E.; Ruiz-Téllez, T. Plant Biodiversity Knowledge Varies by Gender in Sustainable Amazonian Agricultural Systems Called Chacras. Sustainability 2019, 11, 4211. https://doi.org/10.3390/su11154211
Luzuriaga-Quichimbo CX, Hernández del Barco M, Blanco-Salas J, Cerón-Martínez CE, Ruiz-Téllez T. Plant Biodiversity Knowledge Varies by Gender in Sustainable Amazonian Agricultural Systems Called Chacras. Sustainability. 2019; 11(15):4211. https://doi.org/10.3390/su11154211
Chicago/Turabian StyleLuzuriaga-Quichimbo, Carmen X., Míriam Hernández del Barco, José Blanco-Salas, Carlos E. Cerón-Martínez, and Trinidad Ruiz-Téllez. 2019. "Plant Biodiversity Knowledge Varies by Gender in Sustainable Amazonian Agricultural Systems Called Chacras" Sustainability 11, no. 15: 4211. https://doi.org/10.3390/su11154211
APA StyleLuzuriaga-Quichimbo, C. X., Hernández del Barco, M., Blanco-Salas, J., Cerón-Martínez, C. E., & Ruiz-Téllez, T. (2019). Plant Biodiversity Knowledge Varies by Gender in Sustainable Amazonian Agricultural Systems Called Chacras. Sustainability, 11(15), 4211. https://doi.org/10.3390/su11154211








